Abstract
We show that Saccharomyces cerevisiae strains lacking Trm8p/Trm82p tRNA m7G methyltransferase are temperature-sensitive in synthetic media containing glycerol. Bacterial TRM8 orthologs complement the growth defect of trm8-Δ, trm82-Δ, and trm8-Δ trm82-Δ double mutants, suggesting that bacteria employ a single subunit for Trm8p/Trm82p function. The growth phenotype of trm8 mutants correlates with lack of tRNA m7G methyltransferase activity in vitro and in vivo, based on analysis of 10 mutant alleles of trm8 and bacterial orthologs, and suggests that m7G modification is the cellular function important for growth. Initial examination of the roles of the yeast subunits shows that Trm8p has most of the functions required to effect m7G modification, and that a major role of Trm82p is to maintain cellular levels of Trm8p. Trm8p efficiently cross-links to pre-tRNAPhe in vitro in the presence or absence of Trm82p, in addition to its known residual tRNA m7G modification activity and its SAM-binding domain. Surprisingly, the levels of Trm8p, but not its mRNA, are severely reduced in a trm82-Δ strain. Although Trm8p can be produced in the absence of Trm82p by deliberate overproduction, the resulting protein is inactive, suggesting that a second role of Trm82p is to stabilize Trm8p in an active conformation.
Keywords: tRNA modification, S. cerevisiae, S-adenosylmethionine, methyltransferases, Trm8, Trm82, YggH, TM0925, 7-methylguanosine, m7G
INTRODUCTION
A continuing puzzle in RNA biology is the precise role of tRNA modifications. Although more than 79 different modifications have been identified in tRNA (Limbach et al. 1994; Bjork 1995), and 25 of these modifications are found in yeast (Sprinzl et al. 1998), a cellular role for many of them has not yet been defined. The recent identification of a substantial number of genes responsible for tRNA modification (Hopper and Phizicky 2003) has allowed the opportunity to study their roles by examining mutant phenotypes.
Surprisingly, deletion of the majority of enzymes responsible for tRNA modification revealed few obvious defects. Only three tRNA modifying enzymes in yeast are known to be essential, including m1A58 methyltransferase Gcd10p/Gcd14p (Anderson et al. 1998), I34 adenosine deaminase Tad2p/Tad3p (Gerber and Keller 1999), and tRNAHis G−1 guanylyltransferase Thg1p (Gu et al. 2003). Strains lacking each of six other modification enzymes affecting residues in the anticodon region have distinct growth or translation phenotypes, including 2′-O-Me32,34 methyltransferase Trm7p (Pintard et al. 2002), m1G37/m1I37 methyltransferase Trm5p (Bjork et al. 2001), ψ38,39 pseudouridylase Pus3p (Lecointe et al. 1998), mcm5U/mcm5s2U34 carboxyl methyltransferase Trm9p (Kalhor and Clarke 2003), m5C34,40,48,49 methyltransferase Trm4p (Wu et al. 1998), and i6A37 isopentenyl transferase Mod5p (Laten et al. 1978; Janner et al. 1980; Dihanich et al. 1987).
The other 16 known modification enzymes in yeast each modify residues remote from the anticodon region, and mutants lacking these enzymes have only subtle phenotypes. Each of these mutants has little obvious growth or translation defect, and the observation of distinct phenotypes has required more sensitive approaches such as synthetic interaction screens (Grosshans et al. 2001; Johansson and Bystrom 2002; Urbonavicius et al. 2002), or, as shown in Escherichia coli, growth competition experiments (Gutgsell et al. 2000). The lack of obvious phenotype of strains lacking these modification enzymes contrasts sharply with the strong evolutionary conservation of a number of these enzymes and their modifications. Even more unexpectedly, some of the subtle phenotypes associated with defects in tRNA modification, including those of yeast m5U54 meth-yltransferase Trm2p (Johansson and Bystrom 2002) and E. coli ψ55 pseudouridylase TruB (Gutgsell et al. 2000), were complemented with catalytically inactive mutants, suggesting that lack of the modification of tRNA is not the cause of the defect, and that tRNA-modifying enzymes may have other distinct cellular roles.
We focus here on m7G (7-methylguanosine) modification of tRNA. This modification is highly conserved, based on its presence in >40% of all sequenced bacterial and eukaryotic tRNA species. In all but three cases, m7G is found at position 46 in the extra loop, a site known from the crystal structures of tRNAPhe (Kim et al. 1974; Robertus et al. 1974) and tRNAiMet (Basavappa and Sigler 1991) to form tertiary interactions with bases at positions 13 and 22. Additionally, the m7G and m1A modification are the only two yeast modifications that confer a positive charge to the base.
We previously used a biochemical genomics approach to identify two proteins, Trm8p and Trm82p, that copurify with tRNA m7G methyltransferase activity. We showed that these two proteins are both necessary for m7G46 modification of tRNA in vivo and in vitro, form a complex in vivo, and are sufficient for activity in vitro (Alexandrov et al. 2002). Neither Trm8p nor Trm82p is significantly related to yeast Abd1p, which catalyzes m7G formation during capping of mRNAs (Mao et al. 1995), other than within the S-adenosylmethionine (SAM)-binding domain of Trm8p. Trm8p is highly conserved in eukaryotes and bacteria, extending from humans to Mycoplasma genitalium, with less that 500 ORFs (Bahr et al. 1999), and Trm82p orthologs are present in the majority of sequenced eukaryotes. Nevertheless, deletion of TRM8 or TRM82 results in no obvious growth defect under standard laboratory conditions (Alexandrov et al. 2002), raising the question of the role of these proteins in these organisms.
Surprisingly, whereas Trm8p/Trm82p complex appears to be conserved in eukaryotes, it may be a single Trm8p subunit in bacteria, since bacteria appear to lack Trm82p (Michaud et al. 2000). We showed previously that the two-protein mechanism of tRNA m7G formation is conserved in higher eukaryotes, since expression of both human orthologous proteins (METTL1 and WDR4) in yeast was required to restore m7G methyltransferase activity in extracts (Alexandrov et al. 2002). However, it appears that a single bacterial protein may be sufficient for activity, consistent with the implication of a single purified 25-kDa Salmonella typhimurium polypeptide in tRNA m7G methylation (Colonna et al. 1983), and the demonstration of tRNA m7G-modifying activity for E. coli YggH (De Bie et al. 2003), and Aquifex aeolicus aq065 protein purified from E. coli (Okamoto et al. 2004).
As a first step toward determining the physiological roles of TRM8/TRM82 genes and the role of m7G modification of tRNA, we have found and characterized a phenotype associated with loss of either or both of the TRM8 and TRM82 genes. We show that this growth phenotype is strongly correlated with m7G methyltransferase activity in vitro and in vivo. We also provide in vivo evidence that the bacterial Trm8 ortholog does not require a second subunit to function in yeast, raising the issue of the function of each of the two yeast subunits.
We provide evidence that a crucial function of yeast Trm82p is to maintain the levels of active Trm8p in vivo, since deletion of Trm82p results in a severe reduction in the level of Trm8p protein, and Trm8p prepared from yeast in the absence of Trm82p is not detectably active. In contrast, Trm8p appears to possess the requisite functions of the catalytic subunit: it has a SAM-binding domain, has residual catalytic activity when purified from E. coli, and cross-links to pre-tRNAPhe in vitro.
RESULTS
TRM8 and TRM82 are both required for growth at 38°C on glycerol-containing synthetic media
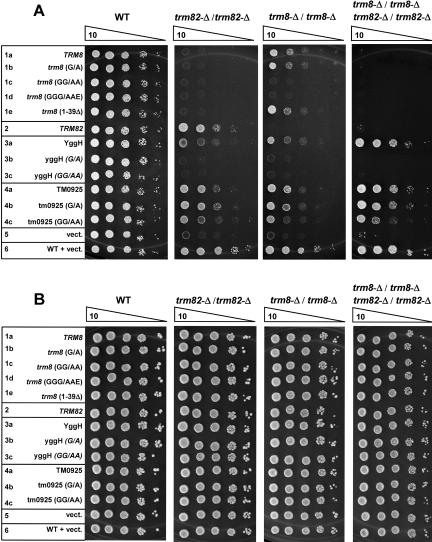
We found a distinct growth defect that results from the loss of TRM8 or TRM82 genes. As demonstrated in Figure 1 ▶ by serial 10-fold dilutions of yeast cells, homozygous diploid strains lacking either Trm8p (relevant genotype: trm8-Δ/ trm8-Δ) or Trm82p (relevant genotype: trm82-Δ/trm82-Δ) grow extremely poorly relative to wild-type strains on synthetic minimal media (Sherman 1991) containing 2% glycerol at 38°C. No obvious growth differences were observed between mutants and the wild-type strain on this media at 30 °C or at 36°C, or on two other media at either 30°C or38°C: synthetic minimal media containing 2% dextrose, and yeast extract-peptone media containing 2% glycerol (data not shown). Since growth of the mutants was also not compromised on acetate or lactate media (data not shown), this phenotype does not originate from a general loss of mitochondrial function. This growth defect is due to loss of Trm8p or Trm82p, because it is restored by a single-copy plasmid containing the corresponding TRM8 or TRM82 under control of its own promoter, but not by the vector control (Fig. 1 ▶).
FIGURE 1.
Growth defect of trm8-Δ/trm8-Δ and trm82-Δ/trm82-Δ strains on glycerol-containing minimal media. Homozygous diploid trm8-Δ/trm8-Δ, trm82-Δ/trm82-Δ, or wild-type strains, containing a CEN URA3 plasmid bearing TRM8 or TRM82 under control of its own promoter as indicated, were grown overnight, and 10-fold serial dilutions were plated on synthetic media lacking uracil. (A) Cells plated on glucose-containing media, incubated for 2 d at 30°C. (B) Cells plated on glycerol-containing media, incubated for 7 d at 38°C.
The similar conditions, magnitude, and temperature dependence of the growth defects of trm8-Δ and trm82-Δ strains, and the absence of an additional effect in the trm8-Δ trm82-Δ double deletion strain (see below) suggest the mutual involvement of TRM8 and TRM82 in the same biological process, implying that the growth phenotype is due to loss of functional Trm8p/Trm82p complex.
We used this phenotype to address two separate issues of Trm8p/Trm82p-catalyzed m7G formation. First, we provide evidence confirming that bacterial orthologs of Trm8p can affect m7G modification alone in vivo. Second, we provide evidence that the catalytic activity of Trm8p/Trm82p m7G methyltransferase is central to its function in vivo. The results of these experiments are shown together in Figures 2 ▶ and 3 ▶, and Table 1 ▶.
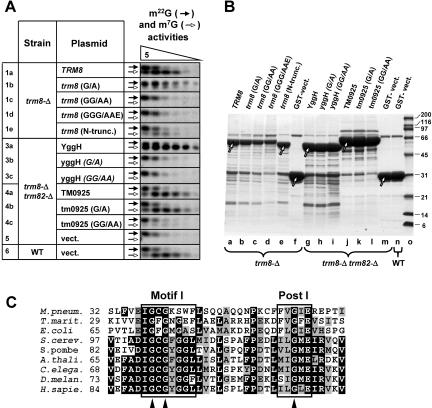
FIGURE 2.
Complementation of the growth defect by variants of TRM8 and of two of its bacterial orthologs. Homozygous diploid strains with genetic background indicated at the top of each titration panel, and containing plasmids expressing GST-fusion proteins under PCUP1 control as indicated at the left, were grown overnight and plated after serial 10-fold dilutions, on synthetic media lacking leucine and uracil, and containing carbon sources as indicated. (A) Cells plated on media containing glycerol, and incubated at 38°C for 7 d. (B) Cells plated on media containing glucose, and incubated at 30°C for 3 d. Rows 1a–1e, plasmids expressing variants of yeast TRM8, as described in the text, as GST-fusion proteins; 2, plasmid expressing TRM82; 3a–3c, plasmids expressing E. coli YggH variants; 4a–4c, plasmids expressing T. maritima TM0925 variants; 5, vector control; 6, wild-type strain control, with vector control.
FIGURE 3.
m7G-methyltransferase activity is altered in cells expressing Trm8p, YggH, and TM0925 mutant variants. (A) Analysis of m7G methyltransferase activity of variants. Activity was assayed with [α-32P]GTP-labeled yeast pre-tRNAPhe substrate and fivefold dilutions of crude extracts beginning with 6 μg of protein, and modified nucleotides were analyzed as described in Materials and Methods. Open arrows, m7G methyltransferase activity; black arrows, m22G methyltransferase activity, as internal control. (B) SDS-PAGE analysis of expressed GST fusion proteins. Expressed GST-fusion proteins were purified by glutathione sepharose chromatography from extracts of yeast strains shown in Figures 2 ▶ and 3A ▶, analyzed by 4%–15% SDS-PAGE, and stained with Coomassie. Arrows indicate protein bands of the expected size. (C) Alignment of Motif I and Post I for putative SAM-binding domains of Trm8p-orthologous proteins. Sites of point mutations used in this study (G103, G105, and G124) are indicated with triangles.
TABLE 1.
Complementation of growth defect correlates with m7G formation in tRNAPhe in vitro and in vivo
| Strain | Plasmid | Growth defect complementationa | m7G activity in vitrob | % of m7G in vivoc | |
| 1a | TRM8 | Yes | +++ | 110 | |
| 1b | trm8 (G/A) | Yes | + | 5.1 | |
| 1c | trm8-Δ | trm8 (GG/AA) | No | − | not detectedd |
| 1d | trm8 (GGG/AAE) | No | − | not detected | |
| 1e | trm8 (1–39Δ) | Yes | ++ | 39 | |
| 3a | YggH | Yes | +++ | 119 | |
| 3b | yggH (G/A) | No | − | not detected | |
| 3c | trm8-Δ | yggH (GG/AA) | No | − | not detected |
| 4a | trm82-Δ | TM0925 | Yes | +++ | 112 |
| 4b | tm0925 (G/A) | Yes | +++ | 28 | |
| 4c | tm0925 (GG/AA) | Yes | + | 5.5 | |
| 5 | vect. | No | − | not detected | |
| 6 | Wild type | vect. | Yes | +++ | 100 |
aAs shown in Figure 2 ▶.
bAs shown in Figure 3A ▶.
cAs determined by HPLC analysis of m7G content of tRNAPhe purified from the corresponding strain. 100% corresponds to m7G content of tRNAPhe purified from the wild-type strain (in this experiment: 0.80 m7G per tRNA).
dDetection limit of m7G was 2% of the wild-type amount.
Bacterial Trm8p orthologs complement the trm8-Δ trm82-Δ growth phenotype and restore m7G-methyltransferase activity in vitro and in vivo
Reports from other investigators indicate that purified bacterial Trm8p orthologs YggH from E. coli (De Bie et al. 2003) and aq065 from Aquifex aeolicus (Okamoto et al. 2004) have tRNA m7G methyltransferase activity. To determine whether and to what extent bacterial proteins could function in yeast, we tested E. coli protein YggH and Thermotoga maritima protein TM0925 for complementation of the growth defect of trm8-Δ and trm82-Δ strains, and for m7G-methyltransferase activity. As demonstrated by serial dilutions (Fig. 2A ▶), YggH and TM0925 each successfully complemented the yeast trm8-Δ phenotype nearly as well as observed with yeast Trm8p (lanes 3a,4a,1a). Strikingly, expression of either of the prokaryotic orthologs also complemented the phenotypes of trm82-Δ mutants and of trm8-Δ trm82-Δ double mutants, whereas yeast TRM8 or TRM82 each complemented only their own deletions strains (Fig. 2A ▶, lanes 1a,2). Moreover, expression of either YggH or TM0925 fully restored m7G-methyltransferase activity in extracts from a trm8-Δ trm82-Δ double mutant strain, as measured using yeast pre-tRNAPhe as substrate (Fig. 3A ▶, lanes 3a,4a). Additionally, expression of either YggH or TM0925 fully restored the in vivo levels of m7G in tRNAPhe in a trm8-Δ trm82-Δ strain to those of the wild-type strain (Table 1 ▶, cf. 3a, 4a, and 6). Thus, bacterial orthologs of Trm8p can rescue the growth defect of yeast strains lacking both proteins of the Trm8p/Trm82p complex and, unlike their eukaryotic counterparts, do not require either a bacterial partner or Trm82p to catalyze formation of m7G in tRNA either in vitro in extracts or in vivo.
The growth defect of trm8 and trm82 mutants is associated with lack of tRNA m7G methyltransferase activity in vitro and in vivo
A growth phenotype such as that described above can occur if the methyltransferase activity of Trm8p/Trm82p is important for function, or if some other unknown activity of the complex is important, as was observed with phenotypes of trm2 yeast strains and truB E. coli strains (Gutgsell et al. 2000; Johansson and Bystrom 2002). To determine whether tRNA m7G modification activity correlates with phenotype, we altered the S-adenosylmethionine (SAM)-binding domain in Trm8p and in its bacterial orthologs, and assayed complementation, biochemical activity, and tRNA m7G content in vivo. As shown in Figure 3C ▶, Trm8p orthologs, like a number of other methyltransferases, contain a Motif I and a Post I motif, regions which have been implicated in SAM binding in other proteins (Cheng et al. 1993; Labahn et al. 1994; Hodel et al. 1996; Djordjevic and Stock 1998; Niewmierzycka and Clarke 1999). Trm8p and variants were altered at three conserved glycines within these motifs, to make a G103A mutation (designated G/A), a G103A G105A double mutation (GG/ AA), and a G103A G105A G124E triple mutation (GGG/AAE). Corresponding G/A and GG/AA mutations in the SAM-binding domain of yeast m7G cap methyltransferase Abd1p (Mao et al. 1996) and m1A methyltransferase Gcd14p (Anderson et al. 2000) impaired their catalytic function. We also tested a trm8 (1–39Δ) mutant because this region is different in eukaryotes and bacteria. All mutants were expressed under PCUP1 control as N-terminal GST fusions.
As shown in Figure 2A ▶, both trm8 (G/ A), and trm8 (1–39Δ) complemented the growth defect of trm8-Δ mutants as 1a,1b,1e), whereas trm8 (GG/AA) and trm8 (GGG/AAE) (lanes 1c,1d) failed to complement. Similarly, tm0925 (G/A) and tm0925 (GG/AA) complemented the growth defect of a trm8-Δ strain (lanes 4b,4c), whereas yggH (G/A) and yggH (GG/AA) mutants did not complement at all (lanes 3b,3c).
As determined by assay of yeast extracts in the presence of excess SAM, m7G methyltransferase activity correlates with complementation (Fig. 3A ▶). Activity was not detected in all extracts derived from mutants in which complementation was not observed, including trm8 (GG/AA), trm8 (GGG/AAE), yggH (G/A), and yggH (GG/AA) (Fig. 3A ▶, lanes 1c,1d,3b,3c). In contrast, m7G methyltransferase activity was detected, albeit at various levels, in all cases where complementation was observed: at high levels in extracts from strains expressing TRM8, trm8 (1–39Δ), YggH, tm0925, and tm0925 (G/A) (Fig. 3A ▶, lanes 1a,1e,3a,4a,4b), and at lower levels in extracts from strains expressing trm8(G/A) and tm0925 (GG/AA) (lanes 1b,4c). Internal controls show that the m22G methyltransferase activity was virtually the same in the extracts (Fig. 3A ▶), and that varying levels of m7G methyltransferase activity were not due to variations in the amount of protein, as measured by yield of Trm8p orthologs or variants after purification of the expressed protein (Fig. 3B ▶).
Examination of tRNAPhe modification levels in vivo demonstrates directly that m7G content correlates with complementation of the growth phenotype of trm8 mutants. To evaluate the extent of tRNAPhe m7G methylation, we purified the tRNA from each of these strains using biotinylated DNA and streptavidin beads, and analyzed the nucleoside content using HPLC. As shown in Table 1 ▶, all strains that did not complement a trm8-Δ mutant had no detectable m7G in their tRNAPhe, whereas strains with wild-type or mutant alleles that complemented the growth defect also had detectable amounts of m7G in tRNAPhe. As with the assay of extracts, there was variability in the amount of m7G detected in vivo; however, there was a very high correlation of m7G modification levels of tRNAPhe found in vivo (Table 1 ▶) with tRNAPhe m7G modification activity observed in extracts in vitro (Fig. 3A ▶; Table 1 ▶).
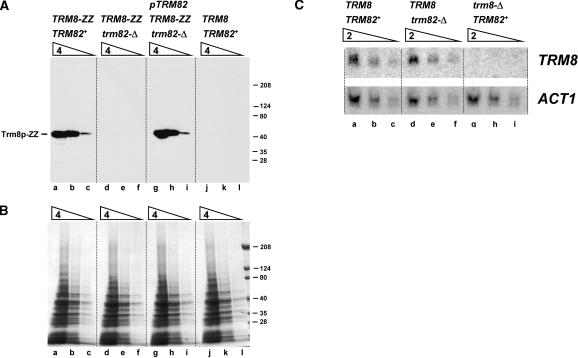
Trm82p controls the cellular levels of Trm8p protein in addition to its requirement for activity
The finding that a two-protein yeast m7G methyltransferase can be functionally substituted by a single subunit bacterial enzyme prompted us to examine the roles of yeast Trm8p and Trm82p subunits. Surprisingly, we found that the level of chromosomally TAP-tagged Trm8p was reduced at least 16-fold in crude yeast SDS lysates from a trm82-Δ strain, relative to that observed in extracts from a TRM82+ strain (Fig. 4A ▶, cf. lanes d–f and a–c). Furthermore, the Trm8p levels were fully restored upon introduction of a single-copy plasmid expressing Trm82p into this trm82-Δ strain (lanes g–i). As demonstrated by Northern blot analysis with a TRM8-specific probe (Fig. 4C ▶), the TRM8 mRNA level is virtually the same in a trm82-Δ strain as in a TRM82+ strain (Fig. 4A ▶, cf. lanes d–f and lanes a–c) and, as expected, is absent in mRNA from a specificity control trm8-Δ strain (lanes g–i). This result suggests that Trm82p does not affect the amount of TRM8 mRNA but, instead, is involved in the regulation or maintenance of Trm8p at the protein level.
FIGURE 4.
Trm8p protein, but not mRNA, is severely reduced in a trm82-Δ strain. (A) Comparison of Trm8p expression in a trm82-Δ and a TRM82+ strain. Otherwise isogenic haploid strains containing wild-type TRM8 or chromosomally epitope-tagged TRM8 (tagged with His6-HA-3Cprotease site-ZZProtein A, abbreviated ZZ), and TRM82+ or trm82-Δ as indicated, were grown, harvested, and lysed with SDS and glass beads as described in Materials and Methods. Lysates were serially diluted fourfold, starting with 2.7 OD600 units of cells, resolved by SDS-PAGE, and analyzed for Trm8-ZZ expression by Western blot analysis, as described in Materials and Methods. Lanes a–c, TRM8-ZZ TRM82+ strain; d–f, TRM8-ZZ trm82-Δ strain; g–i, TRM8-ZZ trm82-Δ strain transformed with plasmid containing CEN LEU2 PTRM82TRM82; j–l, TRM8 TRM82+ strain, with no epitope tag. (B) SDS-PAGE analysis of lysates described in (A) and stained with Coomassie. (C) Comparison of TRM8 mRNA in a trm82-Δ and a TRM82+ strain. RNA from otherwise isogenic wild-type (lanes a–c), trm82-Δ (d–f), and trm8-Δ (g–i) haploid strains was resolved on a 1% agarose gel, transferred to membrane, and hybridized with 32P-labeled TRM8 or ACT1-specific probes, as described in Materials and Methods. Successive lanes contain 15, 7.5, and 3.8 μg RNA.
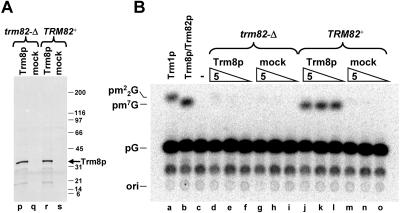
We note that deliberate overproduction of Trm8p in a trm82-Δ strain can bypass the control of Trm8 protein levels exerted by Trm82p, since Trm8p can be overexpressed and purified from a trm82-Δ strain in a yield comparable to that from wild-type cells (Fig. 5A ▶); however, Trm8p produced from a trm82-Δ strain is essentially inactive (Fig. 5B ▶). Presumably the tRNA m7G methyltransferase activity observed with purified Trm8p prepared from TRM82+ cells is due to endogenous Trm82p that copurifies with Trm8p as part of the Trm8p/Trm82p complex observed previously (Alexandrov et al. 2002). The Trm82p is not visible in the Coomassie-stained gel shown in Figure 5A ▶, because only Trm8p is overproduced in these strains.
FIGURE 5.
Trm8p overexpressed in a trm82-Δ strain has much reduced activity. (A) GST-Trm8p or GST alone (mock) were overex-pressed under PCUP1 control in a trm82-Δ or a TRM82+ strain as indicated, and expressed proteins were purified from extracts by chromatography on glutathione sepharose resin, followed by release of the Trm8p moiety by cleavage with GST-3C protease, and then analyzed by 4%–15% SDS PAGE and Coomassie staining. (B) Analysis of m7G methyltransferase activity of purified Trm8p. Trm8p purified as described in A from a trm82-Δ (lanes d–f) or a TRM82+ strain (j–l), or mock-purified controls (lanes g–i, m–o), was analyzed for methyltransferase activity, using serial fivefold dilutions of preparations. Controls: a, yeast m22G tRNA methyltransferase, b, yeast Trm8p/Trm82p m7G methyltransferase, c, buffer only.
Trm8p carries partial catalytic and tRNA-binding determinants
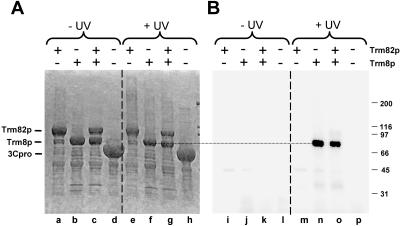
To further examine the function of the subunits, we examined tRNA binding. The presence of a consensus SAM-binding domain in Trm8p, but not in Trm82p, implicates Trm8p in the methyl transfer, but the source of the RNA binding was unknown. To address this, we used in vitro UV cross-linking to identify subunit(s) in contact with the RNA, using pre-tRNAPhe transcribed with [α-32P]UTP (Fig. 6 ▶). As can be seen in Figure 6B ▶, Trm8p efficiently cross-links to pre-tRNAPhe in a UV-dependent manner, and cross-links as efficiently when part of the Trm8p/Trm82p complex (lanes g,o) or when alone (lanes f,n). In contrast, Trm82p does not detectably cross-link to pre-tRNA, whether as part of the Trm8p/Trm82p complex or when present alone (lanes e,m,g,o), and no cross-linking is observed in a control purification (lanes h,p). Thus, Trm8p likely has both the SAM and the tRNA binding sites.
FIGURE 6.
Trm8p cross-links to pre-tRNAPhe in the presence and absence of Trm82p. Preparations of yeast Trm82p, Trm8p, Trm8p/ Trm82p, or similarly purified control protein 3C protease were subjected to UV cross-linking with pre-tRNAPhe as described in Materials and Methods, followed by RNase treatment and resolution by 10% SDS-PAGE. (A) Analysis of proteins after SDS-PAGE and Coomassie staining. (B) Analysis of radioactivity incorporated in gel shown in A.
DISCUSSION
We obtained evidence that lack of functional Trm8p or Trm82p in yeast is associated with a distinct phenotype: temperature-sensitive growth on glycerol-containing synthetic media. This phenotype underscores the importance of these proteins in survival, as implied by the widespread evolutionary conservation of putative orthologs of Trm8p and Trm82p (Bahr et al. 1999; Michaud et al. 2000; Alexandrov et al. 2002). The finding of nearly identical phenotypes of trm8-Δ and trm82-Δ mutants extends our previous genetic and biochemical data implicating TRM8 and TRM82 in the same process, as indicated by their mutual participation in tRNA m7G modification in vitro and in vivo, and by their presence in the same complex (Alexandrov et al. 2002).
We also obtained evidence that the growth defect of trm8-Δ and trm82-Δ mutants is associated with lack of tRNA m7G modification activity. Establishment of this connection was important because of two other cases in which phenotypes associated with tRNA-modifying enzymes were complemented by catalytically inactive enzyme mutants (Gutgsell et al. 2000; Johansson and Bystrom 2002), suggesting a second function for these tRNA-modifying proteins. For each of the 10 tested Trm8p orthologs and variants, we found that complementation of the growth defect correlates with tRNA m7G methyltransferase activity in extracts and with m7G levels in isolated tRNAPhe (Figs. 2 ▶, 3 ▶; Table 1 ▶). However, we showed above that complementation is observed in two cases in which there is as little as 5% m7G modification of tRNAPhe (Table 1 ▶, lines 1b,4c). We propose three different explanations for how such low m7G levels could be sufficient for complementation. First, the cell may only require 5% of the normal amount of m7G-containing tRNA for growth under these conditions. Second, the low observed m7G levels may result from evaluation of m7G under permissive conditions instead of nonpermissive conditions. We note that the narrow temperature window of this phenotype did not permit evaluation of m7G under nonpermissive conditions. Third, the m7G values for tRNAPhe may not be the crucial determinant for growth; since m7G is found in 11 tRNA species in yeast, the m7G content of any one of these species could be the crucial limiting factor.
Although the evidence cited above establishes a correlation of m7G methyltransferase activity with a growth phenotype, it is formally possible that the physiologically important effect is linked to m7G formation in another substrate. We note that yeast Pus1 protein is known to modify tRNA as well as U2 snRNA substrates (Massenet et al. 1999), that mPus1 can also act on Steroid Receptor RNA Activator (Zhao et al. 2004), and that Pus7p can act on tRNA and U2 snRNA (Ma et al. 2003).
We have shown that bacterial Trm8p orthologs YggH of E. coli and TM0925 of T. maritima can act alone to complement the growth defect of trm8-Δ trm82-Δ double mutants (Fig. 2 ▶, lanes 3a,4a), and have the requisite m7G methyltransferase activity (Fig. 3 ▶; Table 1 ▶). This result is consistent with the lack of a Trm82p ortholog detected in any sequenced prokaryotic organism (Michaud et al. 2000), extends previous in vitro results demonstrating m7G methyltransferase activity of purified E. coli protein YggH (De Bie et al. 2003) and Aquifex aeolicus protein aq065 (Okamoto et al. 2004), and is in contrast with the established two-protein mechanism of m7G formation in yeast and likely in humans (Alexandrov et al. 2002). We note that another single-subunit bacterial modifying enzyme, Thermus thermophilus m1A58 methyltransferase (Droogmans et al. 2003), is also represented by a two-subunit protein complex (Gcd10p/ Gcd14p) in yeast (Anderson et al. 2000).
Our results suggest a different partitioning of the roles of the subunits in Trm8p/Trm82p than in the other two known yeast tRNA-modifying complexes. The observation that Trm8p can be cross-linked to tRNA in the absence or presence of Trm82p suggests that the Trm8p subunit, which is known to have a SAM-binding domain, also has tRNA-binding activity. In contrast, in the Gcd10p/Gcd14p m1A58 methyltransferase complex, Gcd14p has a SAM-binding domain, whereas the Gcd10p subunit binds tRNA (Anderson et al. 1998), and in the Tad2p/Tad3p adenosine deaminase complex, association of both subunits is required for its cross-linking to tRNAAla substrate (Gerber and Keller 1999). The presence of cofactor and likely substrate binding domains in the Trm8p subunit, as well as its residual m7G methyltransferase activity (Alexandrov et al. 2002), suggest that Trm8p has most of the required functions for m7G modification, but sheds little light on the role of Trm82p.
Our experiments demonstrate that lack of Trm82p has two dramatic effects on m7G methyltransferase activity. First, Trm82p has an important role in regulating the level of Trm8p in the cell, since trm82-Δ strains have little, if any, endogenous Trm8p (<6% of wild-type amounts, Fig. 4 ▶). Second, Trm82p has an important additional role in promoting m7G methyltransferase activity, since Trm8p produced in the absence of Trm82p, either in E. coli (Alexandrov et al. 2002) or in yeast (Fig. 5 ▶), has <1% of normal activity.
The exact mechanism by which Trm82p exerts these effects is unclear. Trm82p might affect Trm8p levels by affecting the translation of Trm8p, or by controlling aspects of its targeting for degradation, and Trm82p might affect activity in any number of ways. Trm82p might have a chaperone-like function that protects Trm8p from degradation and stabilizes Trm8p in an active conformation. This would explain both the lack of Trm8p in a trm82-Δ strain and the lack of activity of Trm8p purified from a trm82-Δ strain, and would be consistent with our inability to reconstitute activity by mixing appropriate extracts or purified components (Alexandrov et al. 2002). Future experiments will undoubtedly cast more light on the precise roles of Trm82p, and may reveal the regulatory basis for the existence of the two-subunit m7G methyltransferase in eukaryotes, as opposed to the single-subunit protein in bacteria.
Based on the evidence presented here, Trm8p/Trm82p activity has at least one important physiological role that imparts a selective advantage to cells with m7G in their RNA, and which may explain the high degree of evolutionary conservation of both the proteins and the modification. The exact nature of the process impaired by the lack of m7G modification remains to be determined.
MATERIALS AND METHODS
Yeast strains
Homozygous diploid trm8-Δ/trm8-Δ and trm82-Δ/trm82-Δ deletion strains and the corresponding wild-type diploid parent BY4743 were previously described (Alexandrov et al. 2002). Homozygous diploid trm8-Δ/trm8-Δ trm82-Δ/trm82-Δ double deletion strain AA0249 (BY4741/BY4742, trm8-Δ0, trm82-Δ::kanMX) was constructed by PCR-based disruption of TRM8 using URA3 from YEplac182 (Gietz and Sugino 1988) in haploid strains BY4741 (MATa, his3-Δ1, leu2-Δ0, ura3-Δ0, met15-Δ0) and BY4742 (MATΔ, his3-Δ1, leu2-Δ0, ura3-Δ0, lys2-Δ0), followed by selection on 5-fluoroorotic acid media to allow excision of URA3, as previously described (Xing et al. 2002), disruption of TRM82 with the appropriate kanMX cassette, mating, and diploid selection on synthetic media lacking methionine and lysine. Primers were used as follows: PCR of URA3 with flanking sequences, 1st round, Trm8_D1F (5′-ACTAGAACAATTTACCACATATACGCT TTTCAATTCAATTCATC) and Trm8_D1R (5′-CGTTGGTAAT CTTGTGAAACCCGATGATAAGCTGTCAAAC), 2nd round, Trm8_D2F (5′-GTTTATTGTTAAGCATAGATGTATAACTAGA ACAATTTACCACATATACGC) and Trm8_D2R (5′-TTACAA TATGGCTGGCGTTGGTAATCTTGTGAAACCCG), 3rd round Trm8_D3F (5′-CCATAGGATAAAATTTTCAAGCGTTTATTGT TAAGCATAGATGTATAACTAGAAC) and Trm8_D2R; PCR of kanMX with flanking sequences: 165D_F (5′-TTGCGAGAA CATAAGACGACG) and 165D_R (5′-GCTTTAGAATTGGG CCTCAG) using genomic DNA from strain 33523 (BY4743, trm82-Δ::kanMX), obtained from Research Genetics, as a template.
Yeast strain EJG758 (Martzen et al. 1999) was used for Western and Northern blot experiments. PCR-based deletion of trm8 and trm82 with kanMX in this genetic background was done using primer pairs: 201D_F3 (5′-GACTCTCCCCACAAAGCCGG) and 201D_R3 (5′-CCACGTCGTAACATATGGTGATATTGC), and 165D_F and 165D_R (see above), using genomic DNA from strains 33899 (BY4743, trm8-Δ::kanMX) and 33523 (BY4743, trm82-Δ::kanMX), respectively, as templates. Chromosomal tagging of TRM8 at its C terminus was achieved in two rounds of PCR using TAP-cassette pAVA0258 as a template and primers TAP_3 (5′-GAGGGCGGTGTCGTGTACAC) and TAP_32 (5′-CTAGTTATACATCTATGTACGACTCACTATAGGG), TAP_3 and TAP_33 (5′-TGTATATGTGGTAAATTGTTCTAGTTATAC ATCTATGTAC). TAP-cassette pAVA0258, contained in succession TRM8, His6, HA epitope, the rhinovirus 3C protease site, ZZ domain derived from protein A of Staphylococcus aureus, and URA3 gene derived from Kluyveromyces lactis. It was constructed by PCR-amplification of the TRM8-His6-HA-Cprotease site-ZZ region (which will be described in detail elsewhere) with primers TAP_30 [5′-CTTATTGCCATGGGAGGGCGGTGTCGTGTACAC-3′] and TAP_31 [5′-ATAGTAACTGCAGTCACTGATGATTCGGG TCTACTTTCGG-3′] using PGAL10-TRM8-His6-HA-3Cprotease site-ZZ plasmid (Alexandrov et al. 2002) as a template, followed by digestion of the product with NcoI and PstI and ligation into the vector pBS1539 (Puig et al. 2001). Results of all genetic manipulations were confirmed by PCR.
Plasmids for protein expression in yeast
Open reading frames of TRM8 and TRM82 containing 600–650 base pairs of their upstream regions were amplified from yeast genomic DNA using corresponding primer pairs (201_own_F: 5′-TTC ACAT GCATG CCAGC ACAAGACAAGCTGATGGTG-3′, 201_own_R: 5′-GACTAATTCGAGCTCGTTACAATATGGCTGGC GTTGG-3′, 165_own_F: 5′-TTCACATGCATGCCCGCCAAACC AAGCATGTGC-3′, 165_own_R: 5′-GACTAATTCGAGCTCCT GCTATTCAATTCGCCGCCTTC-3′), digested with SphI and SacI and ligated into the URA3 CEN vector yCPlac33 and LEU2 CEN vector yCPlac111 (Gietz and Sugino 1988) to express these ORFs under their own promoters using a single-copy plasmid.
Expression of proteins under control of PCUP1 promoter was achieved in a PCUP1-GST-3Cprotease site-LIC vector pAVA0262, which was constructed from the plasmid pYEX 4T-1 (Martzen et al. 1999) by replacement of the thrombin site/MCS region (EcoRI, PstI) with 3Cprotease site/LIC-cloning region: 5′-GAATTCCTG GAAGTTCTGTTCCAGGGTCCTGGTTCGCGAATATTCTAGCT TTGTTTAAACAGCACGAACAAGTTCTGCAG-3′. Strains expressing proteins under PCUP1 control were grown for extract preparation or protein purification (Figs. 3 ▶, 5 ▶) as previously described (Martzen et al. 1999). Basal level of expression from PCUP1 plasmids was used for complementation experiments (Fig. 2 ▶), since overexpression of some protein variants resulted in inhibition of growth. TRM8 and TRM82 were amplified from yeast genomic DNA; YggH was amplified from E. coli genomic DNA, and T. maritima TM0925 was amplified from American Type Culture Collection clone 633437 using appropriate primer pairs:
(201_F_SG: 5′-GGGTCCTGGTTCGATGAAAGCCAAGCCACT AAGCC;
201_R_SG: 5′-CTTGTTCGTGCTGTTTATTACAATATGGCTG GCGTTGGTAATC;
165_F_SG: 5′-GGGTCCTGGTTCGATGAGCGTCATTCATC CTTTGCAG;
165_R_SG: 5′-CTTGTTCGTGCTGTTTACGCCGCCTTCAGC TAGAAACAGAG;
YGGH_F_LIC: 5′-GGGTCCTGGTTCGATGAAAAACGACGTCA TCTCACCG;
YGGH_R_LIC: 5′-CTTGTTCGTGCTGTTTATTATTTCACCCTC TCGAACATTAAGTCC;
TM_F_SG: 5′-GGGTCCTGGTTCGATGGTTGTAACAGAATAC GAACTG; and
TM_R_SG: 5′-CTTGTTCGTGCTGTTTATTAAGGAGAGCCCT GAGCGGTTAACTC).
ORFs were cloned into NruI and PmeI-digested and T4 DNA polymerase-treated (with 1 mM dATP) pAVA0262 using a standard LIC-cloning procedure (Aslanidis and de Jong 1990). Primer pairs used for mutagenesis:
Trm8_G/A_mut_F (5′-GCTGATATTGGCTGTGCATTCGGTG GGTTGATGATAGATTTATC) and Trm8_G/A_mut_R (5′-CCCACCGAATGCACAGCCAATATCAGCAATCGTCACCTTC);
Trm8_mut_F (5′-GCTGATATTGCCTGTGCATTCGGTGGGTT GATGATAGATTTATC) and Trm8_mut_R (5′-CCCACCGA ATGCACAGGCAATATCAGCAATCGTCACCTTC);
YggH_G/A_mut_F (5′-GCTTGAGATTGGTTTTGCCATGGGG GCGTCGCTGGTG) and YggH_G/A_mut_R (5′-CGCCCC CATGGCAAAACCAATCTCAAGCGTCACCGGCG);
YggH_mut_F (5′-GCTTGAGATTGCTTTTGCCATGGGGGCGT CGCTGGTG) and YggH_mut_R (5′-CGCCCCCATGGCAA AAGCAATCTCAAGCGTCACCGGCG);
TM_G/A_mut_F (5′-GGTTGAGATTGGTTTTGCAAACGGGGA ATTTCTGGCAGAACTTGC) and TM_G/A_mut_R (5′-CCC GTTTGCAAAACCAATCTCAACCACTATCTTTGCCTTTCTG); and
TM_mut_F (5′-GTGGTTGAGATTGCTTTTGCAAACGGGGAA TTTCTGGCAGAACTTGC) and TM_mut_R (5′-CCCGTTTG CAAAAGCAATCTCAACCACTATCTTTGCCTTTCTG).
All the resulting clones were sequenced.
Assay for m7G methyltransferase activity
[α-32P]GTP-labeled S. cerevisiae pre-tRNAPhe was incubated with protein in the presence of 1 mM S-adenosylmethionine, followed by P1 nuclease treatment of tRNA and thin layer chromatography of modified nucleotides, as described (Alexandrov et al. 2002).
Preparation of low-molecular-weight RNA
RNA was prepared by hot phenol extraction of harvested cells, as described (Rubin 1975).
Purification of individual tRNAPhe from RNA and HPLC analysis of modified nucleosides
Purification of tRNA was carried out as described (Alexandrov et al. 2002; Xing et al. 2004) using 5′-biotinylated DNA oligomer 5Bio-F1 (5′/Biotin/GTGGATCGAACACAGGACCT).
Western blot analysis
Fifteen OD600 units of yeast cells were lysed by vigorous shaking with glass beads in the presence of 100 μL of 2 × SDS PAGE loading buffer (100 mM Tris pH 6.8, 200 mM dithiothreitol, 4% SDS, 0.2% bromphenol blue, and 20% glycerol), incubated for 5 min in a boiling water bath, centrifuged for 10 sec at 16,000g, and resolved by 4%–15% SDS-PAGE. Western blot was performed using nitrocellulose membrane with peroxidase antiperoxidase as described (Puig et al. 2001).
Northern blot analysis
Northern blot analysis was performed using TRM8- and ACT1-specific DNA oligomers TRM8_N1R (5′-CATCCCTAAGATAA GATCTTCAGGGAAGGCTGG) and ACT13 (5′-TTAGAAA CACTTGTGGTGAACGATAG) with Bright Star-Plus membrane (Ambion) according to the manufacturer’s protocol.
Cross-linking
Thirty thousand cpm of [α-32P]UTP-labeled intron-containing pre-tRNAPhe (Reyes and Abelson 1987) was incubated for 20 min on ice with metal affinity-purified fractions of His6-MBP-Trm8, His6-MBP-Trm82, His6-MBP-Trm8/His6-MBP-Trm82 (Alexandrov et al. 2004), or control protein His6-MBP-3Cpro, and exposed to 254 nm UV light for another 20 min at 26°C. The reactions were treated with 0.5 μg of DNase-free RNase from bovine pancreas (Roche), and the products were separated by 5%–15% SDS-PAGE. Radioactivity incorporation was visualized using a phosphorimager (Molecular Dynamics).
Acknowledgments
We thank L. Kotelawala for valuable early contributions, and N. Shull, F. Xing, J. Jackman, and Y. Kon for valuable help and advice. This research was supported by NIH grant GM52347 to E.M.P.
Article published online ahead of print. Article and publication date are at http://www.rnajournal.org/cgi/doi/10.1261/rna.2030705.
REFERENCES
- Alexandrov, A., Martzen, M.R., and Phizicky, E.M. 2002. Two proteins that form a complex are required for 7-methylguanosine modification of yeast tRNA. RNA 8: 1253–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexandrov, A., Vignali, M., LaCount, D.J., Quartley, E., de Vries, C., De Rosa, D., Babulski, J., Mitchell, S.F., Schoenfeld, L.W., Fields, S., et al. 2004. A facile method for high-throughput co-expression of protein pairs. Mol. Cell. Proteomics 3: 934–938. [DOI] [PubMed] [Google Scholar]
- Anderson, J., Phan, L., Cuesta, R., Carlson, B.A., Pak, M., Asano, K., Bjork, G.R., Tamame, M., and Hinnebusch, A.G. 1998. The essential Gcd10p-Gcd14p nuclear complex is required for 1-methyl-adenosine modification and maturation of initiator methionyl-tRNA. Genes & Dev. 12: 3650–3662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson, J., Phan, L., and Hinnebusch, A.G. 2000. The Gcd10p/ Gcd14p complex is the essential two-subunit tRNA(1-methyl-adenosine) methyltransferase of Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. 97: 5173–5178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aslanidis, C. and de Jong, P.J. 1990. Ligation-independent cloning of PCR products (LIC-PCR). Nucleic Acids Res. 18: 6069–6074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahr, A., Hankeln, T., Fiedler, T., Hegemann, J., and Schmidt, E.R. 1999. Molecular analysis of METTL1, a novel human methyltransferase-like gene with a high degree of phylogenetic conservation. Genomics 57: 424–428. [DOI] [PubMed] [Google Scholar]
- Basavappa, R. and Sigler, P.B. 1991. The 3 A crystal structure of yeast initiator tRNA: Functional implications in initiator/elongator discrimination. EMBO J. 10: 3105–3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork, G.R. 1995. Biosynthesis and function of modified nucleosides. In tRNA: Structure, Biosynthesis and Function (eds. D. Soll, U.L. RajBhandary), pp. 165–205. ASM Press, Washington, DC.
- Bjork, G.R., Jacobsson, K., Nilsson, K., Johansson, M.J., Bystrom, A.S., and Persson, O.P. 2001. A primordial tRNA modification required for the evolution of life? EMBO J. 20: 231–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng, X., Kumar, S., Posfai, J., Pflugrath, J.W., and Roberts, R.J. 1993. Crystal structure of the HhaI DNA methyltransferase complexed with S-adenosyl-L-methionine. Cell 74: 299–307. [DOI] [PubMed] [Google Scholar]
- Colonna, A., Ciliberto, G., Santamaria, R., Cimino, F., and Salvatore, F. 1983. Isolation and characterization of a tRNA(guanine-7-)-methyltransferase from Salmonella typhimurium. Mol. Cell. Biochem. 52: 97–106. [DOI] [PubMed] [Google Scholar]
- De Bie, L.G., Roovers, M., Oudjama, Y., Wattiez, R., Tricot, C., Stalon, V., Droogmans, L., and Bujnicki, J.M. 2003. The yggH gene of Escherichia coli encodes a tRNA (m7G46) methyltransferase. J. Bacteriol. 185: 3238–3243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dihanich, M.E., Najarian, D., Clark, R., Gillman, E.C., Martin, N.C., and Hopper, A.K. 1987. Isolation and characterization of MOD5, a gene required for isopentenylation of cytoplasmic and mitochondrial tRNAs of Saccharomyces cerevisiae. Mol. Cell. Biol. 7: 177–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djordjevic, S. and Stock, A.M. 1998. Chemotaxis receptor recognition by protein methyltransferase CheR. Nat. Struct. Biol. 5: 446–450. [DOI] [PubMed] [Google Scholar]
- Droogmans, L., Roovers, M., Bujnicki, J.M., Tricot, C., Hartsch, T., Stalon, V., and Grosjean, H. 2003. Cloning and characterization of tRNA (m1A58) methyltransferase (TrmI) from Thermus thermophilus HB27, a protein required for cell growth at extreme temperatures. Nucleic Acids Res. 31: 2148–2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber, A.P. and Keller, W. 1999. An adenosine deaminase that generates inosine at the wobble position of tRNAs. Science 286: 1146–1149. [DOI] [PubMed] [Google Scholar]
- Gietz, R.D. and Sugino, A. 1988. New yeast-Escherichia coli shuttle vectors constructed with in vitro mutagenized yeast genes lacking six-base pair restriction sites. Gene 74: 527–534. [DOI] [PubMed] [Google Scholar]
- Grosshans, H., Lecointe, F., Grosjean, H., Hurt, E., and Simos, G. 2001. Pus1p-dependent tRNA pseudouridinylation becomes essential when tRNA biogenesis is compromised in yeast. J. Biol. Chem. 276: 46333–46339. [DOI] [PubMed] [Google Scholar]
- Gu, W., Jackman, J.E., Lohan, A.J., Gray, M.W., and Phizicky, E.M. 2003. tRNAHis maturation: An essential yeast protein catalyzes addition of a guanine nucleotide to the 5′ end of tRNAHis. Genes & Dev. 17: 2889–2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutgsell, N., Englund, N., Niu, L., Kaya, Y., Lane, B.G., and Ofengand, J. 2000. Deletion of the Escherichia coli pseudouridine synthase gene truB blocks formation of pseudouridine 55 in tRNA in vivo, does not affect exponential growth, but confers a strong selective disadvantage in competition with wild-type cells. RNA 6: 1870–1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodel, A.E., Gershon, P.D., Shi, X., and Quiocho, F.A. 1996. The 1.85 A structure of vaccinia protein VP39: A bifunctional enzyme that participates in the modification of both mRNA ends. Cell 85: 247–256. [DOI] [PubMed] [Google Scholar]
- Hopper, A.K. and Phizicky, E.M. 2003. tRNA transfers to the limelight. Genes & Dev. 17: 162–180. [DOI] [PubMed] [Google Scholar]
- Janner, F., Vogeli, G., and Fluri, R. 1980. The antisuppressor strain sin1 of Schizosaccharomyces pombe lacks the modification isopentenyladenosine in transfer RNA. J. Mol. Biol. 139: 207–219. [DOI] [PubMed] [Google Scholar]
- Johansson, M.J. and Bystrom, A.S. 2002. Dual function of the tRNA (m(5)U54)methyltransferase in tRNA maturation. RNA 8: 324–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalhor, H.R. and Clarke, S. 2003. Novel methyltransferase for modified uridine residues at the wobble position of tRNA. Mol. Cell. Biol. 23: 9283–9292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, S.H., Suddath, F.L., Quigley, G.J., McPherson, A., Sussman, J.L., Wang, A.H., Seeman, N.C., and Rich, A. 1974. Three-dimensional tertiary structure of yeast phenylalanine transfer RNA. Science 185: 435–440. [DOI] [PubMed] [Google Scholar]
- Labahn, J., Granzin, J., Schluckebier, G., Robinson, D.P., Jack, W.E., Schildkraut, I., and Saenger, W. 1994. Three-dimensional structure of the adenine-specific DNA methyltransferase M.Taq I in complex with the cofactor S-adenosylmethionine. Proc. Natl. Acad. Sci. 91: 10957–10961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laten, H., Gorman, J., and Bock, R.M. 1978. Isopentenyladenosine deficient tRNA from an antisuppressor mutant of Saccharomyces cerevisiae. Nucleic Acids Res. 5: 4329–4342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecointe, F., Simos, G., Sauer, A., Hurt, E.C., Motorin, Y., and Grosjean, H. 1998. Characterization of yeast protein Deg1 as pseudouridine synthase (Pus3) catalyzing the formation of ψ 38 and ψ 39 in tRNA anticodon loop. J. Biol. Chem. 273: 1316–1323. [DOI] [PubMed] [Google Scholar]
- Limbach, P.A., Crain, P.F., and McCloskey, J.A. 1994. Summary: The modified nucleosides of RNA. Nucleic Acids Res. 22: 2183–2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, X., Zhao, X., and Yu, Y.T. 2003. Pseudouridylation (Psi) of U2 snRNA in S. cerevisiae is catalyzed by an RNA-independent mechanism. EMBO J. 22: 1889–1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao, X., Schwer, B., and Shuman, S. 1995. Yeast mRNA cap methyltransferase is a 50-kilodalton protein encoded by an essential gene. Mol. Cell. Biol. 15: 4167–4174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ———. 1996. Mutational analysis of the Saccharomyces cerevisiae ABD1 gene: Cap methyltransferase activity is essential for cell growth. Mol. Cell. Biol. 16: 475–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martzen, M.R., McCraith, S.M., Spinelli, S.L., Torres, F.M., Fields, S., Grayhack, E.J., and Phizicky, E.M. 1999. A biochemical genomics approach for identifying genes by the activity of their products. Science 286: 1153–1155. [DOI] [PubMed] [Google Scholar]
- Massenet, S., Motorin, Y., Lafontaine, D.L., Hurt, E.C., Grosjean, H., and Branlant, C. 1999. Pseudouridine mapping in the Saccharomyces cerevisiae spliceosomal U small nuclear RNAs (snRNAs) reveals that pseudouridine synthase pus1p exhibits a dual substrate specificity for U2 snRNA and tRNA. Mol. Cell. Biol. 19: 2142–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaud, J., Kudoh, J., Berry, A., Bonne-Tamir, B., Lalioti, M.D., Rossier, C., Shibuya, K., Kawasaki, K., Asakawa, S., Minoshima, S., et al. 2000. Isolation and characterization of a human chromosome 21q22.3 gene (WDR4) and its mouse homologue that code for a WD-repeat protein. Genomics 68: 71–79. [DOI] [PubMed] [Google Scholar]
- Niewmierzycka, A. and Clarke, S. 1999. S-adenosylmethyionine-dependent methylation in Saccharomyces cerevisiae. J. Biol. Chem. 274: 814–824. [DOI] [PubMed] [Google Scholar]
- Okamoto, H., Watanabe, K., Ikeuchi, Y., Suzuki, T., Endo, Y., and Hori, H. 2004. Substrate tRNA recognition mechanism of tRNA (m7G46) methyltransferase from Aquifex aeolicus. J. Biol. Chem. 279: 49151–49159. [DOI] [PubMed] [Google Scholar]
- Pintard, L., Lecointe, F., Bujnicki, J.M., Bonnerot, C., Grosjean, H., and Lapeyre, B. 2002. Trm7p catalyses the formation of two 2′-O-methylriboses in yeast tRNA anticodon loop. EMBO J. 21: 1811–1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puig, O., Caspary, F., Rigaut, G., Rutz, B., Bouveret, E., Bragado-Nilsson, E., Wilm, M., and Seraphin, B. 2001. The tandem affinity purification (TAP) method: A general procedure of protein complex purification. Methods 24: 218–229. [DOI] [PubMed] [Google Scholar]
- Reyes, V.M. and Abelson, J. 1987. A synthetic substrate for tRNA splicing. Anal. Biochem. 166: 90–106. [DOI] [PubMed] [Google Scholar]
- Robertus, J.D., Ladner, J.E., Finch, J.T., Rhodes, D., Brown, R.S., Clark, B.F., and Klug, A. 1974. Structure of yeast phenylalanine tRNA at 3 A resolution. Nature 250: 546–551. [DOI] [PubMed] [Google Scholar]
- Rubin, G.M. 1975. Preparation of RNA and ribosomes from yeast. Methods Cell. Biol. 12: 45–64. [DOI] [PubMed] [Google Scholar]
- Sherman, F. 1991. Getting started with yeast. Methods Enzymol. 194: 3–21. [DOI] [PubMed] [Google Scholar]
- Sprinzl, M., Horn, C., Brown, M., Ioudovitch, A., and Steinberg, S. 1998. Compilation of tRNA sequences and sequences of tRNA genes. Nucleic Acids Res. 26: 148–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, P., Brockenbrough, J.S., Paddy, M.R., and Aris, J.P. 1998. NCL1, a novel gene for a non-essential nuclear protein in Saccharomyces cerevisiae. Gene 220: 109–117. [DOI] [PubMed] [Google Scholar]
- Xing, F., Martzen, M.R., and Phizicky, E.M. 2002. A conserved family of Saccharomyces cerevisiae synthases effects dihydrouridine modification of tRNA. RNA 8: 370–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, X., Patton, J.R., Davis, S.L., Florence, B., Ames, S.J., and Spanjaard, R.A. 2004. Regulation of nuclear receptor activity by a pseudouridine synthase through posttranscriptional modification of steroid receptor RNA activator. Mol. Cell 15: 549–558. [DOI] [PubMed] [Google Scholar]