Abstract
In (–)-stranded replication intermediates of the potato spindle tuber viroid (PSTVd) a thermodynamically metastable structure containing a specific hairpin structure (HP II) has been proposed to be essential for viroid replication. In the present work a method was devised allowing the direct detection of the HP II structure in vitro and in vivo using a biophysical approach. An RNA oligonucleotide was constructed which specifically binds to the HP II loop region in transient (–)-strand intermediates. Analysis of the resulting oligonucleotide/HP II complexes on temperature-gradient gels enabled us to follow the formation of HP II during in vitro transcription by T7 RNA polymerase. Moreover, we were able to demonstrate the formation of HP II during viroid replication in potato (Solanum tuberosum) cells.
INTRODUCTION
Viroids are plant pathogens distinguished from viruses by the absence of a protein coat, the absence of protein coding capacity and by their small size. They are circular, single-stranded RNA molecules with sizes ranging from 245 to 461 nt. It is generally assumed that most reactions of viroid replication and pathogenesis depend on enzymes of the host (for reviews see 1–3).
The following introduction to the replication of viroids is based mainly on the analyses of the potato spindle tuber viroid (PSTVd), which is the type strain of the major viroid genus of family Pospiviroidae (4). Pospiviroidae are replicated according to an asymmetric rolling circle model (5), whereas other viroids like Avsunviroidae follow a symmetric rolling circle model (6,7). Native PSTVd RNA adopts an unbranched, rod-like structure with a high degree of intramolecular base pairing (8,9). The circular PSTVd [cPSTVd, by definition (+)-strand] is transcribed by the host encoded DNA-dependent RNA polymerase II into oligomeric linear RNA (–)-strands (Fig. 1), which are then transcribed into oligomeric linear RNA (+)-strands by the same enzyme (10,11). The oligomeric (+)-strands are processed to monomeric units and ligated to circles by host-encoded RNase(s) and ligase(s), respectively (12,13). Self-cleavage could be ruled out for Pospiviroidae (14) but was found for Avsunviroidae (6,7). For PSTVd, a specific cleavage–ligation site and corresponding structural motif involved in the processing reaction were elucidated (13,15).
Figure 1.
Replication cycle of viroids of the PSTVd class. The mature circular viroid RNA adopts a rod-shaped secondary structure under native conditions (top). Complementary sequences which can form hairpins I, II and III are indicated correspondingly. In vivo transcription both from (+) to (–) (step 1) and (–) to (+) (step 2) is carried out by RNA polymerase II (11). Sequential folding during synthesis of (–)-stranded oligomers (step 1) leads to metastable conformations including HP II, which is involved in the next step, i.e. synthesis of (+)-stranded oligomers (step 2). The (+)-stranded oligomers rearrange into structures with structural termini (L and R) similar to that of the mature circle. In the central part, however, a metastable structure including extra-stable tetra-loop hairpins (tl) is formed (13). This structure is recognized by host RNase(s) and ligase(s) that process the replication intermediate to circles. Step 3 represents 5′-cleavage, step 4 3′-cleavage and ligation within the tl region. The processing site is marked by a triangle. The (+)-circles rearrange easily to the rod-shaped structure (top). The sequences which are involved in the formation of the HP II stem are shown in detail in the top right part of the Figure.
Well defined structural elements were also found as motifs critical for replication. Recently, the nucleotides A111 and A325 at a distance of 16 and 15 nt, respectively, downstream of GC boxes, have been identified as start sites for (–)-strand synthesis, and the GC boxes were discussed as promotor-like elements (16).
In addition, from site-directed mutagenesis of PSTVd it could be concluded that the formation of a thermodynamically metastable structure including a GC-rich hairpin [the so-called hairpin II (HP II)] is critical for infectivity (17). An independent study reported recently that in non-infectious recombinant viroids the restoration of the correct base pairing in the HP II structure leads to the recovery of infectivity (18). Further analysis suggested that HP II is a functional element of the (–)-strand replication intermediate (19). Current models hold that HP II, as part of a metastable structure within the (–)-stranded oligomer, is probably formed as a result of sequential folding steps during viroid replication. Sequential folding processes leading to structure formation were proposed earlier (20–22). Computer algorithms that approximate the kinetic features of the RNA folding process, including folding during synthesis, have been used to predict such metastable structures (23,24).
Simulations using in vitro transcription of PSTVd cDNA templates with T7 RNA polymerase in combination with temperature-gradient gel electrophoresis (TGGE) (25,26) have revealed the generation of such metastable structures in addition to the formation of the thermodynamic most stable rod-like structure (15,27–29). From experiments where the elongation rate of the RNA polymerase was adjusted to the in vivo rate it was concluded that rearrangement into the rod-like structure is slow and does not abolish the metastable structures in biologically relevant times (29). However, up to now only the existence of metastable structures after in vitro transcription could be demonstrated experimentally (27), although their structural details remained elusive. In particular, the existence of HP II as a structural element of these metastable configurations in oligomeric (–)-strands has not been demonstrated directly. Chemical mapping could not be applied since the structure of interest is probably not present in a high fraction of molecules. To address these questions we developed a method utilizing an RNA oligonucleotide probe which binds and thereby specifically detects any structure containing HP II. We combined the oligonucleotide mapping technique with TGGE and analyzed the formation of HP II during in vitro transcription of monomeric and dimeric PSTVd (–)-strands. We also assayed the effects of initiating transcription at different sites on the model viroid sequences. Furthermore, we were able to extend these studies to extracts from PSTVd-infected cell cultures and in doing so we could show that HP II is indeed formed during in vivo replication of PSTVd.
MATERIALS AND METHODS
Templates for in vitro transcription
Four plasmids, pSH1, pRH716, pRH720 and pRH736, were used as templates for transcription with T7 RNA polymerase. All plasmids are derivatives of pRH701 (27). After restriction with either Sty1, HaeIII or AluI, the sense DNA of PSTVd strain Intermediate (Di) was cloned into the multicloning site of pRH701. After linearization with EcoRI, run-off transcription with T7 RNA polymerase results in: from plasmid pSH1 a 372 nt transcript with the sequence 5′-GGCAAG-(–)PSTVd Intermediate (Di) (337–1/359–338)-GGGAAUU-3′, called PSTVd-Sty1; from plasmid pRH716 a 368 nt transcript with the sequence 5′-GG-(–)PSTVd Intermediate (Di) (146–1/359–147)-GGGAAUU-3′, called PSTVd-HaeIII; from plasmid pRH720 a 727 nt transcript with the sequence 5′-GG-(–)PSTVd Intermediate (Di) [146–1/(359–1)/359–147]-GGGAAUU-3′, called PSTVd-HaeIII/dimer; from plasmid pRH736 a 1086 nt transcript with the sequence 5′-GG-(–)PSTVd Intermediate (Di) [281–1/(359–1)(359–1)/359–282]-GGGAAUU-3′, called PSTVd-AluI/trimer. After linearization of pSH1 with Bsp1286, run-off transcription with T7 RNA polymerase results in a 203 nt transcript with sequence 5′-GGCAAG-(–)PSTVd Intermediate (Di) (337–141)-3′, called PSTVd-Sty1/Bsp1286 (IVTBSP).
T7 RNA polymerase transcription
T7 run-off transcripts were performed at 37°C for 1.5 h (27).
Temperature-gradient gel electrophoresis
Structure distributions after transcription were characterized by TGGE (30). Perpendicular to the direction of electrophoresis a linear temperature gradient was established, resulting in the separation of different secondary structures of the transcripts. Gels are 0.2 mm thick and contained 4 or 5% (w/v) polyacrylamide, 0.17% bisacrylamide, 0.1% (v/v) TEMED, 0.2× TBE and 0.04% ammonium peroxodisulfate for starting the polymerization. Electrophoresis of samples was performed in three steps: (i) electrophoresis for 15 min with 30 V/cm at a constant temperature of 15°C, (ii) establishment of the temperature gradient, and (iii) electrophoresis for 1.5–3 h at 30 V/cm. Gels were stained with silver (31) and/or exposed to X-ray film (Kodak Xomat AR).
Calculation of RNA secondary structures
The calculation of RNA secondary structures and structural transitions was carried out on a DECstation 3800 (Digital Equipment) using the algorithm LinAll published previously (32).
RNA and DNA oligonucleotides
The RNA oligonucleotides 14A, complementary to the PSTVd (–)-sequence 238–251 (5′-UUUGCGCUGUCGCU-3′) and the RNA oligonucleotide 27AB complementary to PSTVd (–)-sequence 318–307/237–251 (5′-CUUACUUGCUUCCU UUGCGCUGUCGCU-3′) were synthesized on a nucleic acid synthesizer (model 381A, Applied Biosystems) using 2′-O-Fpmp-RNA-CE-Phosphoramidites (MWG Biotech). The chemically modified oligo RNA 27AB (2′-OMe-RNA) was purchased from Eurogentec (Maastricht). For reverse transcription DNA primer AS2, complementary to PSTVd (–)-sequence 137–164 (5′-TGCCCAGCGGCCGACAGGAGT AATTCCC-3′) was used. All oligonucleotides were gel-purified before use.
Complex formation between the HP II containing RNA structures and RNA oligonucleotides 14A and 27AB
Complexes were formed by incubating 200 ng of in vitro transcript (IVTBSP, PSTVd-HaeIII or PSTVd-Sty1) for 20 min in buffer HS (500 mM sodium chloride, 4 M urea, 1 mM sodium cacodylate, 0.1 mM EDTA pH 7.0) with 105 c.p.m. 27AB or 14A, respectively. After subsequent dialysis against 0.2× TBE buffer, TGGE analysis in a 5% polyacrylamide gel was performed.
DMS modification analysis
Chemical modifications were carried out in a 200 µl volume containing 1 µg of the RNA transcript IVTBSP or the complexes IVTBSP/27AB, 50 mM HEPES–KOH pH 8.0, 1 mM EDTA, 7 µg of tRNA and 0.1% DMS for 15 min on ice. The reaction was stopped by addition of 14 µg of carrier tRNA and a subsequent ethanol precipitation, which was repeated once after resuspending the pellet. Modification sites were identified by primer extension of 50 ng of modified RNA with labeled primer AS2.
The RNA complexes IVTBSP/27AB were previously established by an incubation of 1 µg of IVTBSP with a 7-fold molar excess of oligo RNA 27AB in HS buffer at 65°C for 20 min. After the reaction mix was snap cooled and dialyzed against TE buffer, the DMS modification reaction was performed as described above. Under these conditions all IVTBSP transcripts are complexed by the RNA oligonucleotide. The modified complexes were separated on a denaturing gel in order to dissociate the complexes. The uncomplexed IVTBSP was eluted from the gel, and the modification sites were identified by primer extension of 50 ng of modified RNA with radiolabeled primer AS2 (107 c.p.m.) and analyzed on denaturing 8% polyacrylamide gels in comparison with the viroid sequence on plasmid DNA.
Preparation of nuclei and native RNA isolation
The preparation of nuclei was performed as described previously (15). It was modified only in respect to the buffers omitting PMSF, leupeptin and pepstatin.
To dissolve the chromatin structure for the RNA isolation under native conditions DNaseI (20 U) was added and incubated for 20 min at room temperature together with 14 µg of tRNA, 800 U RNasin, 80 mM ribonucleoside vanadyl complex (a gift from Mr Bernd Esters) and 0.24% NP-40. Total nucleic acid was extracted by two phenol/chloroform extractions and precipitated by ethanol.
RESULTS
Construction of a short RNA transcript forming HP II
HP II is assumed to be part of a metastable structure formed transiently during viroid replication or during in vitro transcription using viroid cDNA as a template. The lack of naturally occurring stable HP II structures makes the development of a probe for its presence very difficult. Here we present the development of a model system that allows the detection of metastable HP II structures using an oligonucleotide probe.
Using in vitro transcription we synthesized a short model RNA transcript which contains HP II as the thermodynamically most stable structure. This allows an oligonucleotide probe to be validated by tests on this RNA, and this probe can be used to analyze more complicated RNA populations. For the in vitro transcription of this model RNA we linearized a monomeric PSTVd sequence containing plasmid (pSH1) with Bsp1286 for use as template. A (–)-stranded 203 nt run-off in vitro transcript encompassing viroid nucleotides 337–141 (relative to nucleotide 1 in the viroid sequence) was generated from this template and named in vitro transcript BSP (IVTBSP) according to the Bsp1286 restriction site used. The viroid fragment represented by IVTBSP is shown in Figure 2A on a rod-like viroid model.
Figure 2.
Structure of the HP II model RNA transcript (IVTBSP). (A) The 5′ and 3′ ends of the in vitro transcript (–) IVTBSP (viroid residues 337 and 141, respectively) are indicated within the rod-shaped viroid structure. The sequence elements necessary for the formation of HP II are shown as gray bars. (B) The optimal secondary structure of the resulting transcript suggested by thermodynamic calculations using the program LinAll is shown. The HP II stem structure is marked in gray. (C) Silver-stained polyacrylamide temperature-gradient gel. Structures of IVTBSP were analyzed directly after in vitro transcription. Two co-existing structures ‘L’ and ‘D’ are indicated by arrows. The major structure ‘D’ shows two structural transitions which can be interpreted according to thermodynamic calculations as follows: the first transition ‘a’ is attributed to denaturation of base pairs adjoining the HP II loop and the second transition ‘b’ to rearrangements in the 3′ part of IVTBSP. HP II denatures at temperatures higher than 60°C. Structures ‘L’ and ‘D’ differ only in the 3′ part of the RNA but both contain HP II (data not shown).
Thermodynamic calculations using the program LinAll (32) suggested that HP II is formed in the 5′ part of IVTBSP, whereas its 3′ end is folded into a double-stranded helix with internal loops. The thermodynamically most stable secondary structure for IVTBSP resulting from those calculations is shown in Figure 2B. TGGE analyses to examine the secondary structure were carried out either directly after in vitro transcription or after forming the equilibrium structure by heating and slowly cooling the transcript under low salt conditions. Directly after in vitro transcription two structures were detectable on the temperature-gradient gel: a predominant structure (D) and a minor second structure (L) with a reduced mobility at lower temperatures (Fig. 2C). Under equilibrium conditions only structure D could be detected on a temperature-gradient gel. The second minor structure L visible only after in vitro transcription without further treatment was predicted to represent a HP II containing structure with a slightly rearranged 3′-structural segment using LinAll (data not shown). Chemical mapping of the model RNA transcript with DMS was carried out on structure D and the results are in good agreement with the predicted secondary structure in Figure 2B; analysis of structure D by DMS mapping are shown in Figure 4C. Hence, the TGGE and mapping results strongly support the existence of HP II within the IVTBSP model RNA transcript.
Figure 4.
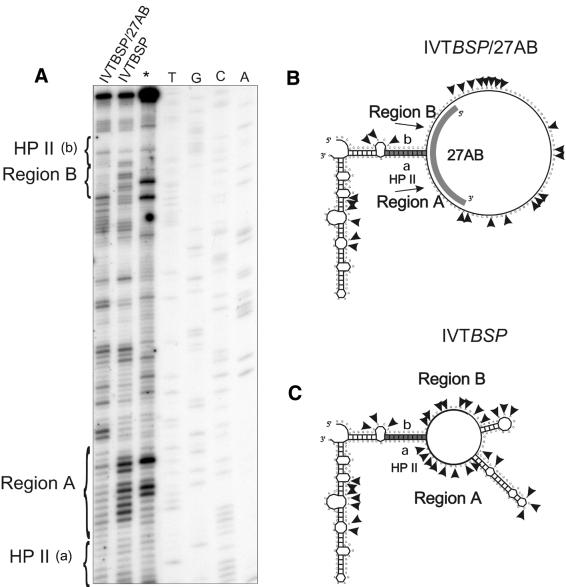
Chemical modification of the HP II model RNA transcript either alone or in complex with RNA oligonucleotide 27AB. (A) Chemical modification of the HP II model RNA transcript either alone or in complex with RNA oligonucleotide 27AB. Complex formation and chemical modification were performed as described in Materials and Methods. The modification sites were identified by primer extension and analyzed on denaturing 8% polyacrylamide gels in comparison with the viroid sequence on plasmid DNA (lanes T, G, C, A). In the lane marked by an asterisk a control reaction with unmodified IVTBSP is shown. The sequence regions ‘a’ (nucleotides 227–236 according to the full-length viroid) and ‘b’ (nucleotides 319–328) involved in the formation of the HP II stem are indicated at the margin. The sequence regions A and B complementary to the RNA oligonucleotide 27AB are also indicated. (B) The DMS modification sites shown on the secondary structure model of a IVTBSP/27AB complex. Since the secondary structure of the HP II loop region after the formation of the IVTBSP/27AB complex is unknown, it is shown single stranded to simplify the model. One should note that due to the complex formation between 27AB and HP II the secondary structure within the loop region of HP II is affected even in the region where the RNA oligonucleotide does not bind. (C) The DMS modification sites shown on the secondary structure of IVTBSP identified in three independent experiments. The RNA oligonucleotide binding region 27AB is accessible in free IVTBSP but inaccessible in IVTBSP/27AB complexes visible as a different modification pattern.
Development of two RNA oligonucleotides directed against the HP II structure
The accessibility of HP II in the stable IVTBSP structure allowed us to develop specific probes for this RNA species. Two RNA oligonucleotides complementary to a sequence within the HP II loop were synthesized. The first RNA oligonucleotide (14A) is complementary to a sequence adjacent to the 3′ end of the HP II stem (Fig. 3, top right). This region should be accessible in HP II containing structures, but inaccessible in the stable rod-like structure of the fully folded viroid. The radiolabeled RNA oligonucleotide was incubated for 20 min at room temperature with IVTBSP under high salt conditions. Products were separated on a temperature-gradient gel and visualized by silver staining (Fig. 3, top left) and autoradiographed (Fig. 3, top middle). The autoradiograph in Figure 3 (top middle) clearly shows that 14A binds to the model RNA. The shifted complex bands (C) consisting of structures D and L (see Fig. 2) migrate with a reduced mobility compared with the uncomplexed structures. The complex is stable up to 33.5°C under the low salt conditions during TGGE. At temperatures above 34°C oligo 14A dissociates from the RNA transcript.
Figure 3.
Complex formation between the HP II model RNA transcript and RNA oligonucleotides 14A and 27AB. The binding sites of the two RNA oligonucleotides 14A (14 nt) and 27AB (27 nt) complementary to the HP II loop region are shown in the right panels. After the radioactively labeled RNA oligonucleotides were incubated with IVTBSP TGGE analysis was performed. The gels were stained with silver (left) and afterwards exposed for autoradiography (middle). The IVTBSP/oligo complexes ‘C’ are marked with an arrow. They show a reduced mobility compared with the uncomplexed HP II model RNA transcripts visible as structures ‘D’ and ‘L’ in the silver-stained gels. The stability of the IVTBSP/27AB complex is significantly higher than the stability of the IVTBSP/14A complex, as predicted.
When full-length viroid (+) and (–)-RNA strands were generated by in vitro transcription followed by heating and cooling to form the fully paired stable rod-like RNA structures no complex formation can be seen with 14A under otherwise identical conditions (data not shown). The fact that 14A binds to IVTBSP but not to the rod-like structure indicates that the complementary nucleotides in IVTBSP are single stranded or located in a region of low stability; it does not, however, directly prove that the RNA oligonucleotide 14A binds to a putative HP II loop structure.
To demonstrate this directly a second RNA oligonucleotide (27AB) was synthesized. This RNA oligonucleotide binds to the HP II loop region A and B adjacent to the hairpin stem forming a double-stranded helix perpendicular to the stem (Fig. 3, bottom right, regions A and B). These two sequence regions A and B are adjacent to each other only when HP II has been formed. The radiolabeled RNA oligonucleotide 27AB was incubated for 20 min at room temperature with IVTBSP as described above. Products were separated on a temperature-gradient gel and visualized by silver staining (Fig. 3, bottom left) and autoradiographed (Fig. 3, bottom middle). The autoradiograph shows that 27AB binds to the IVTBSP with a higher stability compared with 14A under the same conditions. The complexes are stable up to 41°C. These data suggest that RNA oligonucleotide 27AB binds both regions in the loop (see Fig. 3, region A and region B) adjacent to the HP II stem.
Next, we confirmed HP II formation and the binding site of 27AB by assaying protection from DMS modification (Fig. 4). Region B as well as region A in the HP II loop are accessible to DMS in the uncomplexed model RNA transcript (see Fig. 4C and A, lane IVTBSP), whereas they are inaccessible to DMS in the complex with IVTBSP (see Fig. 4B and A, lane IVTBSP/27AB). These experiments confirm that RNA oligonucleotide 27AB binds specifically within the predicted HP II loop region. Taken together, these data demonstrate that oligonucleotide 27AB in combination with TGGE can serve as a reliable structural probe for HP II containing structures. Complexes formed between HP II and oligo 27AB show a characteristic melting temperature in TGGE (41°C; Fig. 3). Monitoring this melting temperature on TGGE allows to distinguish between 27AB/HP II complexes and not fully paired complexes formed between the 3′ part of 27AB and different metastable structures.
Formation of HP II during in vitro transcription of (–)-stranded PSTVd sequences
To address the question if HP II structures are formed during in vitro transcription, different (–)-stranded PSTVd transcripts were generated by in vitro transcription from plasmid templates and probed with 27AB.
Two different plasmid templates were transcribed to generate monomeric (–)-stranded viroid RNAs with different start points of transcription (PSTVd-StyI and PSTVd-HaeII), allowing the influence of the transcriptional start site on the formation of HP II to be assayed. Transcription of the monomeric viroid sequences started either at nucleotide 147 (PSTVd-HaeII, Fig. 5A) or at nucleotide 337 (PSTVd-StyI, Fig. 6A) (27,29).
Figure 5.
Detection of HP II after in vitro transcription of monomeric (–)-stranded PSTVd sequences (PSTVd-HaeIII). (A) The transcriptional start site of PSTVd-HaeIII is shown on a viroid rod-shaped model structure. (B) In vitro transcription was performed using the plasmid vector pRH716 after linearization with EcoRI (see Materials and Methods). The presence of HP II after in vitro transcription was tested by incubation of radiolabeled RNA oligonucleotide 27AB with transcript. TGGE analysis was performed and the gel was stained with silver. On the silver-stained gel several bands are detectable (S*, X, W, M*, B). (C) Autoradiograph of the silver-stained gel. Only the RNA species complexed with RNA oligo 27AB ‘C1’, ‘C2’ and ‘C3’ are visible. See text for details.
Figure 6.
Detection of HP II after in vitro transcription of monomeric (–)-stranded PSTVd sequences (PSTVd-StyI). (A) The transcriptional start site of PSTVd-StyI is shown on a viroid rod-shaped model structure. (B) In vitro transcription was performed using the plasmid vector pSH1 after linearization with EcoRI (see Materials and Methods). The presence of HP II after in vitro transcription was tested by incubation of radiolabeled RNA oligonucleotide 27AB with transcript. TGGE analysis was performed and the gel stained with silver. On the silver-stained gel several bands are detectable (S, M, Q/P, R). (C) Autoradiograph of the silver-stained gel. Only the RNA species complexed with RNA oligo 27AB ‘C4’ and ‘C5’ are visible. See text for details.
Transcription of PSTVd-StyI starts with the synthesis of sequences necessary for the formation of HP II. Therefore HP II containing structures are expected to form due to sequential folding of the transcript. In contrast, the transcript PSTVd-HaeIII starts in the upper right part of the rod-shaped structure fairly distant from HP II, and therefore metastable structures containing HP II are expected to form potentially with lower probabilities.
The analyses of these templates are summarized in Figures 5 and 6. RNA PSTVd-HaeIII (Fig. 5) and PSTVd-StyI (Fig. 6) RNA were synthesized for 1.5 h and probed with radiolabeled 27AB for 20 min immediately after in vitro transcription. Products were separated on a temperature-gradient gel and visualized by silver staining and autoradiography. Thermodynamic and kinetic calculations suggest that HP II structures should still be present under these conditions. However, most of the metastable structures are expected to rearrange into the most stable rod-shaped secondary structure (27,29,33).
In the silver-stained temperature-gradient gel of PSTVd-HaeIII transcripts (Fig. 5B) several bands are detectable after in vitro transcription. The band S* with the highest mobility can be attributed to the rod-shaped structure as reported earlier (29) and bands with reduced mobilities (W, M*, X) have been found to originate from metastable structures with more bulky shape (29). In the upper part of the silver-stained gel bimolecular complexes (B) formed between two monomeric viroid sequences are visible (Fig. 5B). Note that due to the very low amount of radiolabeled RNA oligonucleotide (2 ng) the shifted complex bands are only visible on the autoradiograph but not on the silver-stained gel.
On the autoradiograph (Fig. 5C), several radiolabeled PSTVd-HaeIII/27AB complexes which contain HP II (C1, C2, C3) are visible. These data demonstrate that HP II is formed during in vitro transcription of PSTVd-HaeIII. Still, the amount of HP II in some RNA species seems to be rather low under this experimental condition, as expected. For example, the complexed band C3 is undetectable by silver staining, but it is detected with the labeled 27AB probe (Fig. 5C). Therefore, probing with radiolabeled oligo 27AB proves to be a more sensitive method for the detection of the metastable HP II structure.
Figure 6 presents the results of a similar experiment performed on PSTVd-Sty1 transcripts. In the silver-stained temperature-gradient gel of PSTVd-Sty1 transcripts (Fig. 6B), the rod-shaped structure S and additional bands which most likely contain metastable structures (M, Q/P, R) are visible. From the autoradiograph shown in Figure 6C it is clear that some of these bands do contain HP II structures but due to the shift of the oligonucleotide/transcript complexes (C4, C5) it cannot be unequivocally decided which of the bands M, Q/P and R contain HP II.
Experiments with other monomeric and dimeric (–)-stranded in vitro transcripts starting at different start sites yielded comparable results (data not shown). The amount of HP II structures within the structural ensemble was in the range of 1–10% of all RNA structures.
Adapting the oligonucleotide binding assay to in vivo conditions
Next, we attempted to study the formation of HP II containing structures during PSTVd replication in vivo in nuclei of potato cells. To this aim we modified 27AB to contain a 2′-OMe group instead of the 2′-OH group which provides resistance to RNase cleavage.
As a control, we initially asked whether the HP II/oligonucleotide 2′-OMe 27AB complexes can form in potato nuclei and are stable to degradation or denaturation during the purification procedure from nuclei. To simplify this control experiment we added PSTVd-StyI in vitro transcripts and radiolabeled oligonucleotide 2′-OMe 27AB to nuclei from uninfected potato cells (strain HH258) (34). We also added an excess of tRNA (14 µg), RNase inhibitor (800 U RNasin) and 80 mM ribonucleoside vanadyl complex as RNase protectants and 0.24% NP-40 to enable diffusion of the oligonucleotide into the nuclei in subsequent experiments. After RNA extraction as described in Materials and Methods under native conditions, a TGGE analysis was performed to verify the structural integrity of the isolated complexes (Fig. 7). As reported earlier, we found that a phenol/chloroform extraction and ethanol precipitation does not denature the previously formed HP II structures (35).
Figure 7.

Detection of HP II within PSTVd-StyI transcripts during native preparation of total nucleic acid from nuclei. 106 nuclei from non-infected potato cells were incubated with radiolabeled 2′-OMe-RNA oligonucleotide 27AB (107 c.p.m.). One microgram of PSTVd-StyI transcript was added to the nuclei. Total nucleic acid was isolated under native conditions as described in Materials and Methods. After a subsequent dialysis against 0.2× TBE buffer a 5% polyacrylamide TGGE analysis was performed and the gel exposed for 12 h. The corresponding autoradiograph is shown, and the PSTVd-StyI/27AB complexes are marked by ‘C’.
The results demonstrate that HP II containing structures can form complexes with 27AB 2′-OMe under the above conditions and that the complexes retain structural integrity during the purification procedure. As expected, the modified oligonucleotide 27AB 2′-OMe showed the same binding stability as the non-modified oligonucleotide 27AB. Additional titration experiments show that a limit of 50 pg of HP II containing transcripts can still be detected (data not shown).
Focusing of differently sized PSTVd replication intermediates into one band
Another experimental difficulty we had to overcome is that the total concentration of (–)-stranded replication intermediates in nuclei is very low, and the replication intermediates are distributed over a size range of one up to six PSTVd units (3,27,36–38). For that reason we attempted to focus the different lengths of the natural replication intermediates into one band by using TGGE with three different acrylamide layers to gain higher sensitivity.
As a test system, a mixture of monomeric (M, PSTVd-StyI/monomer), dimeric (D, PSTVd-HaeIII/dimer) and trimeric (T, PSTVd-AluI/trimer) (–)-stranded transcripts was used to determine optimal focus conditions. The transcripts were added with the radiolabeled oligonucleotide 27AB 2′-OMe to the nuclei from uninfected cell cultures and the RNA isolated as described before.
The differently sized RNAs migrate through the first layer (3% polyacryamide, 0.5% agarose) before entering a highly concentrated polyacrylamide layer (15% acrylamide). Only the uncomplexed radiolabeled oligonucleotide 27AB 2′-OMe migrates further due to its small size, whereas the different sized RNA transcripts are stacked between the two gel layers. After entering the third gel layer with lower concentration (3% polyacrylamide), the uncomplexed radiolabeled oligonucleotides 27AB 2′-OMe migrate faster again and can be separated from the complexes between the first and second layer. Figure 8 shows the result of such a focusing TGGE analysis.
Figure 8.
Focusing of heterogeneous PSTVd transcripts. 300 ng of monomeric (M), dimeric (D) and trimeric (T) (–)-stranded PSTVd transcripts obtained by in vitro transcription of pSH1, pRH720 and pRH736 were incubated with radiolabeled 27AB 2′-OMe (105 c.p.m.) for 20 min at room temperature. After subsequent dialysis against 0.2× TBE a three-layer gel analysis was performed and exposed for 12 h. The three different layers of the gel are indicated at the right margin. The monomeric, dimeric and trimeric transcripts were focused between the first and the second layer. In the bottom of the gel the uncomplexed radiolabeled 27AB 2′-OMe oligonucleotides are visible.
Besides the fast moving band of the uncomplexed radiolabeled oligonucleotide 27AB 2′-OMe, the three barely separated bands of the RNA transcripts are detectable at the border between the 15% and the upper 3% gel. These bands vanish at 41°C, the characteristic temperature for the dissociation of the oligo 27AB from the HP II loop region. It should be noted that due to the high polyacrylamide gel concentration of the second layer structural transitions of the long RNA molecules are no longer visible.
Detection of HP II in naturally formed (–)-stranded replication intermediates
After adapting the method described above we attempted to analyze the formation of HP II containing structures during PSTVd replication in vivo in potato cell nuclei. PSTVd-infected nuclei (strain M316) (34) were incubated with radiolabeled oligonucleotide 27AB 2′-OMe under the conditions described above. Under these conditions the radiolabeled oligo 2′-OMe-RNA 27AB should be able to pass the nuclei membranes by diffusion since the concentration of NP-40 (0.24%) has been reported previously to slightly damage the nuclei membranes (39). Total RNA was extracted from these nuclei and a focusing temperature-gradient gel analysis was performed. The 15% highly concentrated polyacrylamide layer was replaced by an 18% layer to further improve the concentration effect (Fig. 9). A radiolabeled band representing the oligonucleotide/HP II complexes is detectable at the boundary between the 3 and 18% layers which vanishes at 41°C, the characteristic temperature at which HP II/27AB 2′-OMe complexes dissociate. In a control experiment using RNA extracted from non-infected cell cultures no radioactive signal could be detected showing that the signal is not derived from unspecific hybridization (data not shown). The presence of this radioactive band stable up to 41°C proves that HP II is formed during viroid replication in vivo. Thus, the oligonucleotide probing method developed in this work allows the specific detection of transient RNA structures in vitro as well as in vivo and clearly shows the presence of the previously postulated transient HP II during PSTVd replication.
Figure 9.
Detection of HP II within metastable replication intermediates formed in vivo. 4 × 108 nuclei from PSTVd-infected potato cells were incubated with radiolabeled 27AB 2′-OMe (3 × 107 c.p.m.). Total nucleic acid was isolated under native conditions as described. After subsequent dialysis against 0.2× TBE buffer a TGGE analysis with focusing properties (see Fig. 8) was performed. The corresponding autoradiograph is shown, and the HP II/2′-OMe oligonucleotide complexes are marked by ‘C’. In the bottom of the gel the uncomplexed radiolabeled 27AB 2′-OMe oligonucleotides are visible.
DISCUSSION
Using an oligonucleotide probing procedure we showed that the HP II structure is present in an PSTVd-infected cell extract. The functional relevance of a HP II containing structure was first demonstrated by site-directed mutagenesis studies (17,19) and recently confirmed in an independent study in which the restoration of the correct base pairing in HP II in a non-infectious recombinant viroid (CECS) leads to the recovery of infectivity (18). When various mutants were compared with respect to their viability and tendency for reversion, it was seen that the double-helical structure of the stem is essential for replication, whereas the exact sequence represents only a fine tuning for optimal replication. Structural considerations led to the conclusion that it is the (–)-strand replication intermediate which contains HP II as part of a metastable structure (19). From a computer simulation of (–)-strand folding it was proposed that the left terminal domain folds first which then guides the folding of HP II (24). All PSTVd isolates retain the capacity to form the HP II structure, supporting its importance. It was proposed that HP II might act as a binding site for host cell transcription factors.
Several factors complicated the analysis of HP II and caused us to adopt the RNA oligonucleotide approach. Difficulties arose mainly due to the very low concentration of HP II in vivo and its metastable nature. The investigation of a metastable structure within an ensemble of different RNA structures is very difficult. Chemical mapping and optical melting experiments are not helpful, because they require one single structure to interpret the results correctly. However, using TGGE it is possible to detect metastable as well as stable structures and even structures of low concentration within an RNA structural ensemble. However, the attribution of the bands in the gel pattern to defined secondary structures is only possible in favorable cases. In our previous study, chemical mapping combined with TGGE or general native electrophoresis allowed co-existing structures to be analyzed (40). However, in the present study, no significant differences in the chemical mapping pattern between the metastable structures (M, Q, P, R) visible after in vitro transcription of PSTVd-Sty1 could be detected (data not shown). The reasons for these results are discussed below.
Different structures of the transcripts after in vitro synthesis were detected in TGGE. Apparently single bands might contain more than one RNA structure in a certain temperature range; for example in Fig. 6 bands Q and P co-migrate at low temperatures but separate from each other prior to complete denaturation. These co-migrating structures contain most probably only small structural variations, so that their hydrodynamic shapes in the electrophoresis coincide in a certain temperature range. Thus, even more structures than visible in TGGE might be present in the structural ensemble. In addition, the concentration of some structures might be so low in the ensemble that they cannot be detected by silver staining. For example, the HP II containing structure C3 is well labeled on the autoradiograph of Figure 5, but not detectable in the silver-stained gel. However, other bands of comparable intensity in the autoradiograph do correspond to bands of the silver-stained gel. We have to conclude that all bands of the autoradiograph but not all or even only a minority of the bands of the silver-stained gel do contain HP II. This complicated situation makes it plausible that chemical mapping of the co-existing structure did not lead to interpretable results in this study.
The design of a specific RNA oligonucleotide probe allowed us to detect HP II containing structures in vivo. The utilization of oligonucleotides to detect single-stranded regions within RNA structures has been reported in various studies (41–45). In addition, RNA folding intermediates have been investigated using oligonucleotide mapping (46), and an oligo DNA probe complementary to the free ends of a stem has been used for the detection of a RNA hairpin structure (47). In order to obtain structure-specific binding and to avoid forcing a metastable structure into a HP II conformation, several RNA oligonucleotides complementary to different regions of the HP II loop from 11 to 30 nt length were tested. Some of them, especially the 3′-end extended version of oligo 14A, were found to bind the rod-shaped structure as well. In comparison, one other oligonucleotide, corresponding to the 11 nt 5′-end region of oligo 27AB (region B, oligo 11B), was not able to bind at all (data not shown).
Since the 3′ part of oligo 27AB (oligonucleotide 14A) binds to the 3′-loop side of the stem of HP II (see Fig. 3), but the corresponding 5′ part of oligo 27AB, studied alone (11B), does not bind as a separate probe as discussed above, we can rule out that the two halves of oligo 27AB bind separately to their complementary regions, thereby forcing the structure into HP II.
Our observations suggest two possible pathways for binding of 27AB. One possible binding mechanism of oligonucleotide 27AB could be an initial binding of its 3′ side followed by spanning the double-helical stem and subsequent binding of its 5′ side, suggesting the sliding mechanism for binding of 27AB. Another possibility is that 27AB binds as a typical example of a three-way junction in which the orientation of the three stems and their stacking will strongly determine the structure and stability of the remainder of the hairpin loop as well as imposing a possible irregularity at the junction, which may also be important for the strength of binding of the probe.
HP II could be detected in a model transcript, in monomeric, dimeric and trimeric transcripts after in vitro synthesis and in a crude RNA extract prepared under native conditions from viroid-infected cell cultures. The amount of HP II structures after in vitro transcription starting from different start sites was found to be in the range of 1–10% and only a negligible influence of the start site on the concentration of HP II containing structures was found under these experimental conditions. Thus, one might ask, if HP II is formed in only 1–10% of all structures after in vitro transcription, can it fulfill a biologically relevant function in vivo?
It should be noted that the in vitro transcription reactions were carried out for 1.5 h with T7 RNA polymerase, prior to the incubation with oligo 27AB. This does not reflect in vivo conditions, since the rate of RNA polymerase II is lower than the rate of T7 RNA polymerase and it had been shown previously that the rate of transcription and the duration strongly influence the structural distribution. With prolonged transcription times and higher transcriptional rates the structural distribution was shown to be shifted to more stable structures (29). Considering this, the formation of HP II containing structures should be higher at earlier time points during in vitro transcription. In addition, PSTVd replication in vivo should lead to higher amounts of HP II structures. It was reported recently that in vivo at least two different start sites exist for the transcription into (–)-strands, A111 and A325 (16). In a forthcoming study the influence of the start sites of transcription including the in vivo sites on the distribution of the alternative structures is analyzed systematically taking into account also the influence of the other parameters mentioned above.
When the HP II containing structures were analyzed in the cell extract (i.e. under in vivo or close to in vivo conditions) we had to overcome, as an additional experimental difficulty, that the RNA molecules containing those structures are not homogeneous in size. Since the structures are metastable, conditions for the preparation of the RNA had to be found which preserve the metastable structures, which means preventing them from switching into the most stable structures. Only the combination of a radioactively labeled structure-specific probe with gel electrophoresis with focusing properties and a temperature-gradient finally allowed us to demonstrate unequivocally HP II containing structures in PSTVd-infected cell cultures. Thus, the direct biophysical analysis confirmed our earlier analysis of infectivity data after site-directed mutagenesis (17,19). Indeed, a principle of viroid structural plasticity was confirmed in the sense that the native (+)-strand circular viroid is active as a stable structure, but the (–)-strand replication intermediate as a metastable structure. The metastability holds not only for the template activity for synthesis of the new oligomeric (+)-strand, but also the new (+)-strand assumes a metastable structure for correct cleavage and ligation (13).
It has been argued several times that polymerase II or transcription factors or a complex of both might bind to HP II containing structures. So far, this problem could not be approached experimentally, since transcript RNA with HP II as the predominant structure in the ensembles of many structures was not available. With the model RNA IVTBSP, however, large amounts of RNA containing the HP II motif in a stable form are available for a search for interacting host factors. The structure-specific oligonucleotide 27AB which binds tightly to two segments separated by a stem–loop structure opens up novel possibilities for selecting optimal antisense constructs.
Acknowledgments
ACKNOWLEDGEMENTS
We thank Drs F. Bushman, T. Baumstark, M. Schmitz, G. Steger and O. Schröder for stimulating discussions and Ms H. Gruber for her help in preparing the manuscript. The work was supported by grants from the Deutsche Forschungsgemeinschaft and Fonds der Chemischen Industrie.
REFERENCES
- 1.Riesner D. and Gross,H.J. (1985) Viroids. Annu. Rev. Biochem., 54, 531–564. [DOI] [PubMed] [Google Scholar]
- 2.Diener T.O. (ed.) (1987) The Viroids. Plenum Publishing Corporation, New York, NY.
- 3.Semancik J.S. (ed.) (1987) Viroids and Viroid-Like Pathogens. CRC Press, Boca Raton, FL.
- 4.Flores R., Randles,J.W., Bar-Joseph,M. and Diener,T.O. (1998) A proposed scheme for viroid classification and nomenclature. Arch. Virol., 143, 623–629. [DOI] [PubMed] [Google Scholar]
- 5.Branch A.D. and Robertson,H.D. (1984) A replication cycle for viroids and other small infectious RNAs. Science, 233, 450–455. [DOI] [PubMed] [Google Scholar]
- 6.Symons R.H. (1992) Small catalytic RNAs. Annu. Rev. Biochem., 61, 641–671. [DOI] [PubMed] [Google Scholar]
- 7.Darós J.A., Marcos,J.F., Hernández,C. and Flores,R. (1994) Replication of avocado sunblotch viroid: evidence for a symmentric pathway with two rolling circles and hammerhead ribozyme processing. Proc. Natl Acad. Sci. USA, 91, 12813–12817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gross H.J., Domdey,H., Lossow,C., Jank,P., Raba,M., Alberty,H. and Sänger,H.L. (1978) Nucleotide sequence and secondary structure of potato spindle tuber viroid. Nature, 273, 203–208. [DOI] [PubMed] [Google Scholar]
- 9.Riesner D., Henco,K., Rokohl,U., Klotz,G., Kleinschmidt,A.K., Domdey,H., Jank,P., Gross,H.J. and Sänger,H.L. (1979) Structure and structure formation of viroids. J. Mol. Biol., 133, 85–115. [DOI] [PubMed] [Google Scholar]
- 10.Mühlbach H.P. and Sänger,H.L. (1979) Viroid replication is inhibited by a-Amanitin. Nature, 278, 185–188. [DOI] [PubMed] [Google Scholar]
- 11.Schindler I.M. and Mühlbach,H.P. (1992) Involvement of nuclear DNA-dependent RNA polymerases in potato spindle tuber viroid replication: a reevalution. Plant Sci., 84, 221–229. [Google Scholar]
- 12.Tsagris M., Tabler,M., Mühlbach,H.P. and Sänger,H.L. (1987) Linear oligomeric potato spindle tuber viroid (PSTV) RNAs are accurately processed in vitro to the monomeric circular viroid proper when incubated with a nuclear extract from healthy potato cells. EMBO J., 6, 2173–2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baumstark T., Schröder,A.R.W. and Riesner,D. (1997) Switch from cleavage to ligation is driven by a change from a tetraloop to a loop E conformation. EMBO J., 16, 599–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsagris M., Tabler,M. and Sänger,H.L. (1987) Oligomeric potato spindle tuber viroid RNA does not process autocatalytically under conditions where other RNAs do. Virology, 157, 227–231. [DOI] [PubMed] [Google Scholar]
- 15.Baumstark T. and Riesner,D. (1995) Only one of four possible secondary structures of the central conserved region of potato spindle tuber viroid is a substrate for processing in a potato nuclear extract. Nucleic Acids Res., 23, 4246–4254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fels A., Hu,K. and Riesner,D. (2001) Transcription of potato spindle tuber viroid by RNA polymerase II starts predominantly at two specific sites. Nucleic Acids Res., 29, 4589–4597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Loss P., Schmitz,M., Steger,G. and Riesner,D. (1991) Formation of a thermodynamically metastable structure containing hairpin II is critical for infectivity of potato spindle tuber viroid RNA. EMBO J., 10, 719–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Candresse T., Góra-Sochacka,A. and Zagórski,W. (2001) Restoration of secondary hairpin II is associated with restoration of infectivity of non-viable recombinant viroid. Virus Res., 75, 29–34. [DOI] [PubMed] [Google Scholar]
- 19.Qu F., Heinrich,C., Loss,P., Steger,G., Tien,P. and Riesner,D. (1993) Multiple pathways of reversion in viroids for conservation of structural elements. EMBO J., 12, 2129–2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boyle J., Robillard,G.T. and Kim,S.H. (1980) Sequential folding of transfer RNA: a nuclear magnetic resonance study of successive longer tRNA fragments with a common 5′ end. J. Mol. Biol., 139, 601–625. [DOI] [PubMed] [Google Scholar]
- 21.Nussinov R. and Tinoco,I.,Jr (1981) Sequential folding of a messenger RNA molecule. J. Mol. Biol., 151, 519–533. [DOI] [PubMed] [Google Scholar]
- 22.Kramer F.R. and Mills,D.R. (1981) Secondary structure formation during RNA synthesis. Nucleic Acids Res., 9, 5109–5124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schmitz M. and Steger,G. (1996) Description of RNA folding by ‘simulated annealing’. J. Mol. Biol., 255, 254–266. [DOI] [PubMed] [Google Scholar]
- 24.Gultyaev A.P., van Batenburg,F.H. and Pleij,C.W. (1998) Dynamic competition between alternative structures in viroid RNA simulated by an RNA folding algorithm. J. Mol. Biol., 276, 43–55. [DOI] [PubMed] [Google Scholar]
- 25.Riesner D., Steger,G., Zimmat,R., Owens,R.A., Wagenhöfer,M., Hillen,W., Vollbach,S. and Henco,K. (1989) Temperature-gradient gel electrophoresis: analysis of conformational transitions, sequence variations and protein–nucleic acid interactions. Electrophoresis, 10, 377–389. [DOI] [PubMed] [Google Scholar]
- 26.Riesner D. (1991) Viroid: from thermodynamics to cellular structure and function. Mol. Plant Microbe Interact., 2, 122–131. [DOI] [PubMed] [Google Scholar]
- 27.Hecker R., Wang,Z., Steger,G. and Riesner,D. (1988) Analysis of RNA structures by temperature-gradient gel electrophoresis: viroid replication and processing. Gene, 72, 59–74. [DOI] [PubMed] [Google Scholar]
- 28.Riesner D., Baumstark,T., Qu,F., Klahn,T., Loss,P., Rosenbaum,V., Schmitz,M. and Steger,G. (1992) Physical basis and biological examples of metastable RNA structures. In Lilley,D.M.J., Heumann,H. and Suck,D. (eds), Structural Tools for the Analysis of Protein–Nucleic Acids Complexes. Birkhäuser Verlag, Basel, pp. 401–436.
- 29.Repsilber D., Wiese,S., Rachen,M., Schröder,A.R.W., Riesner,D. and Steger,G. (1999) Formation of metastable RNA structures by sequential folding during transcription: time-resolved structural analysis of potato spindle tuber viroid (–)-stranded RNA by temperature-gradient gel electrophoresis. RNA, 5, 574–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rosenbaum V. and Riesner,D. (1987) Temperature-gradient gel electrophoresis. Thermodynamic analysis of nucleic acids and proteins in purified form and in cellular extracts. Biophys. Chem., 26, 235–246. [DOI] [PubMed] [Google Scholar]
- 31.Schmitz A. and Riesner,D. (1998) Correlation between bending of the VM region and pathogenicity of different Potato Spindle Tuber Viroid strains. RNA, 4, 1295–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schmitz M. and Steger,G. (1992) Base-pair probability profiles of RNA secondary structures. Comput. Appl. Biosci., 8, 389–399. [DOI] [PubMed] [Google Scholar]
- 33.Steger G., Tabler,M., Brüggemann,W., Colpan,M., Klotz,G., Sänger,H.L. and Riesner,D. (1986) Structure of viroid replicative intermediates: physico-chemical studies on SP6 transcripts of cloned oligomeric potato spindle tuber viroid. Nucleic Acids Res., 14, 9613–9630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mühlbach H.P. and Sänger,H.L. (1981) Continuous replication of potato spindle tuber viroid (PSTV) in permanent cell cultures of potato and tomato. Biosci. Rep., 1, 79–87. [DOI] [PubMed] [Google Scholar]
- 35.Hecker R. (1989) Die Struktur der intermediären Ribonucleinsäuren bei der Replikation von Viroiden. Dissertation, Heinreich-Heine-Universität, Düsseldorf, Germany.
- 36.Branch A.D., Robertson,H.D. and Dickson,E. (1981) Longer than unit length viroid minus strands are present in RNA from infected plants. Proc. Natl Acad. Sci. USA, 85, 6381–6385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Owens R.A. and Diener,T.O. (1982) RNA intermediates in potato spindle tuber viroid replication. Proc. Natl Acad. Sci. USA, 79, 113–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rohde W. and Sänger,H.L. (1981) Detection of complementary RNA intermediates of viroid replication by Northern blot hybridization. Biosci. Rep., 1, 327–336. [DOI] [PubMed] [Google Scholar]
- 39.Schindler L.M. (1989) Untersuchungen zum Replikationsmechanismus des Potato-Spindle-Tuber-Viroids (PSTV) an isolierten Zellkernen und Zellkernextrakten aus Solanum demissum. Dissertation, Universität Stuttgart.
- 40.Schröder A.R.W., Baumstark,T. and Riesner,D. (1998) Chemical mapping of co-existing structures. Nucleic Acids Res., 14, 3449–3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bussière F., Ouellet,J., Cote,F., Levesque,D. and Perreault,J.P. (2000) Mapping in solution shows the peach latent mosaic viroid to possess a new pseudoknot in a complex, branched secondary structure. J. Virol., 74, 2647–2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Uhlenbeck O.C., Baller,J. and Doty,P. (1970) Complementary oligonucleotide binding to the anticodon loop of fMet-transfer RNA. Nature, 225, 508–512. [DOI] [PubMed] [Google Scholar]
- 43.Weller J.W. and Hill,W.E. (1992) Probing dynamic changes in rRNA conformation in the 30S subunit of the Escherichia coli ribosome. Biochemistry, 31, 2748–2757. [DOI] [PubMed] [Google Scholar]
- 44.Le Cuyer K. and Crothers,J.R. (1994) Kinetics of an RNA conformational switch. Proc. Natl Acad. Sci. USA, 91, 3373–3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pinard R., Payant,C. and Brakier-Gingras,L. (1995) Mutations at position 13 and/or 914 in Escherichia coli 16S ribosomal RNA interfere with the initiation of protein synthesis. Biochemistry, 34, 9611–9616. [DOI] [PubMed] [Google Scholar]
- 46.Zarrinkar P.P., Wang,J. and Williamson,J.R. (1996) Slow folding kinetics of RNase P RNA. RNA, 2, 564–573. [PMC free article] [PubMed] [Google Scholar]
- 47.Francois J.-C., Thuong,N.T. and Hélène,C. (1994) Recognition and cleavage of hairpin structures in nucleic acids by oligodeoxynucleotides. Nucleic Acids Res., 22, 3943–3950. [DOI] [PMC free article] [PubMed] [Google Scholar]