Abstract
RluD is the pseudouridine synthase responsible for the formation of Ψ1911, Ψ1915, and Ψ1917 in Escherichia coli 23S rRNA. Previous work from our laboratory demonstrated that disruption of the rluD gene and/or loss of the pseudouridine residues for which it is responsible resulted in a severe growth phenotype. In the current work we have examined further the effect of the loss of the RluD protein and its product pseudouridine residues in a deletion strain lacking the rluD gene. This strain exhibits defects in ribosome assembly, biogenesis, and function. Specifically, there is a deficit of 70S ribosomes, an increase in 50S and 30S subunits, and the appearance of new 62S and 39S particles. Analysis of the 39S particles indicates that they are immature precursors of the 50S subunits, whereas the 62S particles are derived from the breakdown of unstable 70S ribosomes. In addition, purified mutant 70S ribosomes were found to be somewhat less efficient than wild type in protein synthesis. The defect in ribosome assembly and resulting growth phenotype of the mutant could be restored by expression of wild-type RluD and synthesis of Ψ1911, Ψ1915, and Ψ1917 residues, but not by catalytically inactive mutant RluD proteins, incapable of pseudouridine formation. The data suggest that the loss of the pseudouridine residues can account for all aspects of the mutant phenotype; however, a possible second function of the RluD synthase is also discussed.
Keywords: RluD pseudouridine synthase, pseudouridine residues, ribosome assembly, 39S particles, immature precursors, protein synthesis
INTRODUCTION
Cellular RNA contains a number of post-transcriptionally modified nucleosides, the most common of which is pseudouridine (Ψ; 5-ribosyl-uracil). Ψ is found in RNAs for which the stability of their tertiary structure is important for function, such as rRNA, tRNA, and snRNA (Ofengand and Rudd 2000). Pseudouridine synthases are the enzymes responsible for formation of the Ψ residues. In Escherichia coli there are 11 synthases that can be grouped into five families that account for all the known rRNA and tRNA Ψ residues. RsuA, RluE, RluB, and RluF belong to the RsuA family; RluA, RluC, RluD, and TruC are in the RluA family; and TruA, TruB, and TruD are the sole members of their respective families. Each of the synthases has proven to be site specific and no overlapping function has been detected. The contribution to cell physiology by individual pseudouridine synthases and the Ψ residues for which each is responsible has been investigated in our laboratory by deletion of their respective genes. Mutant E. coli strains lacking the individual synthases, RsuA (Conrad et al. 1999), RluA (Raychaudhuri et al. 1999), RluC (Conrad et al. 1998), TruB (Gutgsell et al. 2000), RluB, RluE, RluF, and TruC (Del Campo et al. 2001), and TruD (Kaya and Ofengand 2003) have been characterized. Of these, only the loss of the rluA (Ψ746 23S rRNA and Ψ32 tRNAphe) and the truB (Ψ55 all tRNAs) synthase genes were shown to result in a demonstrable growth phenotype as evidenced by reduced survival in competition with wild-type E. coli. In the case of the truB mutant, the loss of the TruB synthase, but not the Ψ55 residue, was shown to be responsible for this phenotype.
RluD is the synthase responsible for formation of Ψ1911, Ψ1915, and Ψ1917, which are located in a stem-loop structure of domain IV, helix 69, of 23S rRNA. This region has been shown to be part of the peptidyltransferase center (PTC). Based on the crystal structures of Thermus thermophilus 70S ribosomes (Yusupov et al. 2001) and Deinococcus radiodurans 50S rRNA (Bashan et al. 2003; Yonath and Bashan 2004) and cryo-electron microscopy mapping of E. coli 70S ribosomes (Agrawal et al. 2004) complexed with various substrates, domain IV and helix 69 (including Ψ1915; Agrawal et al. 2004) are known to interact with mRNA, tRNAs, 16S rRNA, and Ribosomal Release Factor, and may be involved in proper tRNA positioning, in translocation, and in release of mRNA from the post-termination complex. Taken together, these findings suggest that helix 69 and the modified residues of 23S rRNA are important for efficient protein biosynthesis by the ribosome.
Previously, disruption of the gene encoding RluD, by insertion of a miniTn10(cam) cassette, resulted in the most profound phenotype of any synthase mutant (Raychaudhuri et al. 1998; Gutgsell et al. 2001). The resulting disruption strain was shown to lack the aforementioned Ψ modifications, and grew half as fast as wild type. However, since the disruption mutant retained the potential to produce a truncated RluD protein consisting of the N-terminal 196 amino acids, an rluD deletion mutant was sought (see Discussion). Here, we report on the deletion of the rluD gene and the resulting severe growth defect (doubling time 6× that of wild type in rich media at 37°C). We further show a marked defect in ribosome assembly in the mutant strain, which likely accounts for its growth phenotype. In addition, second site suppressor revertants and transformants of the deletion strain, which still lack the three Ψ residues, either in the absence of RluD or in the presence of an inactive mutant RluD, were characterized. Taken together, these studies demonstrate that Ψ 1911, 1915, and 1917 are essential for completely wild-type growth in E. coli, and that their effect is likely to be at the level of ribosome assembly.
RESULTS
Generation of a ΔrluD::kan mutant
Deletion of the gene for the E. coli pseudouridine synthase, RluD, was accomplished by gene replacement resulting in a ΔrluD::kan mutant strain (Datsenko and Wanner 2000). As will be shown, most of the rluD gene in this strain is replaced by a 1015-bp kanr gene insertion. However, 39 bp of the 5′ and 67 bp of the 3′ rluD gene sequence are retained. The 5′ rluD sequence is followed by a stop codon so that at most, only a 13 amino acid RluD protein fragment could be produced. Upon introduction of this mutation into E. coli MG1655, only very small colonies were obtained. Twenty-seven clones of this mutant strain were isolated, all of which were kanamycin resistant.
Deletion of the rluD gene was confirmed by analysis of wild-type and mutant genomic DNA by PCR and by Southern hybridization. All 27 mutant isolates were examined and they showed identical properties; however, the data for only one of the strains are shown (Fig. 1). The PCR product from mutant genomic DNA was 1.1 kb compared to just 1.0 kb for the wild-type product (Fig. 1A, lanes 1,2). The presence of the kanamycin insert introduces a HindIII site into the mutant genome that is not present in the wild-type rluD gene. Consequently, digestion of material from the PCR reactions shown in lanes 1 and 2 with HindIII resulted in two bands for the mutant product (Fig. 1A, lane 4), but only the original undigested band for the wild type (Fig. 1A, lane 3).
FIGURE 1.
PCR, Southern transfer and hybridization, and Ψ sequence analysis of wild-type and ΔrluD::kan mutant E. coli strains. (A) PCR reaction products were generated using genomic DNA as template. Primers used contain sequences homologous to the 5′ and 3′ ends of the rluD gene retained in the ΔrluD::kan mutant strain (forward primer 5′-GAAGCAGTATATATGGCACAACGAGTACAG CTC and reverse primer 5′-GGGAAGCTTTCATAACCAGTCCAC TTCATC). (Lanes 1,3) wild-type MG1655; (lanes 2,4), ΔrluD::kan. (Lanes 1,2) undigested; (lanes 3,4) reaction products digested with 20 units of HindIII (NEB) at 37°C for ≥16 h. Left side of the graph, sizes (kb) of the molecular weight markers. Right side of the graph, sizes (kb) of the DNA bands for each strain. (B) Southern transfer and hybridization were performed using genomic DNA incubated with 20 units of BamHI (NEB) at 37°C for 3 h to achieve partial digestion. Probes were generated from the wild-type MG1655 PCR product by digestion with 10 units NruI (NEB) and 5 units SphI (NEB) to generate the 806-nt probe specific for the center of rluD and a mixed probe specific for the 5′ and 3′ ends of rluD, respectively. Wild-type MG1655 (lanes 1); ΔrluD::kan (lanes 2); (panel I) hybridization with the 806-nt probe; (panel II) the same filter stripped; (panel III) the filter subsequently hybridized with the mixed 5′ and 3′ probes. (C) Pseudouridine sequencing analysis of 23S rRNA from MG1655 and ΔrluD::kan. Total RNA was isolated from the indicated strains and examined for Ψ (Ofengand et al. 2001).
For Southern analysis, wild-type and mutant genomic DNAs were digested with BamHI (Fig. 1B). The probes used were derived from the center of the rluD gene, present only in the wild-type (806-nt probe), and from the 5′ (39 nt) and 3′ (67 nt) ends of the rluD gene that are retained in the mutant genome. As expected, the 806 nt probe hybridized only with the wild-type DNA (Fig. 1B, panel I, lane 1). After stripping this filter (Fig. 1B, panel II), the pooled 5′/3′ probe reacted with both wild-type and mutant genomic DNA (Fig. 1B, panel III, lanes 1 and 2).
To confirm that the resulting mutant was not a pseudorevertant of a still slower growing strain, a phage P1-mediated backcross to wild type was done. Of the 180 tiny colonies obtained, none were clearly smaller than any other, and all were shown to be rluD deletion mutants by PCR analysis, as in Figure 1A (data not shown). The cotransduction frequency for a hypothetical second site suppressor mutation would have to be >99% (<1 in 180 transductants or <1% separable), or <202 bp away from rluD according to the Wu formula (Bachman et al. 1976). Based on all of these data, we conclude that the ΔrluD::kan mutant is a deletion of rluD.
One isolate was chosen for further characterization. DNA sequencing was carried out using genomic DNA from the mutant strain and sequencing primers immediately 5′and 3′of the remaining rluD sequence (data not shown). The sequencing showed that the kanr gene had inserted at the expected position in the genome. The sequencing also confirmed that a stop codon is present in the 5′ rluD sequence at bp 40–42, and, therefore, that only a 13 amino acid RluD fragment could be produced in the mutant strain. Absence of a functional RluD protein was further assessed by loss of the Ψ residues previously shown to be made by this synthase (Raychaudhuri et al. 1998). As expected, Ψ 1911 and 1917 are missing in the deletion strain (Fig. 1C). (It was not possible to determine whether Ψ1915 was present using the CMC modification method due to N3 methylation of this residue in vivo; Gutgsell et al. 2001.)
Consequences of the absence of RluD and Ψ 1911, 1915, and 1917
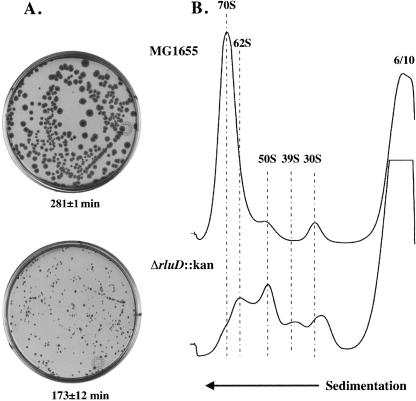
The loss of RluD synthase and/or its Ψ products results in a severe growth phenotype. This is evident from the markedly reduced colony size (Fig. 2A) and the prolonged doubling time (173 min for the mutant vs. 28 min for wild type) in rich media at 37°C. In addition, sucrose gradient analysis revealed a major ribosome defect in the deletion mutant, which likely accounts for the observed growth phenotype (Fig. 2B). Lysates prepared from wild-type cells display a typical ribosome pattern, i.e., a large peak of 70S ribosomes and minor peaks of 50S and 30S subunits. Under these same conditions, a major defect was observed in the mutant profile characterized by the accumulation of 50S and 30S subunits, the appearance of abnormal particles (62S and 39S), and the concomitant loss of 70S ribosomes. This mutant ribosome profile was consistently detected in multiple experiments, with the exception of some variation in the sedimentation position of the mutant 39S and 30S species, suggesting some degree of heterogeneity in the composition of these particles (cf. Figs. 2 and 3; 6/10 mM). The overall recovery of ribosomal material based on A260 was comparable across the gradient for wild type and mutant in all experiments. Therefore, the difference in the distribution of mutant particles, as compared with wild-type, was not due to the loss of ribosomal material during processing or analysis. This abnormal ribosome profile could be due to either a defect in ribosome assembly or to the accelerated breakdown of misassembled ribosomes due to their instability.
FIGURE 2.
Colony morphology, exponential growth rates and ribosome profiles of wild-type and ΔrluD::kan mutant E. coli strains. (A) LB plates without drug (wild type) or with 34 μg/mL kanamycin (mutant only) were incubated 41 h at 37°C. Dilutions were prepared from suspensions of wild-type and mutant cells and 6.8 × 108 cells/mL for each strain were spread on plates and incubated. Exponential growth rates were measured by monitoring cell density at 600 nm with a Perkin Elmer Lambda 25 UV/VIS Spectrophotometer. Doubling time was determined from a semilogarithmic plot of A600 versus time. Each plot consisted of five to seven time points. Doubling times were the average of two determinations (wild type) or six determinations (mutant), and are shown under the plates. (B) Ribosome profiles of wild-type and ΔrluD::kan cells were performed as described in Materials and Methods. Wild-type and mutant cells were lysed at 6 mM Mg++ and gradients were run at 10 mM Mg++. Twelve A260 units were layered onto 14%–32% sucrose gradients and centrifuged at 22,000 rpm for 19 h at 4°C.
FIGURE 3.
Ribosome profiles of wild-type and ΔrluD::kan mutant strains analyzed at various Mg++ concentrations. Ribosome profiles were performed as described in Materials and Methods. Wild-type and mutant cells were lysed, and gradients run at the Mg++ concentrations (mM) shown for lysis/sedimentation. Twelve A260 units were layered onto 14%–32% sucrose gradients and centrifuged at 22,000 rpm for 19 h at 4°C.
Magnesium ion concentration is known to be critical to the maintenance of ribosome structure in vitro. At 4 mM Mg++ even the tightly coupled 70S ribosomes of wild-type MG1655 begin to separate into 50S and 30S subunits (data not shown). Therefore, analyses in which Mg++ concentrations are varied during both lysis and centrifugation may be useful to examine the stability of ribosome particles during processing, and may provide some insight into the nature of the ΔrluD::kan mutant defect.
The MG1655 wild-type ribosome profiles are essentially unchanged when compared at the lowest (6/10 mM lysis/sedimentation) or the highest (20/20 mM) Mg++ concentrations tested (Fig. 3). In contrast, the mutant ribosome profiles vary dramatically within this range of Mg++ concentrations, and suggest how the abnormal 62S and 39S particles may arise. First, mutant 70S ribosomes are stabilized when high Mg++ is present throughout or just during the 19 h of sedimentation (20/20 or 6/20 mM); 62S particles are not present. However, when Mg++ concentrations are decreased during centrifugation (20/10 mM) or throughout the analysis (6/10 mM) there is a destabilization of the 70S ribosomes, resulting in either a slight downward shift in sedimentation or a nearly complete conversion to 62S particles, respectively. Thus, 62S particles appear to derive from unstable 70S particles that readily dissociate when Mg++ concentrations are lowered to ≤10 mM.
On the other hand, in contrast to 62S particles, the appearance of aberrant 39S particles may not be the result of simple instability of mutant 50S ribosomes. This can be seen by a comparison of 20/20 and 20/10 mM with 6/20 and 6/10 mM Mg++, which reveals that the concentration of Mg++ at the time of lysis seems most important for the maintenance of mutant 50S subunits or the appearance of 39S particles. This suggests that, unlike 70S ribosomes, 50S subunits, when initially introduced into a high Mg++ concentration at the time of lysis (20/20 or 20/10 mM), are able to remain stably associated; however, once dissociated (6/20 mM) they cannot be easily reassembled simply by increasing the Mg++ concentration. Moreover, we provide evidence below that 39S particles actually are immature forms of the 50S subunit.
Further characterization of the ΔrluD::kan mutant ribosomes
The previous data indicated that particles that sediment as 70S ribosomes are stabilized in the ΔrluD::kan mutant extract by the presence of 20 mM Mg++ during lysis and/or centrifugation. Similar profiles are seen when lysis and sedimentation are performed at 6/15 mM and 15/10 mM Mg++, except that somewhat fewer 70S particles are recovered (data not shown). However, these analyses do not address the functionality of those mutant 70S particles. Therefore, poly(U)-dependent polyphenylalanine synthesis experiments were performed to determine whether mutant 70S ribosomes were as efficient in protein synthesis as wild-type ribosomes. For these analyses, RNaseI− derivatives of MG1655 and ΔrluD::kan were used to decrease the probability of RNA degradation during incubation. Ribosome profiles of the MG1655/I−::tet and ΔrluD::kan/I−::tet strains were shown to be unchanged as compared to their respective parental strains (data not shown). Wild-type and mutant lysates were prepared and sedimented at 15 mM Mg++ and 70S particles were collected for use in polyPhe synthesis.
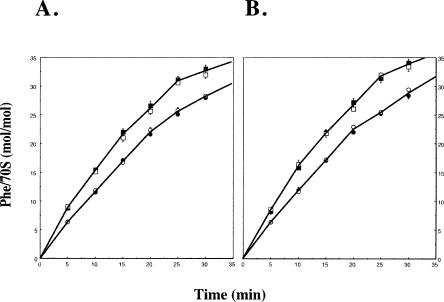
This analysis revealed that when equal amounts (0.5 or 1.0 A260 units) of 70S ribosomes were used, ΔrluD::kan/I−::tet ribosomes were nearly as efficient in protein synthesis (−80%) as wild type (Fig. 4A). In addition, the moles of polyPhe produced per mole of 70S ribosomes used were unchanged at either ribosome concentration for both the wild-type and mutant strains. Therefore, the presence of twice the amount of mutant ribosomes had no inhibitory effect on protein synthesis. These data represent the average of multiple experiments performed using six wild-type and 10 individual mutant ribosome preparations. Consequently, this result is not likely to be due to some peculiarity of any one mutant culture or ribosome preparation. In a second experiment, wild-type and mutant 70S ribosomes were collected after lysis in 6 mM Mg++ followed by sedimentation at 15 mM Mg++, and 0.5 or 1.0 A260 units were again used for protein synthesis. The results revealed that both wild-type and mutant ribosomes collected under conditions that would allow the disassociation of mutant 70S particles during lysis followed by stabilization during sedimentation are functionally indistinguishable from ribosomes maintained at high Mg++ throughout processing. Thus, though many fewer 70S particles are recovered in the mutant at the lower Mg++ concentrations (see Fig. 2B), those that are produced are equally efficient in protein synthesis as assessed by elongation of polyPhe peptide fragments.
FIGURE 4.
Polyphenyalanine synthesis by wild-type and mutant 70S ribosomes. Polyphenylalanine synthesis was performed as described in Materials and Methods. (A) 70S ribosome fractions were isolated from sucrose gradients at 15 mM Mg++ concentration during lysis and sedimentation. Nine individual experiments were performed using 70S fractions from four wild-type and seven mutant ribosome preparations; MG1655/I−::tet 0.5 A260 (closed squares) or ΔrluD::kan/I−::tet 0.5 A260 units (closed circles). Four individual experiments were performed using 70S fractions from two wild-type and four mutant ribo-some preparations; MG1655/I−::tet 1.0 A260 unit (open squares) or ΔrluD::kan/I−::tet 1.0 A260 unit (open circles). (B) Three individual experiments were performed using 70S fractions from two wild-type and three mutant ribosome preparations (lysis 6 mM and sedimentation 15 mM Mg++). Symbols are the same as for A.
The accumulation of abnormal ribosomal particles in the ΔrluD::kan mutant strain suggests defects in rRNA processing, either due to breakdown of unstable particles or to the presence of under- or unprocessed rRNA precursors. In E. coli, the rRNAs are cotranscribed as a single 30S transcript that is initially cleaved by the endonuclease RNase III to release precursors of the 23S, 16S, and 5S rRNAs. The p23S precursor that results from RNase III cleavage contains three to seven extra nucleotides at its 5′ end and seven to nine extra nucleotides at its 3′ end. The 16S precursor (17S) resulting from this primary cleavage contains 115 extra 5′ nucleotides and 33 additional nucleotides at the 3′ end. Previous studies have determined that final maturation of 23S rRNA occurs within polysomes (Sirdeshmukh and Schlessinger 1985; Srivastava and Schlessinger 1988). In addition, it has been shown that precursors of 16S rRNA are present in particles that cosediment with mature 30S subunits, and that these precursor particles are found only as subunits, not in 70S ribosomes (Lindahl 1975). Therefore, the rRNA content and the state of maturation of the 23S and 16S rRNA present in ΔrluD::kan mutant particles was assessed.
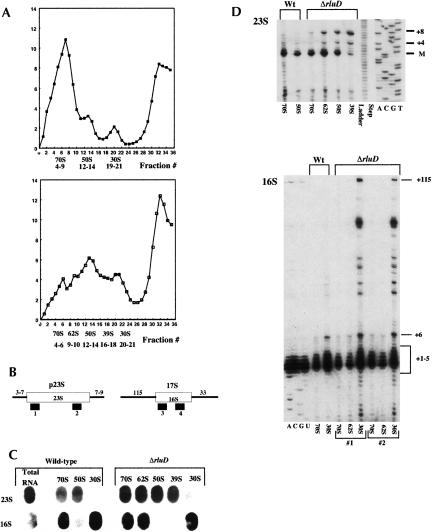
Lysates were prepared at 6 mM Mg++ and sedimentation was done at 10 mM Mg++ to allow for isolation of 62S and 39S particles. Fractions were then collected across the entire ribosome profile for wild-type and mutant, and A260 values were determined (Fig. 5A). The total amount of ribosomal material based on A260 units of the collected fractions was calculated for wild type and mutant and proved to be equivalent for both strains. Those fractions that represent 70S, 50S, and 30S particles for wild type and mutant, as well as mutant 62S and 39S particles, were pooled for further analysis. These pooled fractions were analyzed by slot blot hybridization using oligonucleotide probes specific for internal 23S (probe 2) and 16S (probe 4) sequences (Fig. 5B) and 5S sequences (data not shown). The same filter was hybridized with each of the oligonucleotide probes. The filter was stripped between each hybridization, and loss of signal was confirmed by autoradiography prior to hybridization with the next probe. The 23S probe revealed the presence of 23S rRNA within mutant and wild-type 70S and 50S particles as well as mutant 62S and 39S particles (Fig. 5C). The 16S probe hybridized with both wild-type and mutant 70S and 30S particles as well as mutant 62S particles (Fig. 5C). 5S rRNA was detected in wild-type and mutant 70S and 50S particles as well as mutant 62S and 39S particles (data not shown). Therefore, the additional particles detected in mutant ribosome profiles, 62S and 39S, each had the same content of rRNA as mutant and wild-type 70S and 50S particles, respectively.
FIGURE 5.
Fraction collection, slot blot, and primer extension analysis (see Materials and Methods). (A) A260 units/mL of ribosome fractions collected from sucrose gradients (Mg++ concentration 6 mM/10 mM at lysis/sedimentation) for wild-type MG1655/I-::tet (closed squares) and ΔrluD::kan/I-::tet mutant (open squares). (Numbers below each graph are the fractions pooled for the ribosomal subunits indicated.) (B) Open boxes represent mature rRNA species, lines represent precursor sequences, and solid bars indicate probes used for slot blot hybridization (2 and 4) and primer extension analysis (1 and 3). (C) Equal amounts (5 μg) of RNA from pooled ribosomal fractions were transferred to nitrocellulose membranes and probed with the indicated oligonucleotides. (D) Equal amounts (0.5 μg) of RNA from the same pooled fractions were analyzed by primer extension using the indicated oligonucleotides.
To determine the state of maturation of rRNA present in mutant particles, the number of extra 5′ nucleotides was determined by primer extension (Fig. 5D). The extent of maturation of 23S rRNA was determined using an oligo probe (probe 1, Fig. 5B) complementary to residues 35–55 of mature 23S rRNA (Charollais et al. 2003). Using this probe, we find that wild-type 70S and 50S particles contain mature rRNA and a +1 species, as has previously been reported (Li et al. 1999; Charollais et al. 2003). In the ΔrluD::kan mutant, 70S, 62S, and 50S particles also contain predominantly the mature and the +1 species. However, the 62S and 50S particles also contain a small amount of a +4 species as well as an unprocessed +8 species, indicating that a portion of rRNAs in these particles is immature.
Sucrose gradient analysis suggested that mutant 70S ribosomes are unstable during processing. Assuming this instability reflects the in vivo situation, a fraction of mutant 23S rRNA may not be part of a polysome complex long enough for final maturation to occur. It is also possible that not all mutant 70S complexes would be able to function in protein synthesis. This would be in keeping with the polyPhe synthesis experiments in which stabilized mutant 70S ribosomes were found to be 20% less efficient. Finally, mutant 39S particles contain mostly under- (+4) and unprocessed (+8) precursors (Fig. 5D). The small amount of an apparently mature species is likely due to minor contamination of the 39S fraction pool with 50S subunits since the intensity of this band was always low and varied in individual experiments. The predominance of the +8 species indicates a complete lack of processing for most rRNA within this particle and further substantiates our conclusion that the 39S particle is an immature form of the 50S subunit (see Fig. 3).
In previous studies of a 40S particle from a ΔsrmB mutant, a predominant immature species of +7 was detected. However, unexpected +8 species were also seen (Charollais et al. 2003). The accurate numbering of the unprocessed +8 RNA species detected in our experiments was confirmed by comparison of the primer extension products with the single nucleotide step ladder and the 23S DNA sequence run on the same gel (Fig. 5D). In addition, the presence of predominantly the +8 species for the ΔrluD::kan mutant was reproducible in several experiments using two types of reverse transcriptases, AMV (data not shown) and M-MLV (Fig. 5D). The mechanism of RNase III action in maturation of rRNA has long been studied. However, its substrate specificity is still not well understood (Sirdeshmukh and Schlessinger 1985; Srivastava and Schlessinger 1990; Evguenieva-Hackenberg and Klug 2000; Lamontagne and Elela 2004). Therefore, the reaction that results in a +8 species in the 39S particles cannot be evaluated beyond indicating a complete lack of final processing for most 23S rRNA molecules.
Maturation of 16S rRNA within wild-type and ΔrluD mutant 30S particles also was assessed. Primer extension analysis was performed using an oligo probe complementary to residues 15–35 of the mature 16S sequence (probe 3, Fig. 5B). In the mutant 30S particles, many strong bands of up to +115 nt were detected, indicative of the presence of a large population of precursors to 16S rRNA (Fig. 5D). In wild-type 30S particles the same bands were detected; however, their intensity was much less, indicating that most of the rRNA contained within wild-type 30S particles is the mature 16S species. Quantification of the immature species present in wild-type and mutant 30S particles demonstrated that in both, immature species with up to five extra nucleotides are present at similar levels relative to the mature species. However, immature RNAs with 6–115 extra 5′ nucleotides are present in mutant 30S particles at much higher levels (30%) compared to wild type (1%). Therefore, high levels of the least mature 30S precursor species accumulate in the ΔrluD::kan mutant strain. In contrast, wild-type 70S, as well as mutant 70S and 62S particles, lack these additional 5′ nucleotides, and contain largely mature 16S rRNA (Fig 5D). This result provides further evidence to support the idea that mutant 62S particles are breakdown products of unstable 70S particles, rather than precursor particles. While it is unclear why the absence of some Ψ residues in the large subunit should affect maturation of 16S rRNA, this appears to be a common finding. For example, accumulation of 16S precursor rRNA was also detected in the ΔsrmB mutant, in which the primary defect is in 50S ribosome biogenesis (Charollais et al. 2003). In this case, accumulation of 16S precursors was believed to be an indirect consequence of 50S deficiency, rather than a direct result attributable to the loss of the SrmB protein.
Growth properties of the ΔrluD::kan strain supplemented with wild-type or mutant RluD
To address the question of whether the observed phenotype of the ΔrluD::kan mutant is due solely to the loss of the rluD gene, the mutant strain was transformed with plasmid-borne wild-type or mutated rluD genes. Expression of wild-type RluD restored normal growth on solid media to ΔrluD::kan cells either with or without induction (data not shown). However, expression of two RluD proteins that have been mutated at an aspartate residue previously shown to be necessary for synthase activity (D139N and D139T) had no effect on mutant colony size either with or without induction (data not shown). Exponential growth rates were determined for all transformed strains in the presence of inducer (0.05% L-arabinose). Expression of the wild-type RluD protein fully restores normal growth (doubling time 22 ± 1 min), while cells expressing the D139N (143 ± 10 min) or D139T (143 ± 4 min) proteins have generation times comparable to cells that contain empty plasmid (143 ± 7 min).
To determine whether the lack of rescue by mutant proteins might be due to lack of protein resulting from decreased expression or increased proteolysis of aberrantly folded proteins, SDS-PAGE analysis was performed. Lysates were made from cells that were scraped from the plates with or without inducer. This analysis demonstrated that induction results in the expression of equivalent amounts of wild-type and mutant proteins and that they are of the expected size, 39 kDa (data not shown). This analysis also showed that even without induction sufficient wild-type RluD protein was produced to rescue growth despite protein levels that are too low to visualize on SDS-PAGE. Pseudouridine sequence analysis revealed that the expression of wild-type RluD, which rescues growth of the ΔrluD::kan mutant, also restores synthesis of Ψ1911, 1915, and 1917, whereas induced levels of mutant proteins that fail to rescue the mutant growth phenotype also fail to restore Ψ synthesis (data not shown). Sucrose gradient analysis further revealed that only the expression of wild-type RluD restored a normal ribosome profile to the ΔrluD::kan mutant (data not shown). The ribosome profile of the mutant carrying plasmid-borne wild-type rluD gene was indistinguishable from wild-type cells carrying empty plasmid. The ribosome abnormalities of the mutant strain persist in cells expressing plasmid-borne mutant RluD proteins or empty plasmids (refer to Fig. 2B). Therefore, only the presence of the wild-type RluD protein and/or the Ψ residues synthesized by it are able to fully restore normal ribosome biogenesis and wild-type exponential growth in the ΔrluD::kan strain.
Second site suppressor mutant(s)
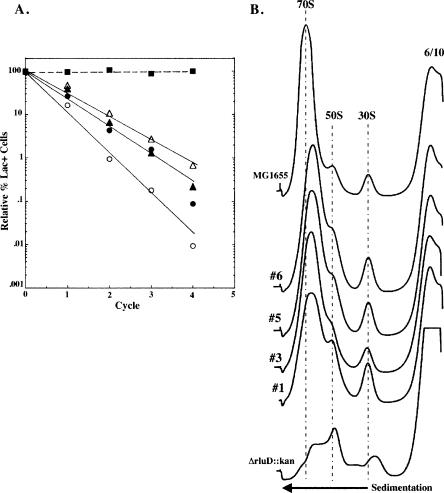
Due to the severity of the growth phenotype, the ΔrluD::kan mutant readily generates colonies that appear to grow normally on solid media (data not shown). Six such colonies were selected, and they proved to be pseudorevertants by molecular and functional assays including, PCR, Southern transfer, and hybridization and by their inability to form Ψ1911, 1915, and 1917 (data not shown). In addition, these six strains retained the ability to grow on plates containing kanamycin, indicating that the kan insert was still present (data not shown). Four of these isolates were chosen for further characterization of their growth phenotype. Though apparently wild type in colony size, exponential growth rates were depressed compared with the true wild-type strain, MG1655 (doubling time 23 ± 1 min), ranging from 25 ± 2 to 29 ± 2 min in rich media at 37°C. In addition, when grown in competition with wild-type cells through all phases of the growth cycle, pseudorevertant cells were present at just 0.01%–1.0% of their original numbers after only four cycles of competitive growth (Fig. 6A). The differences in exponential growth rates and the relative competitiveness of the individual pseudorevertants suggest that separate mutations may be responsible for their improved growth. Studies to formally address what, if any, genetic difference(s) exist among the individual pseudorevertants are currently underway in our laboratory. Not surprisingly, sucrose gradient analysis demonstrated that there is also a modest and somewhat variable defect in ribosome biogenesis when compared with the true wild-type strain, MG1655, with more than normal 50S particles and fewer fully assembled 70S particles (Fig. 6B). Therefore, a mutated state that does not restore the RluD synthase or the Ψ residues for which it is responsible does not fully compensate for the loss of the rluD gene and the resulting phenotype of the ΔrluD::kan mutant.
FIGURE 6.
Competition studies, exponential growth rates, and ribo-some profiles of MG1655 (wild type) and four pseudorevertant isolates of the ΔrluD::kan mutant. (A) Growth competition studies were done as described previously (Gutgsell et al. 2000). MG1655 (wild type) (closed squares), four individual pseudorevertant isolates (open and closed triangles and circles). Exponential growth rates were done as described in Materials and Methods. Doubling times were determined as described in Figure 2. Each plot consisted of seven to nine time points. Doubling times were the average of two determinants. (B) Ribosome profiles were performed as described in Materials and Methods. Wild-type, pseudorevertant, and mutant cells were lysed at 6 mM Mg++ and gradients were run at 10 mM Mg++. Twelve A260 units were layered onto 14%–32% sucrose gradients and centrifuged at 22,000 rpm for 19 h at 4°C.
DISCUSSION
In this work we demonstrate that the pseudouridine synthase RluD and/or the Ψ1911, Ψ1915, and Ψ1917 residues formed by it are necessary for normal ribosome biogenesis, assembly, and function in E. coli. The ribosome defect and the subsequent severe growth phenotype resulting from deletion of the rluD gene were corrected only when plasmid-borne wild-type protein, but not D139 mutant proteins, were reintroduced into the ΔrluD::kan strain. This is in contrast to the results of previous work from our laboratory using two RluD-minus strains (Tiny and Dust), generated by disruption of the rluD gene (Gutgsell et al. 2001). Expression of mutant RluD proteins, D139N and D139T, restored wild-type growth in both disruption strains, but restored Ψ formation only in the Dust strain. The restoration of growth in the absence of Ψ formation in the Tiny strain suggested a second separable function for the RluD protein. However, that disruption mutant strain was created by insertion of a mini Tn10cam cassette between residues 588 and 589 in the rluD gene sequence. Therefore, introduction of plasmid-borne mutated rluD gene sequences into the disruption mutant allowed for the potential of homologous recombination, which could have resulted in restoration of the wild-type rluD gene in either the genome or on the plasmid. There was also the potential for formation of functional D139/D139N or D139/D139T truncated/full-length heterodimer proteins, assuming RluD forms dimers, as was shown previously for TruA (Foster et al. 2000). The wild-type growth and Ψ formation seen with the Dust strain are in fact consistent with one of these scenarios. However, interpretation of the results for the Tiny strain is complicated by the fact that Tiny was shown to arise from Dust as a result of an unidentified second site mutation. Without knowledge of the nature of this second site mutation, it is not yet possible to explain how Tiny cells were able to grow despite the absence of the Ψ residues. Nevertheless, in the present work, expression of the D139 mutant proteins in the background of a true rluD deletion strain avoided these complications, and clearly demonstrated the importance of the Ψs.
In the current study, the lack of rescue in the absence of pseudouridine formation suggests a specific role for the Ψ1911, 1915, and 1917 residues and argues against a second separable function for the RluD protein. If the RluD protein has multiple functions, the D139 mutant proteins might be expected to retain their ability to perform such a function secondary to Ψ formation. SDS-PAGE analysis suggests no obvious effect, such as misfolding or increased proteolysis, as a result of mutation of these RluD proteins. However, subtle changes in protein structure that could affect such a function may not be detectable by this method. Crystallization of the D139 mutant proteins is currently underway, which might help to clarify this issue (P.P. Vaidyanathan and A. Malholtra, unpubl.; Del Campo et al. 2004).
Is there then, based on current evidence, any reason to believe there is a second function for the RluD protein? There is precedence for the idea that the ribosome assembly defect seen in the ΔrluD::kan mutant could be attributed, at least in part, to the lack of the RluD protein. First, several recent studies have demonstrated that nonribosomal proteins, which do not have RNA modification functions, have a role in ribosome biogenesis. Mutation of the genes encoding the DEAD-box RNA helicases SrmB (Charollais et al. 2003) and CsdA (Charollais et al. 2004), the heat shock-induced methyltransferase RrmJ (Hager et al. 2002), as well as the DnaK chaperone (El Hage and Alix 2004) all result in ribosome and growth defects in vivo and phenotypes similar to the ΔrluD mutant. Also, other studies have demonstrated additional functions for yeast and E. coli modifying enzymes apart from their role in pseudouridylation or methylation. The yeast tRNA pseudouridine synthase, Rib2/Pus8p, was shown to have a second catalytic domain that is involved in a completely unrelated metabolic function (Behm-Ansmant et al. 2004). The E. coli trmA (Persson et al. 1992) and rlmAI (Liu et al. 2004) gene products, but not methylation of tRNA U54 and 23S rRNA G745, respectively, are necessary for cell viability, suggesting a second important function for the TrmA and RlmAI proteins. Interestingly, like ΔrluD, the rlmAI mutant strain also produces spontaneous pseudorevertants, still lacking mG745, but with wild-type growth restored, suggesting that methylation of this residue is not necessary for growth. In contrast, in the background of the ΔrluD::kan strain, the subtle defect in exponential growth, ribosome profiles, and the inability of the ΔrluD pseudorevertants to compete with true wild type suggests a role for the Ψ residues.
Taken together the results of the present study suggest the following scenario: The primary defect in the ΔrluD mutant is at the level of rRNA assembly and processing, which results in the characteristic mutant ribosome profile. Instability of mutant 70S ribosomes leads to an accumulation of 62S, 50S, and 30S particles. The accumulation of 39S particles containing precursor rRNA sequences indicates problems with large subunit assembly. The ribosome assembly and processing defect results in a severe growth phenotype reflected by a generation time sixfold that of wild type. In addition, the in vitro studies demonstrated that mutant 70S ribosomes stabilized by high Mg++ concentrations were reproducibly 20% less active in protein synthesis compared to wild-type ribosomes, indicating that there is a modest functional defect as well. What then can be said about the RNP species isolated from mutant lysates that sediment as 70S particles? Primer extension analysis revealed that like wild type, they contain only mature 23S rRNA and the +1 species. Such complete processing of 23S rRNA suggests that mutant 70S particles must have been engaged in protein synthesis because translation is needed to complete maturation (Sirdeshmukh and Schlessinger 1985; Srivastava and Schlessinger 1988). The fact that these mutant particles sediment like wild-type 70S ribosomes also suggests that proper folding and the association of the expected ribosomal proteins has occurred. Therefore, though few 70S particles are recovered in lysates from the mutant strain, those that are present apparently are compositionally similar to wild type. Assuming that the recovery of 70S particles closely reflects the in vivo situation, the pronounced growth defect is likely due to the low amount of functional 70S ribosomes in mutant cells. Studies to further address mutant ribosome composition and function are currently under way.
MATERIALS AND METHODS
Strains
MG1655 was the wild-type E. coli strain. DH5α was the strain used for all initial transformation experiments. MG1655ΔrluD:: kan was constructed by a modification of the gene replacement method of Datsenko and Wanner (2000). A deletion allele containing the amino- and carboxy-terminal fragments of rluD linked to a kanamycin cassette was constructed by PCR amplification from plasmid pUC4K (Amersham). The 5′ primer (5′-GCACAACGAGTA CAGCTCACTGCAACGGTGTCCGAATAATGATTACGAATTCC CCGGATC-3′) was the rluD sequence of residues +4 to +39, followed by a stop codon (bold type) that overlaps the A of the initiating AUG (italics) followed by the complement of residues 1689–1670 of pUC4K (underlined). The 3′primer (5′-TTCGAAATCGGCGCGCATCAVCTCAATCAGCTCCACCCAATTCTGATTAGAAAAACTC-3′) was the complement of rluD residues +951 to +916, followed by the sequence of residues 710–731 of pUC4K (underlined). After PCR amplification of the allele, the wild-type rluD gene was replaced with the deletion allele as described (Datsenko and Wanner 2000).
P1-mediated backcross of four isolates of the ΔrluD::kan strain to wild type was done as described (Miller 1992). Cells were then spread on LB plates containing 34 μg/mL kanamycin. P1 lysates were prepared from six ΔrluD::kan isolates obtained from this primary backcross into MG1655, for a second P1 mediated transductional backcross to MG1655. Cells were again spread on LB/kanamycin plates from which individual colonies were replated onto M9/kanamycin plates to avoid selection of nutritional auxotrophs.
The RNase I− mutation was introduced into MG1655 and ΔrluD::kan strains by P1 mediated transduction of an rna::tet strain, CAN 20-12E (Deutscher et al. 1984).
Transduced cells were plated on M9/tet or M9/tet, kan plates and individual colonies were transferred to a second set of M9 plates as before and subsequently streaked onto LB plates containing the same drugs. The resulting drug resistant colonies were first screened for loss of RNase I activity by Methyl Green Colorimetric Analysis as described (Wright 1971). Loss of RNase I− activity was confirmed for selected clones by determination of acid-soluble radioactivity released from [3H]poly(A) substrate, as described (Deutscher et al. 1984).
Liquid culture
To avoid the emergence of pseudorevertants in ΔrluD::kan cultures, all strains, including wild-type controls, were cultured by inoculating LB or YT media with single colonies. Colonies were picked with sterile pipette tips to standardize the amount of inoculum, and cultures were grown with aeration by shaking at 37°C to an A600 ≤0.5. Aliquots were removed at the time of harvest for dilution and plating to ascertain that revertants had not accumulated at the low density to which cells were grown (<0.01% revertants was considered acceptable). As was necessary for individual experiments, cultures contained 34 μg/mL kanamycin (ΔrluD::kan); 5 μg/mL tetracycline (Rnase I−); 0.5% L-arabinose and/or 100 μg/mL carbenicillin (induction and maintenance of pBAD/Myc-His A plasmids).
Plasmids and transformations
The wild-type rluD gene was PCR amplified from MG1655 genomic DNA using Taq DNA polymerase (Promega) and subcloned into the NcoI and HindIII sites of pBAD/Myc-His A (Invitrogen). The amino-terminal primer was (5′-GAAGAAGGGTCTCACATGGCACAACGAGTACAGCTC-3′), incorporating a BsaI site (italics) 1 nt before the AUG start codon (underlined). The carboxy-terminal primer was (5′-GGGAAGCTTTCATAACCAGTC CACTTCATC-3′), incorporating a HindIII site (italics) followed by the complement of residues 981–961 (numbering begins with the TGA stop codon). As a result of inclusion of the rluD stop codon, the C-terminal polyhistidine tag is not expressed from this plasmid.
To make the D139N and D139T mutants of rluD, the wild-type rluD gene was amplified from MG1655 genomic DNA as described above and subcloned into the NcoI and HindIII sites of pTrc99A (Pharmacia). The pTrc99A/rluD construct was used as template in PCR reactions using pfu turbo polymerase (Stratagene) to incorporate the mutation and amplify the mutated gene. For rluD(D139N) the mutagenic primers were (5′-CATCG TCCATCGTCTGAATAAAGACACCACTGGCC-3′) and (5′-GGCC AGTGGTGTCTTTATTCAGACGATGGACGATG-3′), which incorporated the necessary base change (underlined in the primer sequences) to mutate the aspartate residue to asparagine. For rluD(D139T), the mutagenic primers were (5′-GGATCGCCATCGTCTGACTAAAGACACCACTGGCC-3′) and (5′-GGCCAGTGGTGTCTTTAGTCAGACGATGGACGTGCC-3′), which incorporated the necessary base changes (underlined in the primer sequences) to mutate the aspartate residue to threonine. The pTrc99ArluD/D139N and pTrc99ArluD/D139T plasmids were used as template in PCR reactions to amplify mutated rluD genes as described above for the wild-type rluD gene for subcloning into the NcoI and HindIII sites of pBAD/Myc-His A. All of the mutant plasmids were verified by DNA sequencing.
The ΔrluD::kan mutant was transformed with empty pBAD/Myc-His A plasmid or with wild-type or mutant rluD-containing plasmids by 42°C heat shock (Sambrook et al. 1989) with modifications in order to avoid pseudorevertants as described (see Liquid culture). The following day, colonies were picked from the transformation plates and inoculated into 1 mL medium containing LB kanamycin (34 μg/mL) and carbenicillin (100 μg/mL) for incubation ≤4 h at 37°C. From these short-term cultures glycerol stocks were prepared and LB (kan/carb) plates were streaked in order to determine colony size of transformants and to again ensure that no revertants had emerged. From each 4-h culture, 0.1 mL was retained and 4 mL LB (kan/carb) were added for overnight incubation for plasmid DNA isolation and characterization to insure the presence of the expected construct. Briefly, the pBAD/Myc-His A plasmid and the wild-type rluD gene contain a single PflMI site, while both the D139N and D139T mutants have lost this site as a direct consequence of the nucleotide change(s) that create(s) the aspartic acid mutations. Therefore, upon PflMI digestion, mutant plasmids are linearized and wild-type plasmids appear as two bands on agarose gels.
Ribosome analysis
All strains tested were grown in YT or LB medium as described (see Liquid culture). Cells were cooled by incubating 10 min on ice in precooled centrifuge bottles or by pouring cultures into centrifuge bottles that contained frozen, crushed medium stored at −20°C. Cells were collected by centrifugation and pellets resuspended in lysis buffer (50 mM Tris-HCl at pH 8, 60 mM KCl, 60 mM NH4Cl, 1 mM DTT, 16% sucrose, and various concentrations [6–20 mM] MgOAc) plus 1 mg/mL lysozyme (Sigma) and 40 U/mL DNaseI (Sigma) to begin three freeze–thaw cycles. After the third thaw cycle, 0.5% Brij 58 (Sigma) and 0.5% deoxycholic acid (MC & B) were added, and the sample was incubated on ice for 20 min. All lysates were clarified by centrifugation at 14,000 rpm for 20 min at 4°C. The extract concentration was determined by measuring the A260. To analyze ribosome profiles, 12 A260 units of lysate were layered onto 14%–32% sucrose gradients in buffer (20 mM Tris-HCl at pH 8; 60 mM KCl, 60 mM NH4Cl, 1 mM DTT, and various concentrations [10–20 mM] MgOAc,) and centrifuged at 22,000 rpm for 19 h at 4°C in a Beckman SW 28 rotor. Gradients were analyzed with a Gilson Holochrome with continuous monitoring at A260 nm and a Bromma LKB 2 channel recorder.
To prepare S150 enzymes, 1 or 2 L of LBtet or LBtet/kan medium were inoculated with single colonies of MG1655/I−::tet or MG1655ΔrluD::kan/I−::tet (RNase I− strains), respectively, and grown as described (see Liquid culture). Cells were harvested and lysates were prepared and fractionated to obtain the S150 enzymes fraction as described (Nierhaus and Dohme 1979).
Polyphenylalanine synthesis experiments were performed as described with some modifications (Cunningham et al. 1992). The reaction mixture for polyPhe synthesis contained 45 mM Bicine (pH 8.5), 5 mM HEPES (pH 7.5), 50 mM NH4Cl, 15 mM Mg acetate, 10 mM phospho(enol)pyruvate, 2 mM ATP, 5 mM DTT, 1 mM GTP, 80 μg/mL pyruvate kinase, 0.022 mM cold phenylalanine, and 0.25 μCi [3H] phenylalanine. Reaction mixtures (100 μL) contained 53 μL of this master mix plus S150 enzymes and tRNA (Sigma, E. coli Strain W) at concentrations calculated to provide 100 pmol tRNAPhe based on 2% representation in a total tRNA pool. This reaction mix was incubated 7 min at 37°C to precharge the tRNA. Poly(U) (100 μg/mL) and ribosomes (0.5 or 1.0 A260 units) were added and incubation at 37°C continued. At the indicated times, 35-μL aliquots of the reaction mixture were removed to 13 × 100 mm glass tubes. A solution containing 10% TCA, 2% Casamino Acids was added to ~ 1/4 tube volume, vortexed briefly, and incubated 15 min at 90°C. A solution containing 5% TCA, 2% Casamino Acids was added to ~1/2 tube volume. Solutions were vortexed briefly, cooled, and passed through nitrocellulose filters (Schleicher & Schuell Grade BA85) with vacuum. Filters were dried, dissolved in scintillation fluid (Filtron X), and counted (Beckman LS 3801 Liquid Scintillation System). Values were corrected for the small amount of reaction found in the absence of ribosomes.
RNA analysis
Wild-type MG1655/I-::tet and mutant ΔrluD::kan/I-::tet lysates were prepared as described for ribosome analysis. rRNA fractions (1 mL) were collected from across the resulting sucrose gradients and A260 measurements were taken (PerkinElmer Lambda 25 spectrophotometer). Fractions that represent 70S, 50S, and 30S for wild-type and mutant cells and fractions for mutant 62S and 39S were pooled. The pooled fractions were extracted with phenol:chloroform (1:1), and chloroform:isoamyl alcohol (24:1), and the isolated RNA was precipitated with ethanol and dissolved in distilled H2O. For further analysis, RNA (5 μg) was mixed with 2.5% Ficoll, 0.075% bromophenol blue, 48% formide, 6.5% formaldehyde, 1× MOPS gel-loading buffer. Samples were heated at 65°C for 5 min, mixed with an equal volume of 20× SSC, and placed on ice. RNAs were transfered to Nytran nylon membranes (Schleicher & Schuell) using a slot blot manifold (Bethesda Research Laboratories). RNA was fixed to the membrane by cross-linking (Stratalinker) and membranes were prehybridized ≥4 h at 50°C in hybridization buffer (Clontech ExpressHyb Hybridization Solution) followed by overnight hybridization at 50°C with [32P]-labeled probes. After hybridization, membranes were washed first in 6× SSC for 30 min at 30°C followed by a second wash in 3× SSC 0.5% SDS for 15 min at 50°C. Membranes were analyzed by autoradiography (Kodak Biomax MR film). The filters were hybridized with multiple probes and were stripped between each hybridization by washing ≥3× in boiling 0.1× SSC 0.1% SDS. Complete stripping was verified by autoradiography. Oligonucleotide probes used were 23S (5′- GCACCTGCTCGCGCCGTC-3′), complementary to residues 2359–2376 of mature 23S rRNA; 16S (5′-GGTTGAGCCCGGG GATTTCAC-3′), complementary to residues 604–624 of 16S rRNA; and a pool of two 5S (5′-AGGTGGGACCACCTCTC TAC-3′) and (5′-GGCGCTACGGCGTTTCACTTC-3′), complementary to residues 14–35 and 54–75 of mature 5S rRNA, respectively.
The same RNAs (0.5 μg of each) were used for primer extension using oligonucleotides 23S (5′-CCTTCATCGCCTCTGACTGCC-3′) (Charollais et al. 2003) and 16S (5′-CGTTCAATCTGAGCCAT GATC-3′) (Sigma Genosys), complementary to residues 39–58 and 15–35 of 23S and 16S rRNAs, respectively (numbering is from the mature 5′ end). Oligonucleotide primers were [32P] 5′ end labeled using T4 polynucleotide kinase (NEB). 23S primer extensions were done using Superscript II RNase H− M-MLV reverse transcriptase (Invitrogen) according to the manufacturer’s specifications. 16S and 23S primer extensions were done using AMV reverse transcriptase (Promega) as described previously (Ofengand et al. 2001) except that annealing was at 50°C. The extension products were separated on 8% polyacrylamide 7 M urea gels and detected by autoradiography. The 23S DNA sequencing ladder and the single nucleotide step ladder were obtained using the same primer and the plasmid pCW1 as template (Weitzmann et al. 1990). The 16S sequence was done in parallel with the primer extension reactions using the same primer and MG1655/I−::tet total RNA as template. Intensity of the bands resulting from the 16S primer extension reactions was determined using a PhosphorImager (Molecular Dynamics).
Other methods and materials
Isolation of total RNA and Ψ sequencing were performed as described (Ofengand et al. 2001). Primers were obtained from Gibco-BRL and used without further purification. SDS polyacrylamide gel electrophoresis was performed as described previously (Raychaudhuri et al. 1998). Competition studies were done as described previously (Gutgsell et al. 2000). DNA sequencing was done with a Sequenase Version 2.0 DNA Sequencing Kit (USB Corporation) according to the manufacturer’s directions using primers described for amplification and subcloning of the rluD gene.
Acknowledgments
We are grateful to professors Kenn E. Rudd and Chaitanya Jain for helpful discussions and editorial assistance. We thank Yusuf Kaya for technical assistance with the primer extension analysis shown in Figure 5D.
Article published online ahead of print. Article and publication date are at http://www.rnajournal.org/cgi/doi/10.1261/rna.2550105.
REFERENCES
- Agrawal, R.K., Sharma, M.R., Kiel, M.C., Hirokawa, G., Booth, T.M., Spahn, C.M., Grassucci, R.A., Kaji, A., and Frank, J. 2004. Visualization of ribosome-recycling factor on the Escherichia coli 70S ribosome: functional implications. Proc. Natl. Acad. Sci. 101: 8900–8905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachman, B.J., Low, K.B., and Taylor, A.L. 1976. Recalibrated linkage map of Escherichia coli K-12. Bacteriol. Rev. 40: 116–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bashan, A., Agmon, I., Zarivach, R., Schluenzen, F., Harms, J., Berisio, R., Bartels, H., Franceschi, F., Auerach, T., Hansen, H.A.S., et al. 2003. Structural basis of the ribosomal machinery for peptide bond formation, translocation, and nascent chain progression. Mol. Cell 11: 91–102. [DOI] [PubMed] [Google Scholar]
- Behm-Ansmant, I., Grosjean, H., Massenet, S., and Motorin, Y. 2004. Pseudouridylation at position 32 of mitochondrial and cytoplasmic tRNAs requires two distinct enzymes in Saccharomyces cerevisiae. J. Biol. Chem. 279: 52998–53006. [DOI] [PubMed] [Google Scholar]
- Charollais, J., Pflieger, D., Vinh, J., Dreyfus, M., and Iost, I. 2003. The DEAD-box RNA helicase SrmB is involved in the assembly of 50S ribosomal subunits in Escherichia coli. Mol. Microbiol. 48: 1253–1265. [DOI] [PubMed] [Google Scholar]
- Charollais, J., Dreyfus, M., and Iost, I. 2004. CsdA, a cold-shock RNA helicase from Escherichia coli., is involved in the biogenesis of 50S ribosomal subunit. Nucleic Acids Res. 32: 2751–2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad, J., Sun, D., Englund, N., and Ofengand, J. 1998. The rluC gene of Escherichia coli codes for a pseudouridine synthase that is solely responsible for synthesis of pseudouridine at positions 955, 2504, and 2580 in 23S ribosomal RNA. J. Biol. Chem. 273: 18562–18566. [DOI] [PubMed] [Google Scholar]
- Conrad, J., Niu, L., Rudd, K., Lane, B.G., and Ofengand, J. 1999. 16S ribosomal RNA pseudouridine synthase RsuA of Escherichia coli: Deletion, mutation of the conserved Asp102 residue, and sequence comparison among all other pseudouridine synthases. RNA 5: 751–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham, P.R., Nurse, K., Bakin, A., Weitzmann, C.J., Pflumm, M., and Ofengand, J. 1992. Interaction between the two conserved singlestranded regions at the decoding site of small subunit ribosomal RNA is essential for ribosome function. Biochemistry 31: 12012–12022. [DOI] [PubMed] [Google Scholar]
- Datsenko, K.A. and Wanner, B.L. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. 97: 6640–6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Campo, M., Kaya, Y., and Ofengand, J. 2001. Identification and site of action of the remaining four putative pseudouridine synthases in Escherichia coli. RNA 7: 1603–1615. [PMC free article] [PubMed] [Google Scholar]
- Del Campo, M., Ofengand, J., and Malhotra, A. 2004. Crystal structure of the catalytic domain of RluD, the only rRNA pseudouridine synthase required for normal growth of Escherichia coli. RNA 10: 231–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutscher, M.P., Marlor, C.W., and Zaniewski, R. 1984. Ribonuclease T: New exoribonuclease possibly involved in end-turnover of tRNA. Proc. Natl. Acad. Sci. 81: 4290–4293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Hage, A. and Alix, J.-H. 2004. Authentic precursors to ribosomal subunits accumulate in Escherichia coli in the absence of functional DnaK chaperone. Mol. Microbiol. 51: 189–201. [DOI] [PubMed] [Google Scholar]
- Evguenieva-Hackenberg, E. and Klug, G. 2000. RNase III processing of intervening sequences found in helix 9 of 23S rRNA in the α subclass of Proteobacteria. J. Bacteriol. 182: 4719–4729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster, P.G., Lixuan, H., Santi, D.V., and Stroud, R.M. 2000. The structural basis for tRNA recognition and pseudouridine formation by pseudouridine synthase I. Nat. Struct. Biol. 7: 23–27. [DOI] [PubMed] [Google Scholar]
- Gutgsell, N.S., Englund, N., Niu, L., Kaya, Y., Lane, B.G., and Ofengand, J. 2000. Deletion of the Escherichia coli pseudouridine synthase gene truB blocks formation of pseudouridine 55 in tRNA in vivo, does not affect exponential growth, but confers a strong selective disadvantage in competition with wild-type cells. RNA 6: 1870–1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutgsell, N.S., Del Campo, M., Raychaudhuri, S., and Ofengand, J. 2001. A second function for pseudouridine synthases: A point mutant of RluD unable to form pseudouridines 1911, 1915, and 1917 in Escherichia coli 23S ribosomal RNA restores normal growth to an RluD-minus strain. RNA 7: 990–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hager, J., Staker, B.L., Bügl, H., and Jakob, U. 2002. Active site in RrmJ, a heat shock-induced methyltransferase. J. Biol. Chem. 277: 41978–41986. [DOI] [PubMed] [Google Scholar]
- Kaya, Y. and Ofengand, J. 2003. A novel unanticipated type of pseudouridine synthase with homologs in bacteria, archaea, and eukarya. RNA 9: 711–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamontagne, B. and Elela, S.A. 2004. Evaluation of the RNA determinants for bacterial and yeast RNase III binding and cleavage. J. Biol. Chem. 279: 2231–2241. [DOI] [PubMed] [Google Scholar]
- Li, Z., Pandit, S., and Deutscher, M.P. 1999. Maturation of 23S ribosomal RNA requires the exoribonuclease RNase T. RNA 5: 139–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl, L. 1975. Intermediates and time kinetics of the in vivo assembly of Escherichia coli ribosomes. J. Mol. Biol. 92: 15–37. [DOI] [PubMed] [Google Scholar]
- Liu, M., Novotny, G.W., and Douthwaite, S. 2004. Methylation of 23S rRNA nucleotide G745 is a secondary function of the RlmAI methyltransferase. RNA 10: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, J.H. 1992. A short course in bacterial genetics: A laboratory manual and handbook for Escherichia coli and related bacteria, Chapter 28, pp. 357–364. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- Nierhaus, K.H. and Dohme, F. 1979. Total reconstitution of 50S subunits from Escherichia coli ribosomes. Methods Enzymol. LIX: 443–449. [DOI] [PubMed] [Google Scholar]
- Ofengand, J. and Rudd, K.E. 2000. The ribosome: Structure, function, antibiotics and cellular interactions. In The ribosome: Structure, function, antibiotics, and cellular interactions (eds. R.A. Garrett et al.), pp. 175–189. ASM Press, Washington, DC.
- Ofengand, J., Del Campo, M., and Kaya, Y. 2001. Mapping pseudouridines in RNA molecules. Methods 25: 365–373. [DOI] [PubMed] [Google Scholar]
- Persson, B.C., Gustafsson, C., Berg, D.E., and Bjork, G.R. 1992. The gene for a tRNA modifying enzyme, m5U54-methyltransferase, is essential for viability in Escherichia coli. Proc. Natl. Acad. Sci.. 89: 3995–3998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raychaudhuri, S., Conrad, J., Hall, B.G., and Ofengand, J., 1998. A pseudouridine synthase required for the formation of two universally conserved pseudouridines in ribosomal RNA is essential for normal growth of Escherichia coli. RNA 4: 1407–1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raychaudhuri, S., Niu, L., Conrad, J., Lane, B.G., and Ofengand, J. 1999. Functional effect of deletion and mutation of the Escherichia coli ribosomal RNA and tRNA pseudourindine synthase RluA. J. Biol. Chem. 274: 18880–18886. [DOI] [PubMed] [Google Scholar]
- Sambrook, J., Fritsch, E.F., and Maniatis, T. 1989. Molecular cloning: A laboratory manual, 2d ed., pp, 1.82–1.83. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- Sirdeshmukh, R. and Schlessinger, D. 1985. Ordered processing of Escherichia coli 23S rRNA in vitro. Nucleic Acids Res. 13: 5041– 5054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava, A.K. and Schlessinger, D. 1988. Coregulation of processing and translation: Mature 5′ termini of Escherichia coli 23S ribosomal RNA form in polysomes. Proc. Natl. Acad. Sci. 85: 7144–7148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ———. 1990. Mechanism and regulation of bacterial ribosomal RNA processing. Annu. Rev. Microbiol. 44: 105–129. [DOI] [PubMed] [Google Scholar]
- Weitzmann, C.J., Cunningham, P.R., and Ofengand, J. 1990. Cloning in vitro transcription, and biological activity of Escherichia coli 23S ribosomal RNA. Nuc. Acids Res. 18: 3515–3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright, M. 1971. Mutants of Escherichia coli lacking endonuclease I, ribonuclease I, or ribonuclease II. J. Bacteriol. 107: 87–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonath, A. and Bashan, A. 2004. Ribosomal crystallography: Initiation, peptide bond formation, and amino acid polymerization are hampered by antibiotics. Annu. Rev. Microbiol. 58: 233–251. [DOI] [PubMed] [Google Scholar]
- Yusupov, M.M., Yusupova, G.Z., Baucom, A., Lieberman, K., Earnest, T.N., Cate, J.H.D., and Noller, H.F. 2001. Crystal structure of the ribosome at 5.5 Å resolution. Science 292: 883–896. [DOI] [PubMed] [Google Scholar]