Abstract
The piwi/argonaute family of proteins is involved in key developmental processes such as stem cell maintenance and axis specification through molecular mechanisms that may involve RNA silencing. Here we report on the cloning and characterization of the sea urchin piwi/argonaute family member seawi. Seawi is a major component of microtubule-ribonucleoprotein (MT-RNP) complexes isolated from two different species of sea urchin, Strongylocentrotus purpuratus and Paracentrotus lividus. Seawi co-isolates with purified ribosomes, cosediments with 80S ribosomes in sucrose density gradients, and binds microtubules. Seawi possesses the RNA binding motif common to piwi family members and binds P. lividus bep4 mRNA, a transcript that co-isolates with MT-RNP complexes and whose translation product has been shown to play a role in patterning the animal–vegetal axis. Indirect immunofluorescence studies localized seawi to the cortex of unfertilized eggs within granule-like particles, the mitotic spindle during cell division, and the small micromeres where its levels were enriched during the early cleavage stage. Lastly, we discuss how seawi, as a piwi/argonaute family member, may play a fundamentally important role in sea urchin animal–vegetal axis formation and stem cell maintenance.
Keywords: axis specification, piwi, argonaute, mRNA localization, microtubule, mRNP
INTRODUCTION
Spatial segregation of “morphogenic determinants” within oocytes and eggs plays a fundamentally important role in the specification of cell fate during early development (Wilson 1925; Davidson 1986). In many organisms, the cortex of unfertilized eggs/oocytes serves as a repository of spatiotemporal information by sequestering translationally repressed RNP complexes containing specific mRNAs and ribosomal components (Sardet et al. 2002). Biochemical and morphological studies indicate that segregation of localized mRNAs often involves assemblages of localized mRNAs, polysomes, and accessory proteins such as motors, adaptors, and/or RNA binding proteins that form granule-like ribonucleoprotein structures that colocalize with cytoskeletal elements (Barbarese et al. 1995; Pokrywka and Stephenson 1995; Knowles et al. 1996; Lipshitz and Smibert 2000).
Unfertilized sea urchin eggs possess a preexisting, maternally specified gradient of morphogen that sets the future orientation of the embryonic animal–vegetal axis (Runnstrom 1929). During early cleavage stages three distinct cell fate domains are established along the animal–vegetal axis in the sea urchin embryo. Near the animal pole, orthologs of the transcription factors SoxB1, SoxB2, and Ets4 exhibit a nuclear localization pattern in cells destined to form ectoderm (Angerer and Angerer 2000). Near the vegetal pole, β-catenin is localized to the nuclei of cells destined to become coelomic and skeletogenic mesoderm (Logan et al. 1999). In the cells between these two regions both transcriptional systems are present, resulting in the production of mesodermal, endodermal, and ectodermal cell types (Angerer and Angerer 2000; Brandhorst and Klein 2002). During the early cleavage stage of sea urchin embryogenesis, these regulatory pathways are activated cell autonomously, suggesting that the molecular factors needed for early pattern development are maternally inherited.
In Strongylocentrotus purpuratus eggs and two-cell embryos, a reconstituted microtubule-ribonucleoprotein (MT-RNP) complex consisting of ribosomes, microtubules, mRNA, 107-kDa major vault protein, 77-kDa EMAP, 80-and 66-kDa poly-A binding proteins, and an uncharacterized 100-kDa protein was isolated by temperature- and pH-dependent polymerization cycling of MTs in cell extracts (Suprenant et al. 1989; Hamill et al. 1994; Hamill and Suprenant 1997). By electron microscopy, the MT-RNP complexes consisted of microtubule arrays studded with ribosomes that appeared to be connected via a salt-extractable, protease-sensitive stalk (Hamill et al. 1994). Similar studding of ribosomes on MTs has been reported to occur in vivo along the MT arrays of the mitotic spindle (Hirokawa et al. 1985; Suprenant et al. 1989) and ciliary platform rootlets of epithelial cells at the primary mesenchyme blastula stage of sea urchin embryogenesis (Gibbins et al. 1969).
When isolated by phenol/chloroform extraction, the mRNA associated with the MT-RNP complex is translationally competent, yielding a specific set of polypeptides by in vitro translation (Hamill et al. 1994). When isolated MT-RNP complexes were placed into the same in vitro translation system, the associated mRNAs failed to translate into protein (Hamill et al. 1994), suggesting the complex existed in a translationally arrested state. MT-RNP complexes isolated from the sea urchin Paracentrotus lividus contain bep4 mRNA. Bep4 mRNA is asymmetrically segregated in unfertilized eggs and embryos in a microtubule-dependent manner (Romancino et al. 1998; Romancino and DiCarlo 1999) and Bep4 protein is functionally linked to determination of the animal–vegetal axis (Romancino et al. 2001). The aforementioned are hallmark properties for a cellular component that functions in establishment and maintenance of the animal–vegetal axis during sea urchin development.
In this report, we characterize seawi—a sea urchin 100-kDa member of the piwi/argonaute family of proteins. Seawi is a component of MT-RNP complexes possessing a subset of mRNA, including one (bep4) known to have a role in axis formation. Identification of seawi association with sea urchin MT-RNP complexes establishes and extends our understanding of the biochemical basis of how piwi/argonaute family members may function in the spatial and temporal translation of specific mRNAs during development (Carmell et al. 2002). Consequently, the findings reported here provide insight into the potential cellular mechanisms involved in specifying axis formation during early sea urchin development.
RESULTS
Cloning of sea urchin 100-kDa protein by screening a cDNA library
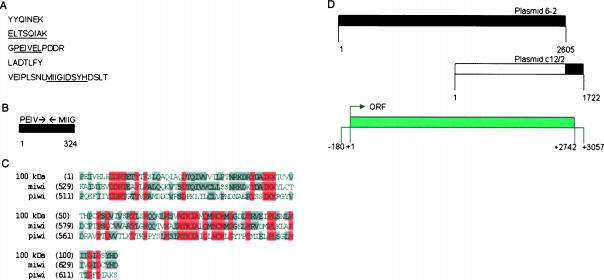
Electrophoretically purified 100-kDa tubulin dimer binding protein was enzymatically digested and microsequences from five different peptides were obtained (Fig. 1A ▶). Degenerate sense and antisense primers for RT-PCR were designed based on the peptide microsequences from three of the five peptides (Fig. 1A ▶, underlined residues). A cDNA of 1730 bp was amplified and partially sequenced using degenerate primers for ELTSQIAK and MIIGIDSYH. Six-frame translation of the obtained sequence identified the presence of the GPEIV microsequence nested between ELTS and MIIG. Specific primers to the PEIVEL portion of the GPEIV microsequence and the MIIGIDSYH portion of the VEIP microsequence were synthesized and used in RT-PCR to amplify a 324-bp product from total egg RNA (Fig. 1B ▶). Searching the GenBank database using the translated sequence of the 324-bp fragment identified 46% and 36% identity between the amplified fragment and the corresponding regions of miwi and piwi, respectively (Fig. 1C ▶).
FIGURE 1.
Molecular cloning of seawi. (A) The five peptide microsequences obtained following proteolytic degradation of the 100-kDa protein. The underlined residues highlight the sequences used for synthesis of degenerate primers. (B) Schematic representation of the result of RT-PCR of total egg RNA with PEIV and MIIG primers with the orientation of the primers shown with arrows. (C) Alignment of the translated sequence of the 324-bp product with piwi and miwi generated using Vector NTI Suite 6.0 software (InforMax, Inc.) AlignX program. Residues identical in all three sequences are shown in red while residues identical in two of the sequences are shown in gray. (D) Schematic representation of the products from cDNA library screening. The black areas in plasmid 6-2 and plasmid c12/2 show the areas used to determine the consensus sequence of the 100-kDa protein. The bottom diagram shows key features of the consensus sequence.
A digoxygenin (DIG)-labeled probe complementary to the amplified 324-bp fragment was prepared and used to screen an S. purpuratus oocyte λ ZAP cDNA library. The initial screen identified plasmid 6-2 that contained a 2605-bp insert (Fig. 1D ▶; GenBank accession no. AY014900) that upon sequence analysis revealed the presence of a consensus Kozak sequence surrounding a start codon followed by an uninterrupted open reading frame with no downstream stop codon. A second DIG-labeled probe was synthesized using primer sequences derived from the 3′ end of plasmid 6-2 (specific sequences in Materials and Methods). Screening the cDNA library with the second probe resulted in the isolation of plasmid c12/2 (GenBank accession no. AY014901). Plasmid c12/2 contained a 1722-bp insert containing a long open reading frame with a stop codon near its 3′ end and had over 1000 bp of overlapping sequence with plasmid 6-2 (Fig. 1D ▶). Splicing together the plasmid sequences produced a single contiguous sequence of 3057 bp containing a 2562-bp open reading frame (Fig. 1D ▶) coding for a 854-amino-acid protein with a calculated molecular weight of 96,715 Da (Fig. 2A ▶). As anticipated, all five of the peptide microsequences identified from the digests of the 100-kDa protein were present within the deduced amino acid sequence of the cloned 100-kDa protein (Fig. 2A ▶, see asterisks).
FIGURE 2.
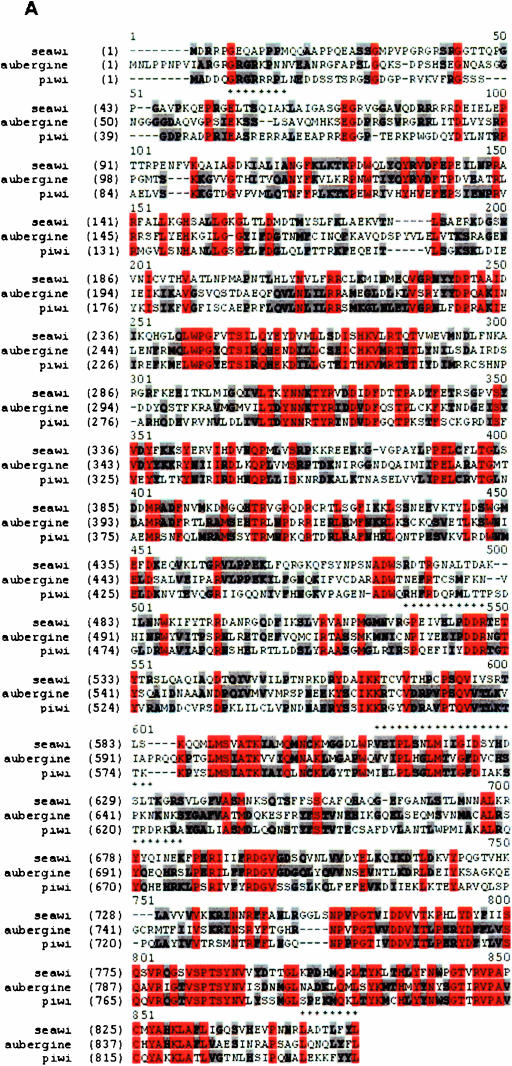
Classification of the 100-kDa protein (seawi) as a member of the piwi family. (A) Alignment of the full-length sequence of the 100-kDa protein with piwi and aubergine. Residues identical in all three sequences are shown in red while residues identical in two of the sequences are shown in gray. The asterisks above the seawi sequence identify the five peptide microsequences. (B) Domain organization of seawi. The level of identity of each of the domains with the corresponding regions from piwi and aubergine are shown. The asterisks above the piwi domain within seawi identify the putative mRNA binding domain based on sequence homology with miwi’s mRNA binding sequence. (C) Phylogenetic analysis generated using Vector NTI Suite Version 6.0 software AlignX program. Note, seawi clusters most closely to hiwi and miwi.
Sequence analysis of sea urchin 100-kDa protein
A search of the GenBank database revealed that the cloned sequences were most closely related to sequences from members of the piwi/argonaute family of proteins. Overall, the amino acid sequence of the 100-kDa protein was 35.7% identical and 52.5% similar to aubergine and 35.6% identical and 53.3% similar to piwi (Fig. 2A ▶). Analysis of the 100-kDa protein’s deduced amino acid sequence using the Pfam database (St. Louis, MO) identified the presence of PAZ and piwi domains that are signature motifs (Cerutti et al. 2000) for the piwi/argonaute family of proteins (Fig. 2B ▶). The PAZ domain extended from residues 236 to 402 and the piwi domain was located at the C terminus at residues 547–840 (Fig. 2B ▶). PAZ domains of both piwi and argonaute family members have been shown to preferentially bind RNA, in particular siRNAs (Lingel et al. 2003, 2004; Ma et al. 2004). Kuramochi-Miyagawa et al. (2001) identified a 138-amino-acid stretch located at the 5′ end of the mouse piwi domain (amino acids 541–678) that is necessary and sufficient for miwi’s RNA binding property. Interestingly, the corresponding stretch (amino acids 532–669; Fig. 2B ▶, see asterisks) for the 100-kDa protein shares 55% identity and 69% similarity with the mouse miwi RNA binding sequence; such a significant level of identity is probably indicative of functional equivalence as an RNA binding region in the 100-kDa protein.
Phylogenetic analysis of 16 members of the piwi/argonaute family placed the 100-kDa protein’s amino acid sequence as being closely related to mouse miwi and human hiwi proteins (Fig. 2C ▶), which cluster together within the piwi subgroup of the piwi/argonaute family. In keeping with the piwi nomenclature, the 100-kDa sea urchin protein was named seawi, for sea urchin piwi (GenBank accession no. AY014899).
Seawi is a major component of S. purpuratus MT-RNP complexes
Sea urchin MT-RNP complexes, prepared by multiple rounds of temperature- and pH-dependent cycling, contain a prominent 100-kDa protein (Fig. 3A ▶, lane 1) that appears to comigrate in SDS-PAGE with purified seawi samples (Fig. 3A ▶, lane 2). Rabbit antisera were prepared using purified seawi and the 100-kDa MT-RNP protein and used for immunoblot analysis to determine whether seawi is the 100-kDa protein in sea urchin MT-RNP complexes. Both anti-seawi (serum 4I4) and anti-100 kDa (serum A59) polyclonal sera bound to a single band of 100 kDa in protein samples of S. purpuratus eggs (Fig. 3B ▶, lanes 3,5). There was no detectable binding of preimmune sera for 4I4 or A59 to any specific protein band in total egg protein (Fig. 3B ▶, lanes 4 and 6, respectively) verifying the monospecificity of the two antisera.
FIGURE 3.
Seawi is the 100-kDa protein of S. purpuratus MT-RNP complexes. (A) SDS-PAGE gel of MT-RNP complex proteins and purified seawi. (Lane 1) Isolated MT-RNP complex. (Lane 2) Purified seawi protein. (B) Characterization of seawi antisera. (Lane 1) Bio-Rad prestained high range SDS-PAGE standards (molecular weights are the same as those shown in A). (Lane 2) SDS-PAGE of total protein from S. purpuratus egg. (Lane 3) Immunoblot of total egg protein with anti-100-kDa protein polyclonal antiserum (4I4). (Lane 4) Immunoblot of total egg protein with preimmune serum for 4I4. (Lane 5) Immunoblot of total egg protein with anti-100-kDa MT-RNP complex protein polyclonal antiserum (A59). (Lane 6) Immunoblot of total egg protein with preimmune serum for A59. (C) Companion immunoblots to the SDS-PAGE of the MT-RNP complex (left lane) and purified seawi (right lane) shown in Panel A. Blots were probed using either anti-100-kDa protein polyclonal antiserum (4I4) or anti-100-kDa MT-RNP complex protein polyclonal antiserum (A59); only the relevant portions of the blots are shown. Each antiserum binds to both of the 100-kDa proteins, thus establishing their immuno-relatedness.
To further assess the relatedness of the two 100-kDa proteins, reciprocal immunoblots were performed using the monospecific antisera generated against the respective 100-kDa polypeptides. Polyclonal antiserum 4I4 (raised against purified seawi) recognized both 100-kDa proteins when used to probe a blot of the MT-RNP complex proteins and purified seawi transferred to nitrocellulose (Fig. 3C ▶). Polyclonal antiserum A59 (prepared against the MT-RNP 100-kDa protein) also recognized both 100-kDa proteins (Fig. 3C ▶). Additionally, both antisera crossreacted with a poly-peptide of the expected molecular weight in lysates from bacteria overexpressing recombinant seawi from plasmid 6-2 but not in lysates from non-plasmid-carrying bacteria (data not shown). Combined, the above data establish that the two 100-kDa proteins are immunologically related and that the 100-kDa protein in MT-RNP complexes is seawi.
Seawi interacts with intact ribosomes
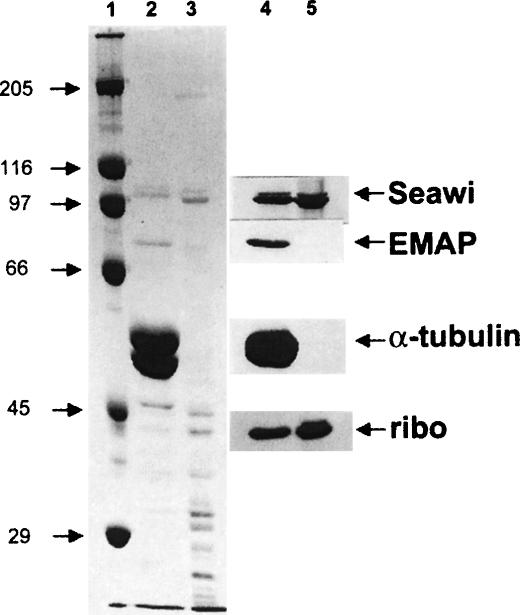
Preparations of sea urchin ribosomes, isolated by preparative sucrose step-gradients, contain a 100-kDa polypeptide that comigrates with the seawi polypeptide in MT-RNP complexes (Fig. 4 ▶, cf. lanes 2 and 3). MT-RNP complexes and ribosome preparations were immunoblotted using antibodies raised against seawi, EMAP, alpha-tubulin, and a 40-kDa protein of the ribosomal 40S subunit. Anti-seawi antibodies crossreacted strongly with the 100-kDa protein present in both MT-RNP and ribosome preparations (Fig. 4 ▶, lanes 4,5). Neither EMAP nor tubulin were detected in preparations of ribosomes (Fig. 4 ▶, lane 5), indicating that the association of seawi with biochemically purified ribosomes was not due to nonspecific association with contaminating MT-RNP complex proteins. As expected, antibodies against the 40-kDa protein of the ribosomal 40S subunit recognized a protein in both the MT-RNP preparation and the purified ribosome preparation.
FIGURE 4.
Seawi is a component of purified ribosome preparations. Coomassie blue stained gel of S. purpuratus MT-RNP complex and purified ribosome preparation are shown in lanes 2 and 3, respectively. Companion immunoblots (MT-RNP complex, lane 4; purified ribosome preparation, lane 5) were probed using antisera against the 100-kDa protein/seawi (A59), EMAP, α-tubulin, and a 40-kDa component of ribosomes (ribo). Molecular weight standards are shown in lane 1.
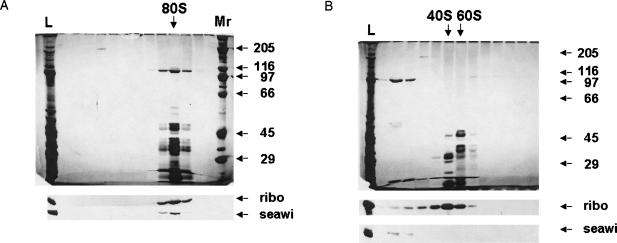
To further establish the specific association of seawi with ribosomes, preparations of isolated ribosomes were sedimented through linear 15–40% sucrose gradients and the resultant gradient fractions were analyzed by SDS-PAGE and immunoblotting. Using salt conditions (80 mM KCl) that maintained the assembly of 80S ribosomes, seawi sedimented in the same gradient fractions as 80S ribosomes (Fig. 5A ▶). When the entire gradient was examined by immunoblotting using anti-seawi antibodies, seawi was only detected in the fractions that contained the 40-kDa ribosomal protein, suggesting a physical association between seawi and intact 80S ribosomes (Fig. 5A ▶). To further test this possibility, ribosome preparations were placed into buffer containing 500 mM KCl and then subjected to sedimentation analysis. Under these conditions, seawi sedimented toward the top of the gradient well removed from the gradient fractions that contain 40 or 60S ribosomes (Fig. 5B ▶). Immunoblotting with anti-seawi and anti-40-kDa ribosomal protein antibodies confirmed this conclusion (Fig. 5B ▶). Thus, seawi is not only a major component of MT-RNP complexes but is also co-isolated with preparations of purified sea urchin egg ribosomes.
FIGURE 5.
Sucrose density centrifugation analysis of S. purpuratus ribosome preparations. (A) Silver stained SDS-PAGE gel of the results of sucrose gradient centrifugation of purified egg ribosomes (lane L) with the corresponding regions of the companion immunoblots probed with A59 antiserum (seawi) and anti-40-kDa ribosomal protein (ribo). The position of the 80S ribosome is indicated, demonstrating that seawi and the 80S ribosome cosediment. The top of the gradient is at the left. (B) Silver stained SDS-PAGE gel of the results of sucrose gradient centrifugation of purified egg ribosomes following high salt treatment. The arrows indicate the positions of the dissociated 40S and 60S ribosomal subunits. Notice that seawi fails to cosediment with either of the ribosomal subunits under these conditions.
Seawi is a component of P. lividus MT-RNP complexes
A common functional role among many piwi/argonaute family members is their participation in axis formation and/or stem cell maintenance through a pathway reliant upon interaction with RNA (Carmell et al. 2002). Hamill et al. (1994) demonstrated that MT-RNP complexes appear to contain a specific subset of mRNAs that are not available for in vitro translation when associated with the MT-RNP complex. SDS-PAGE comparison of the protein profiles of MT-RNP complexes prepared from P. lividus and S. purpuratus indicates that in addition to tubulin they share a remarkably similar protein composition with major bands at 107, 100, and 77 kDa (Fig. 6 ▶, cf. lanes 1 and 2). Immunoblot analysis using anti-seawi antibodies was performed on P. lividus MT-RNP complexes (Romancino et al. 1998) to determine whether the 100-kDa protein in the complexes was seawi. Anti-seawi antibodies crossreacted with a single band at 100 kDa indicating that seawi is a component of P. lividus MT-RNP complexes (Fig. 6 ▶, lane 3). Interestingly, P. lividus MT-RNP complexes are known to carry bep4 mRNA, whose translation product, Bep4, has been shown to be differentially expressed and is believed to play an important role in P. lividus developmental axis formation (Romancino et al. 1998, 2001). Recently, we identified the association of bep4 mRNA with MT-RNP complexes isolated from S. purpuratus eggs (D.J. Desai, S.A. Seipel, A.J. Rodriquez, and E.M. Bonder, unpubl. observations).
FIGURE 6.
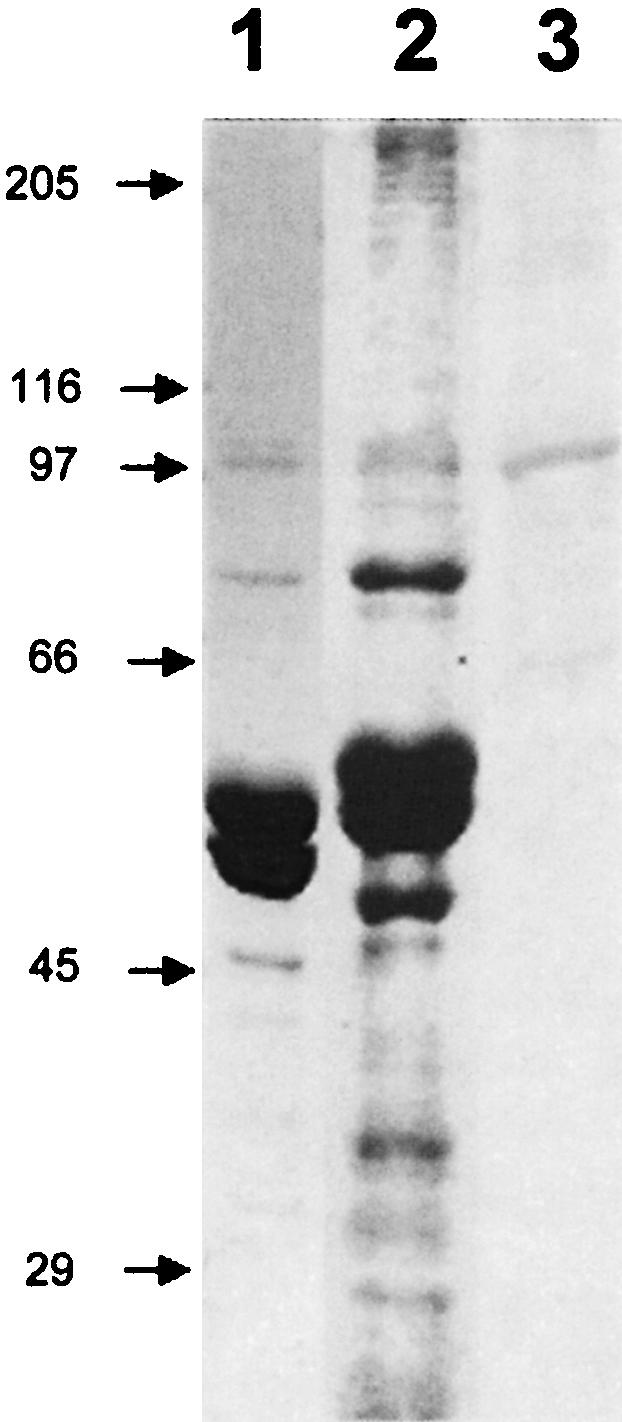
Comparison of S. purpuratus and P. lividus MT-RNP complexes. Coomassie blue stained SDS-PAGE gels of S. purpuratus (lane 1) and P. lividus (lane 2) MT-RNP complex preparation. (Lane 3) Result of an immunoblot of the P. lividus MT-RNP complex probed with anti-seawi antibodies (A59).
Identification of seawi binding to bep4 message
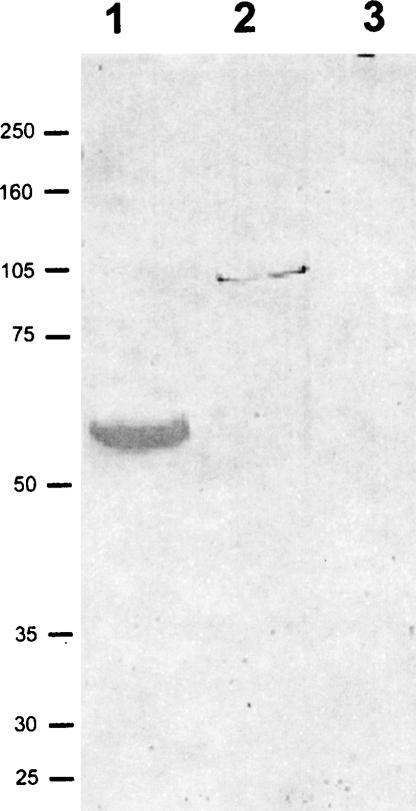
Previously, Montana et al. (1997, 1998) demonstrated that LP54, an RNA binding protein present in MT-RNP, was the only protein within MT-RNP complexes capable of binding the 3′ UTR of bep3 RNA. Since seawi possesses both a PAZ and a piwi domain RNA binding motif (Kuramochi-Miyagawa et al. 2001; Lingel et al. 2003 Lingel et al. 2004; Yan et al. 2003; Ma et al. 2004), we sought to determine whether seawi binds directly to bep4 mRNA, which was previously shown to co-isolate with MT-RNP complexes (Romancino et al. 1998). P. lividus LP54, seawi, and fibronectin were transferred to nitrocellulose and the blotted protein was probed with DIG-labeled bep4 mRNA (Montana et al. 1997, 1998). Using this blotting method, both LP54 (Fig. 7 ▶, lane 1; positive control) and seawi (Fig. 7 ▶, lane 2) exhibited binding to bep4 message while the negative fibronectin control (Fig. 7 ▶, lane 3) demonstrated no binding to bep4 message.
FIGURE 7.
Seawi binds bep4 mRNAs. A Northwestern blot of purified LP54 (lane 1), purified seawi (lane 2), and purified fibronectin (lane 3) probed with full-length bep4 message. Notice that LP54 (previously shown to be an mRNA binding protein); (Montana et al. 1997) and seawi bind bep4 message while the control protein, fibronectin, does not.
Seawi’s mRNA and protein expression is developmentally regulated
Relative RT-PCR and immunoblotting were used to examine the developmental expression pattern of seawi protein and mRNA during early S. purpuratus embryogenesis. PCR primer pairs for seawi and 18S rRNA were PCR amplified through 21 cycles, which corresponded to the linear portion of the amplification cycle for the seawi product. The amplified product was normalized relative to the 18S rRNA product and this ratio was compared to the equivalent value obtained for unfertilized eggs. To examine levels of protein across early embryogenesis, equal concentrations of total embryonic protein from staged embryos from a single SDS-PAGE gel were transferred to nitrocellulose, probed with anti-seawi antibodies, and the results quantitated densitometrically relative to unfertilized eggs.
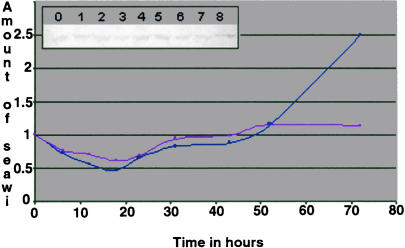
Both seawi mRNA and protein are maternally stored in the unfertilized egg. After fertilization, seawi mRNA levels gradually declined during the initial cleavage stages followed by a relatively rapid burst of expression that returned mRNA levels back to those of the unfertilized egg by the mesenchyme blastula stage (Fig. 8 ▶). A second rise in message level was detected at the prism stage, by which time the embryo has formed its basic body plan that will carry it up to metamorphosis. The pattern for protein expression essentially corresponded to the mRNA profile with temporally overlapping decreases and increases in the protein levels across development (Fig. 8 ▶).
FIGURE 8.
Developmental expression profile of seawi. Levels of seawi message (red) and protein (blue) present at different stages of development were normalized to the amount of message or protein found in the unfertilized eggs. (inset) Representative immunoblot of total S. purpuratus protein probed with A59 antiserum. The following stages were investigated: unfertilized eggs (lane 0); 6 h, 4 divisions (lane 1); 12 h, 8 divisions (lane 2); 18 h, late blastula (lane 3); 23 h, primary mesenchyme ingression (lane 4); 31 h, gastrula (lane 5); 42 h, archenteron formation (lane 6); 52 h, prism (lane 7); 72 h, pluteus larva (lane 8).
Immunolocalization of seawi
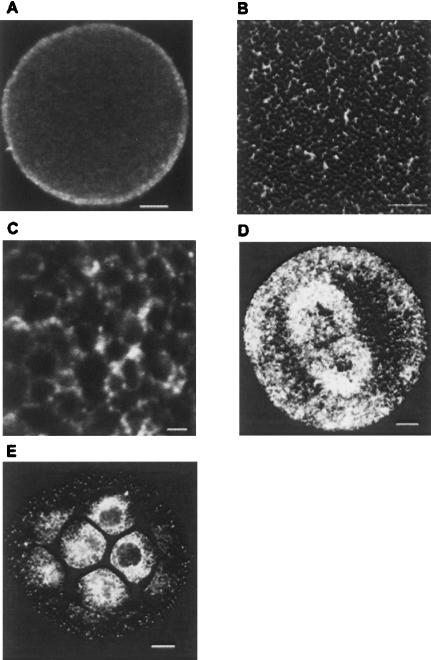
Whole S. purpuratus eggs were stained for indirect immunofluorescence using anti-seawi antibodies and laser scanning confocal microscopy was used to examine the labeled eggs. Confocal microscopy images of labeled eggs detected the presence of a distinct 3–5-μm-thick layer of enhanced fluorescence localized to the cortex of the unfertilized eggs (Fig. 9A ▶). To further characterize this staining pattern, cortices of unfertilized eggs were prepared on glass coverslips and used for immunofluorescence labeling followed by confocal microscopy. Images of the cortical region of the unfertilized egg revealed a characteristic honeycomb pattern of labeling consistent with staining the cortical cytoplasm surrounding cortical granules docked at the plasma membrane (Fig. 9B ▶). While the pattern appeared to be continuous at low magnification, at higher magnification the reticular pattern presented itself as closely spaced distinct puncta having dimensions of ~250 nm (Fig. 9C ▶). Interestingly, the cortex of sea urchin eggs is enriched with ribosomes and this cortical enrichment has been proposed to be a vehicle for differential segregation of mRNAs during division (Sardet et al. 2002). In first mitotic cycle embryos, seawi localization was not as restricted to the cortex of the cells as it was in unfertilized eggs (Fig. 9D ▶). In addition to cytoplasmic staining, there was prominent seawi staining of the mitotic spindle. Inspection of spindle staining gives the impression that seawi is enriched at opposite poles of the mitotic spindle as compared to the equatorial region (Fig. 9D ▶). To determine if seawi protein expression was spatially regulated during development, embryos were raised to the 16/32-cell stage and stained for seawi in order to examine the staining pattern along the animal–vegetal axis. Interestingly, there was a dramatic increase in the levels of seawi labeling within the four micromeres at the vegetal pole of the embryo (Fig. 9E ▶).
FIGURE 9.
Immunolocalization of seawi. (A) Laser scanning confocal micrograph of an unfertilized S. purpuratus egg stained with A59 antiserum. (B) Laser scanning confocal micrograph of a cortical preparation of an unfertilized egg stained with 4I4 antiserum. (C) Higher magnification of a laser scanning confocal micrograph of a cortical preparation of an unfertilized egg stained with 4I4 antiserum. (D) Laser scanning confocal micrograph of a first division S. purpuratus embryo stained with A59 antiserum. (E) Laser scanning confocal micrograph of an early cleavage stage S. purpuratus embryo stained with A59 antiserum. Notice the enrichment of seawi in the four micromeres present at the vegetal pole of the embryo. The bars in A,B,D,E are 10 μm, while the bar in C is 1 μm.
DISCUSSION
Seawi is a member of the piwi/argonaute protein family
In this report, we establish that the previously isolated sea urchin egg 100-kDa microtubule binding protein (seawi) is the sea urchin ortholog of Drosophila piwi. Seawi contains the signature motifs of the piwi/argonaute protein family, including a C-terminal piwi domain, a centrally located PAZ domain, and a N-terminal variable domain (Cerutti et al. 2000). The piwi domain is highly conserved across the entire family and recent findings suggest that a 138-aminoacid stretch within this domain is necessary and sufficient for miwi’s RNA binding (Kuramochi-Miyagawa et al. 2001). Seawi and miwi are 55% identical and 69% similar over those 138 amino acids, suggesting that this segment of seawi may mediate an interaction with mRNA. Recent crystal structures have identified that both the piwi and PAZ domains participate in RNA binding (Parker et al. 2004; Song et al. 2004). The PAZ domain was originally proposed to function as a protein–protein binding domain during translational silencing (Hammond et al. 2001; Moss 2001) and more recently it has been shown to bind to RNAs, including siRNA with a 1:1 stoichiometry (Lingel et al. 2003, 2004; Yan et al. 2003). This binding interaction is fascinating and informative given the emerging importance of piwi/argonaute family members and si- and micro-RNAs in RNA silencing processes (Carmell et al. 2002; Rand et al. 2004). The highly variable N-terminal domain may reflect functional specialization of individual piwi/argonaute family members, thereby providing the observed breadth of functionality such as stem cell establishment and maintenance (piwi, Cox et al. 1998; miwi, Kuramochi-Miyagawa et al. 2001; hiwi, Sharma et al. 2001), RNAi (argonaute-2, Hammond et al. 2001; RDE-1, Tabara et al. 1999), post-transcriptional silencing (argonaute-1, Fagard et al. 2000; aubergine, Wilson et al. 1996; Harris and Macdonald 2001), and localization of transcripts (aubergine, Wilson et al. 1996). Lastly, phylogenetic analysis of the piwi/argonaute family shows that seawi clusters within the piwi subgroup whose members are functionally associated with the maintenance of stem cell populations and/or axis formation.
Seawi and its potential role in sea urchin embryogenesis
Could seawi have a role in sea urchin axis formation and establishment of set-aside cells similar to the role of piwi/argonaute family members during Drosophila development? Drosophila piwi family member aubergine is required for the proper translation and localization of gurken, oskar, and nanos mRNAs whose translation products are subsequently needed for determining dorsal–ventral and anterior–posterior patterning (Wilson et al. 1996). Also, aubergine localizes to polar granules, the cytoplasmic site that will give rise to pole cells, a group of stem cells required for germline development in Drosophila (Harris and Macdonald 2001). Interestingly, aubergine is also linked to RNAi activity in oocytes (Kennerdell et al. 2002). Similar localization patterns for aubergine and vasa, an eIF-4A homolog, coupled with the observation that vasa recruits the Drosophila homolog of eIF-2 to certain mRNAs, suggests that subsets of mRNAs are preassembled into translational initiation complexes (Carrera et al. 2000; Harris and Macdonald 2001). Activation of such preassembled complexes might require site-specific recruitment of other “translation activation factors” making it possible to spatially and temporally regulate protein expression of developmentally important transcripts such as oskar and nanos (Lipshitz and Smibert 2000).
During the initial stages of sea urchin embryogenesis three distinct cell fate domains are established along the animal–vegetal axis. At the vegetal pole, nuclear β-catenin interacts with Tcf/Lef transcriptional regulators to initiate the transcription of numerous genes including Wnt8, Krl, Krox, and Pmar1 (Vonica et al. 2000; Davidson et al. 2002). These factors initiate a cascade of transcription that establishes the “vegetal signaling mechanism,” which patterns vegetal blastomeres and specifies mesenchyme and endoderm (Angerer and Angerer 2000; Davidson et al. 2002). The molecular mechanism regulating the spatial and temporal entry of β-catenin into the nucleus is cell autonomous and requires factors of maternal origin (Davidson et al. 2002).
In P. lividus embryos, Bep4 protein colocalizes with cad-herin and β-catenin at cell–cell junctions of cells at the animal pole while in micromeres there is no junctional Bep4 or β-catenin; instead there is an enhanced level of nuclear β-catenin (Romancino et al. 2001). Using anti-Bep4 antibodies, it was possible to disrupt normal axis specification by allowing the domain of nuclear β-catenin to expand toward the animal pole, suggesting that inhibition of Bep4 function effected the subcellular localization and physiological activity of β-catenin (Romancino et al. 2001). Bep4 may maintain β-catenin in the cytoplasm by participating in junctional assembly, thus preventing β-catenin entry into the nucleus and subsequent initiation of vegetal transcriptional signaling (Aplin and Juliano 2001).
By analogy with the role of aubergine in the translational regulation of oskar and the role of Drosophila argonaute-1 in the regulation of the β-catenin pathway, seawi-containing RNP complexes could regulate the spatial/temporal expression of RNP associated mRNA including the bep4 transcript. After fertilization, the levels of seawi message and protein initially decrease followed by an increase in seawi expression that correlates temporally with the loss of β-catenin from adherens junctions in cells destined to ingress into the blastocoel during gastrulation (Miller and McClay 1997). The second embryonic rise in seawi expression correlates in time with the establishment of the coelomic pouches, a group of set-aside (“stem”) cells that eventually comprise a significant portion of the adult rudiment (Peterson et al. 1997). Confocal microscopy of embryos stained by indirect immunofluorescence document an enhanced staining for seawi within the micromeres as compared to the other blastomeres. A recent preliminary report identified that sea urchin vasa (Voronina et al. 2004), whose Drosophila homolog is involved in regulating developmental transcripts oskar and nanos, shares an enhanced micro-mere localization pattern similar to what we identify for seawi. Further, the observed increase in seawi protein within micromeres temporally coincides with a decrease in micromere expression of Bep4 protein (Romancino et al. 2001), loss of β-catenin from micromere junctions (Miller and McClay 1997), and an increase in β-catenin within nuclei of micromeres (Logan et al. 1999; Romancino et al. 2001). Importantly, the timing of these events correlates with the beginning of nuclear β-catenin signaling during fate specification of the micromeres and the entire vegetal region of the embryo (Davidson et al. 2002). Consequently, the observed timing of expression and localization of seawi within the developing embryo coupled with its biochemical properties provide strong evidence that seawi might participate in modulating embryonic axis specification and formation.
The observation that seawi and ribosomes (Suprenant et al. 1989) are associated with the microtubules of the mitotic spindle provides an avenue for spatial segregation and anchorage of RNP complexes within the developing embryo. Once spatially segregated, translational activation of localized and arrested complexes may require subsequent assembly and activation of the translational machinery as elegantly shown in Drosophila (Carrera et al. 2000; Lipshitz and Smibert 2000; Harris and Macdonald 2001). Segregation and assembly of seawi- (and possibly vasa) associated translationally silencing RNP complexes to micromeres provides a mechanism for temporal and spatial regulation of Bep4 protein expression, which in turn would effect β-catenin localization.
Recently, RNP complexes containing piwi/argonaute family members and miRNAs have been shown to associate with polyribosomes (Nelson et al. 2004). This is interesting in light of miRNAs having a central role in the regulation of protein levels in many organisms through the degradation of message via RNAi or through the translation silencing of specific mRNAs via a complex between the miRNAs and the 3′ UTRs of the silenced message (Carmell et al. 2002). The regulation of expression of specific messages via miRNA is most clearly demonstrated by the role of this pathway in lineage specification during vertebrate hematopoiesis (Chen et al. 2004). Whether similar pathways are in play during sea urchin embryogenesis remains a matter for speculation and is presently under investigation.
MATERIALS AND METHODS
cDNA library screening
The 100-kDa dynamin-like protein was purified from unfertilized S. purpuratus eggs utilizing tubulin dimer affinity chromatography (Faire and Bonder 1993). Purified protein was electrophoretically transferred to nitrocellulose and processed for microsequence analysis at the Univerisity of Massachusetts Medical School Proteomic Mass Spectrometry Lab (formerly the W.M. Keck Protein Facility at the Worcester Foundation for Experimental Biology, Shrewsbury, MA). Degenerate primers were prepared based on the peptide microsequences and used for initial reverse transcription-polymerase chain reaction (RT-PCR) amplification and nucleotide sequencing of PCR products. Partial nucleotide sequence information was used to prepare sequence-specific primers for the GPEIV (5′-gaccagaaatagtggaacttcc-3′) and MIIGcom (5′-gtggtag gagtcgatgccgatgatcat-3′) sequences. RT-PCR was performed using total RNA isolated from unfertilized eggs and the GPEIV and MIIGcom primers. The resulting cDNA was gel purified using a QIAquick gel extraction kit (Qiagen Inc.) and sequenced using a Perkin Elmer DNA sequencing kit, the GPEIV and MIIGcom primers, and an Applied Biosystems ABI Prism 373 automated sequencer. GPEIV and MIIGcom primers were used to prepare a DIG-labeled probe for screening a λZAP cDNA library as previously described (Sirotkin et al. 2000). A second DIG-labeled probe was synthesized using PCR primers C-term (5′- cggacactctgactgat gatgaag-3′) and C-termcom (5′-caacgagaagtttcctgagcgtatc-3′) from the C-terminal portion of plasmid 6-2. Isolated plasmids were sequenced by several rounds of “primer walking.”
Relative RT-PCR analysis of staged embryos
Sea urchin eggs and staged embryos were cultured as previously described (Sirotkin et al. 2000) and total RNA was isolated using the Biotecx Ultraspec RNA Isolation System. RNA from each stage was subjected to relative RT-PCR analysis as described by Ambion using the primers n5 seawi (5′-ccgggatccgaacaagcacccccaccgatg-3′) and n3 seawi (5′-caccaccctcgagttaagacagcagcatcacgtcgtac-3′). Two percent agarose gels of 21 cycle PCR products were stained with SYBR Gold Nucleic Acid Gel Stain (Molecular Probes) for 40 min and the resultant 18S/Seawi bands were quantitated using Metamorph software (Universal Imaging Corp.).
MT-RNP complex and ribosome preparations
MT-RNP complexes were purified from unfertilized S. purpuratus or P. lividus eggs by three cycles of pH- and temperature-dependent MT assembly and disassembly as previously described (Suprenant and Marsh 1987). Monoribosomes were purified from unfertilized eggs exactly as described by Hille and Danilchik (1986) with the exception that 200 μM phenylmethlylsulfonyl fluoride (PMSF) was used instead of soybean trypsin inhibitor.
Sucrose density centrifugation
Linear 15%–40% (w/v) sucrose gradients were prepared in buffer containing 80 or 500 mM KCl, 100 mM potassium acetate, 5 mM magnesium acetate, 9.9 mM K2HPO4, 10.1 mM KH2PO4 (pH 6.8), and 200 μM PMSF. After brief centrifugation (10 min, 39,000g, 4°C) 1 mL of purified monoribosomes (1–2 mg) was overlayed onto a 12-mL gradient and centrifuged in a SW41Ti swinging bucket rotor (Beckman Instruments) at 32,500 rpm (150,000g) for 8 h at 4°C. One-milliliter fractions were collected and analyzed by SDS-PAGE and immunoblotting.
Polyclonal antibody production and characterization
Preparation and characterization of polyclonal anti-100-kDa serum (4I4) was previously described (Faire and Bonder 1993). Briefly, egg 100-kDa protein purified from unfertilized Lytechinus pictus eggs (Faire and Bonder 1993) was run on 7.5%–15% SDS-PAGE and lightly stained with Coomassie brilliant blue. The 100-kDa protein was excised, frozen in liquid N2, finely ground with a mortar and pestle, emulsified with an equal volume of Freund’s complete adjuvant, and used to immunize a New Zealand white rabbit.
Polyclonal antiserum (A59) against the 100-kDa MT-RNP complex protein was generated against third-cycle microtubule preparations. The MT-RNP complex proteins were separated by SDS-PAGE and briefly stained with Coomassie brilliant blue. The 100-kDa protein was excised, electroeluted, dialyzed into PBS, emulsified with Freund’s complete adjuvant, and used to immunize a New Zealand white rabbit.
Protein–RNA (Northwestern) blotting
Purified LP54 (100 ng; as a positive control), purified seawi (100 ng), and fibronectin (200 ng; as a negative control) were electrophoresed in an 8% SDS-polyacrylamide gel and blotted onto nitrocellulose. The blot was incubated in blocking buffer (5× Denhardt’s, 20 μg/mL tRNA, 1 nM dUTP) for 1 h at 25°C. RNA binding was carried out in blocking buffer with 3 ng/mL of DIG-labeled bep4 RNA for 2 h at 25°C. The filter was subsequently washed for 15 min each in binding buffer (BB) (10 mM Tris at pH 7.5, 50 mM NaCl, 1 mM EDTA), 4× BB, and 8× BB. The RNA was bound to membrane using a UV lamp (254 nm) and processed as described by Montana et al. (1997).
Immunocytochemistry and immunoblotting studies
Indirect immunofluorescence localization was conducted on eggs, embryos (Bonder et al. 1989), and isolated cortical lawns (Faire and Bonder 1993). Fixed cells, embryos, and cortical lawns were incubated in a 1:50 dilution of primary antibody (4I4 or A59) followed by a 1:500 dilution of Texas Red conjugated goat anti-rabbit secondary antibodies (Molecular Probes) and images were collected using a BioRad MRC 1024 laser scanning confocal microscope equipped with Nikon optics. Immunoblots with anti-seawi antibodies (antisera 4I4 and A59) were performed at a 1: 5000 dilution of primary antibody followed by incubation in a 1:20,000 dilution of alkaline phosphatase conjugated goat anti-rabbit secondary antibodies. Developmental expression profiles of seawi protein were obtained by transferring equivalent concentrations of total egg/embryo protein to nitrocellulose and probing with antiserum A59. The intensities of the bands were quantitated using Metamorph software. Recombinant seawi protein was produced by overexpression in SOLR cells containing pBluescript plasmids containing the 6.2 seawi insert. Cultures were grown at 37°C to an OD600 of ~0.6 and brought to 10 mM IPTG for 3 h before centrifugation at 7000 rpm in an SS34 rotor to harvest the bacteria. Gel samples were prepared by resuspending the pellet in 20 volumes of 1× SDS-PAGE sample buffer. Control plasmids not containing the insert were overexpressed as described above.
Acknowledgments
We gratefully acknowledge the many members of our labs that contributed moral support, extra sets of hands, lively discussion, and senses of humor during the long process of characterizing seawi. This work was supported by grants from the National Science Foundation RTG Program, National Institutes of Health-MBRS Program, Italian National Council of Research, and a Johnson and Johnson Discovery Award.
Article and publication are at http://www.rnajournal.org/cgi/doi/10.1261/rna.7198205.
REFERENCES
- Angerer, L.M. and Angerer, R.C. 2000. Animal–vegetal axis patterning mechanisms in the early sea urchin embryo. Dev. Biol. 218: 1–12. [DOI] [PubMed] [Google Scholar]
- Aplin, A.E. and Juliano, R.L. 2001. Regulation of nucleocytoplasmic trafficking by cell adhesion receptors and the cytoskeleton. J. Cell Biol. 155: 187–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbarese, E., Koppel, D.E., Deutscher, M.P., Smith, C.L., Ainger, K., Morgan, F., and Carson, J.H. 1995. Protein translation components are colocalized in granules in oligodendrocytes. J. Cell Sci. 108: 2781–2790. [DOI] [PubMed] [Google Scholar]
- Bonder, E.M., Fishkind, D.J., Cotran, N.M., and Begg, D.A. 1989. The cortical actin-membrane cytoskeleton of unfertilized sea urchin eggs: Analysis of the spatial organization and relationship of filamentous actin, nonfilamentous actin, and egg spectrin. Dev. Biol. 134: 327–341. [DOI] [PubMed] [Google Scholar]
- Brandhorst, B.P. and Klein, W.H. 2002. Molecular patterning along the sea urchin animal–vegetal axis. Int. Rev. Cytol. 213: 183–232. [DOI] [PubMed] [Google Scholar]
- Carmell, M.A., Xuan, Z., Zhang, M.Q., and Hannon, G.J. 2002. The Argonaute family: Tentacles that reach into RNAi, developmental control, and tumorigenesis. Genes & Dev. 16: 2733–2742. [DOI] [PubMed] [Google Scholar]
- Carrera, P., Johnstone, O., Nakamura, A., Casanova, J., Jackle, H., and Lasko, P., 2000. VASA mediates translation through interaction with a Drosophila yIF2 homolog. Mol. Cell 5: 181–187. [DOI] [PubMed] [Google Scholar]
- Cerutti, L., Mian, N., and Bateman, A. 2000. Domains in gene silencing and cell differentiation proteins: The novel PAZ domain and redefinition of the Piwi domain. Trends Biochem. Sci. 25: 481–482. [DOI] [PubMed] [Google Scholar]
- Chen, C.Z., Li, L., Lodish, H.F., and Bartel, D.P. 2004. MicroRNAs modulate hematopoietic lineage differentiation. Science 303: 83–86. [DOI] [PubMed] [Google Scholar]
- Cox, D.N., Chao, A., Baker, J., Chang, L., Qiao, D., and Lin, H. 1998. A novel class of evolutionarily conserved genes defined by piwi are essential for stem cell self-renewal. Genes & Dev. 12: 3715–3727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson, E.H. 1986. Gene Activity in Early Development, 4th ed. Academic Press, Inc., Orlando.
- Davidson, E.H., Rast, J.P., Oliveri, P., Ransick, A., Calestani, C., Yuh, C.H., Minokawa, T., Amore, G., Hinman, V., Arenas-Mena, C., et al. 2002. A genomic regulatory network for development. Science 295: 1669–1678. [DOI] [PubMed] [Google Scholar]
- Fagard, M., Boutet, S., Morel, J.B., Bellini, C., and Vaucheret, H. 2000. AGO1, QDE-2, and RDE-1 are related proteins required for post-transcriptional gene silencing in plants, quelling in fungi, and RNA interference in animals. Proc. Natl. Acad. Sci. 97: 11650–11654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faire, K. and Bonder, E.M. 1993. Sea urchin egg 100-kDa dynamin-related protein: Identification of and localization to intracellular vesicles. Dev. Biol. 159: 581–594. [DOI] [PubMed] [Google Scholar]
- Gibbins, J.R., Tilney, L.G., and Porter, K.R. 1969. Microtubules in the formation and development of the primary mesenchyme in Arbacia punctulata. I. The distribution of microtubules. J. Cell Biol. 41: 201–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill, D.R., and Suprenant, K.A. 1997. Characterization of the sea urchin major vault protein: A possible role for vault ribonucleo-protein particles in nucleocytoplasmic transport. Dev. Biol. 190: 117–128. [DOI] [PubMed] [Google Scholar]
- Hamill, D., Davis, J., Drawbridge, J., and Suprenant, K.A. 1994. Polyribosome targeting to microtubules: Enrichment of specific mRNAs in a reconstituted microtubule preparation from sea urchin embryos. J. Cell Biol. 127: 973–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond, S.M., Boettcher, S., Caudy, A.A., Kobayashi, R., and Hannon, G.J. 2001. Argonaute2, a link between genetic and biochemical analyses of RNAi. Science 293: 1146–1150. [DOI] [PubMed] [Google Scholar]
- Harris, A.N. and Macdonald, P.M. 2001. Aubergine encodes a Drosophila polar granule component required for pole cell formation and related to eIF2C. Development 128: 2823–2832. [DOI] [PubMed] [Google Scholar]
- Hille, M.B. and Danilchik, M.V. 1986. The protein synthetic machinery: Ribosomes and cell-free systems. Methods Cell Biol. 27: 175–188. [DOI] [PubMed] [Google Scholar]
- Hirokawa, N., Takemura, R., and Hisanaga, S. 1985. Cytoskeletal architecture of isolated mitotic spindle with special reference to microtubule-associated proteins and cytoplasmic dynein. J. Cell Biol. 101: 1858–1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennerdell, J.R., Yamaguchi, S., and Carthew, R.W. 2002. RNAi is activated during Drosophila oocyte maturation in a manner dependent on aubergine and spindle-E. Genes & Dev. 16: 1884–1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles, R.B., Sabry, J.H., Martone, M.E., Deerinck, T.J., Ellisman, M.H., Bassell, G.J., and Kosik, K.S. 1996. Translocation of RNA granules in living neurons. J. Neurosci. 16: 7812–7820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuramochi-Miyagawa, S., Kimura, T., Yomogida, K., Kuroiwa, A., Tadokoro, Y., Fujita, Y., Sato, M., Matsuda, Y., and Nakano, T. 2001. Two mouse piwi-related genes: miwi and mili. Mech. Dev. 108: 121–133. [DOI] [PubMed] [Google Scholar]
- Lingel, A., Simon, B., Izaurralde, E., and Sattler, M. 2003. Structure and nucleic-acid binding of the Drosophila Argonaute 2 PAZ domain. Nature 426: 465–469. [DOI] [PubMed] [Google Scholar]
- ———. 2004. Nucleic acid 3′-end recognition by the Argonaute2 PAZ domain. Nat. Struct. Mol. Biol. 426: 576–577. [DOI] [PubMed] [Google Scholar]
- Lipshitz, H.D. and Smibert, C.A. 2000. Mechanisms of RNA localization and translational regulation. Curr. Opin. Genet. Dev. 10: 476–488. [DOI] [PubMed] [Google Scholar]
- Logan, C.Y., Miller, J.R., Ferkowicz, M.J., and McClay, D.R. 1999. Nuclear beta-catenin is required to specify vegetal cell fates in the sea urchin embryo. Development 126: 345–357. [DOI] [PubMed] [Google Scholar]
- Ma, J.B., Ye, K., and Patel, D.J. 2004. Structural basis for overhang-specific small interfering RNA recognition by the PAZ domain. Nature 429: 318–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, J.R. and McClay, D.R. 1997. Changes in the pattern of adherens junction-associated beta-catenin accompany morphogenesis in the sea urchin embryo. Dev. Biol. 192: 310–322. [DOI] [PubMed] [Google Scholar]
- Montana, G., Bonura, A., Romancino, D.P., Sbisa, E., and DiCarlo, M. 1997. A 54 kDa protein specifically associates the 3′ untranslated region of three maternal mRNAs with the cytoskeleton of the animal part of the Paracentrotus lividus egg. Eur. J. Biochem. 247: 183–198. [DOI] [PubMed] [Google Scholar]
- Montana, G., Sbisa, E., Romancino, D.P., Bonura, A., and DiCarlo, M. 1998. Folding and binding activity of the 3′ UTRs of Paracentrotus lividus bep messengers. FEBS Lett. 425: 157–160. [DOI] [PubMed] [Google Scholar]
- Moss, E.G. 2001. RNA interference: It’s a small world. Curr. Biol. 11: R772–R775. [DOI] [PubMed] [Google Scholar]
- Nelson, P.T., Hatzigeorgiou, A.G., and Mourelatos, Z. 2004. miRNP: mRNA association in polysomes in human neuronal cell line. RNA 10: 387–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker, J.S., Roe, S.M., and Barford, D. 2004. Crystal structure of a PIWI protein suggests mechanisms for siRNA recognition and slicer activity. EMBO J. 23: 4727–4737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson, K.J., Cameron, R.A., and Davidson, E.H. 1997. Set-aside cells in maximal indirect development: Evolutionary and developmental significance. Bioessays 19: 623–631. [DOI] [PubMed] [Google Scholar]
- Pokrywka, N.J. and Stephenson, E.C. 1995. Microtubules are a general component of mRNA localization systems in Drosophila oocytes. Dev. Biol. 167: 363–370. [DOI] [PubMed] [Google Scholar]
- Rand, T.A., Ginalski, K., Grishin, N.V., and Wang, X. 2004. Biochemical identification of Argonaute 2 as the sole protein required for RNA-induced silencing complex activity. Proc. Natl. Acad. Sci. 101: 14385–14389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romancino, D.P. and Di Carlo, M. 1999. Asymmetrical localization and segregation of Paracentrotus lividus Bep4 maternal protein. Mech. Dev. 87: 3–9. [DOI] [PubMed] [Google Scholar]
- Romancino, D.P., Montana, G., and Di Carlo, M. 1998. Involvement of the cytoskeleton in localization of Paracentrotus lividus maternal BEP mRNAs and proteins. Exp. Cell Res. 238: 101–109. [DOI] [PubMed] [Google Scholar]
- Romancino, D.P., Montana, G., Dalmazio, S., and Di Carlo, M. 2001. Bep4 protein is involved in patterning along the animal–vegetal axis in the Paracentrotus lividus embryo. Dev. Biol. 234: 107–119. [DOI] [PubMed] [Google Scholar]
- Runnstrom, J. 1929. Uber selbstfifferenzierung and induction bei dem seeigelkeim. With. Roux Arch. 117: 123–145. [DOI] [PubMed] [Google Scholar]
- Sardet, C., Prodon, F., Dumollard, R., Chang, P., and Chenevert, J. 2002. Structure and function of the egg cortex from oogenesis through fertilization. Dev. Biol. 241: 1–23. [DOI] [PubMed] [Google Scholar]
- Sirotkin, V., Seipel, S., Krendel, M., and Bonder, E.M. 2000. Characterization of sea urchin unconventional myosins and analysis of their patterns of expression during early embryogenesis. Mol. Reprod. Dev. 57: 111–126. [DOI] [PubMed] [Google Scholar]
- Song, J.J., Smith, S.K., Hannon, G.J., and Joshua-Tor, L. 2004. Crystal structure of Argonaute and its implications for RISC slicer activity. Science. 305: 1434–1437. [DOI] [PubMed] [Google Scholar]
- Suprenant, K.A. and Marsh, J.C. 1987. Temperature and pH govern the self-assembly of microtubules from unfertilized sea-urchin egg extracts. J. Cell Sci. 87: 71–84. [DOI] [PubMed] [Google Scholar]
- Suprenant, K.A., Tempero, L.B., and Hammer, L.E. 1989. Association of ribosomes with in vitro assembled microtubules. Cell Motil. Cytoskeleton 14: 401–415. [DOI] [PubMed] [Google Scholar]
- Tabara, H., Sarkissian, M., Kelly, W.G., Fleenor, J., Grishok, A., Timmons, L., Fire, A., and Mello, C.C. 1999. The rde-1 gene, RNA interference, and transposon silencing in C. elegans. Cell 99: 123–132. [DOI] [PubMed] [Google Scholar]
- Vonica, A., Weng, W., Gumbiner, B.M., and Venuti, J.M. 2000. TCF is the nuclear effector of the beta-catenin signal that patterns the sea urchin animal–vegetal axis. Dev. Biol. 217: 230–243. [DOI] [PubMed] [Google Scholar]
- Voronina, E., Lopez, M.E., Song, J., Gustafson, E., Oliveri, P., Ransick, A., McClay, D.R., and Wessel, G.M. 2004. Germ cell formation in the sea urchin. Mol. Biol. Cell (Suppl.) 15: 135a.
- Wilson, E.B. 1925. The cell in development and heredity, 3d ed. Macmillan, New York.
- Wilson, J.E., Connell, J.E., and Macdonald, P.M. 1996. aubergine enhances oskar translation in the Drosophila ovary. Development 122: 1631–1639. [DOI] [PubMed] [Google Scholar]
- Yan, K.S., Yan, S., Farooq, A., Han, A., Zeng, L., and Zhou, M.M. 2003. Structure and conserved RNA binding of the PAZ domain. Nature 426: 468–474. [DOI] [PubMed] [Google Scholar]