Abstract
We identify and characterize an end-healing enzyme, CthPnkp, from Clostridium thermocellum that catalyzes the phosphorylation of 5′-OH termini of DNA or RNA polynucleotides and the dephosphorylation of 2′,3′ cyclic phosphate, 2′-phosphate, and 3′-phosphate ribonucleotides. CthPnkp also catalyzes an autoadenylylation reaction via a polynucleotide ligase-type mechanism. These characteristics are consistent with a role in end-healing during RNA or DNA repair. CthPnkp is a homodimer of an 870-amino-acid polypeptide composed of three catalytic domains: an N-terminal module that resembles the polynucleotide kinase domain of bacteriophage T4 Pnkp, a central metal-dependent phosphoesterase module, and a C-terminal module that resembles the nucleotidyl transferase domain of polynucleotide ligases. The distinctive feature of CthPnkp vis-à-vis known RNA repair enzymes is that its 3′ end modification component belongs to the calcineurin-type phosphatase superfamily. It contains putative counterparts of the amino acids that form the dinuclear metal-binding site and the phosphate-binding site of bacteriophage λ phosphatase. As with λ phosphatase, the 2′,3′ cAMP phosphatase activity of CthPnkp is specifically dependent on nickel or manganese. We identify homologs of CthPnkp in other bacterial proteomes.
Keywords: RNA repair, DNA repair, end-healing, phosphoesterase, polynucleotide kinase
INTRODUCTION
When breakage of a 3′-5′ phosphodiester in DNA or RNA results in the formation of 5′-PO4 and 3′-OH termini at the break site, the ends can be rejoined to each other (or to new partner strands) by the action of DNA-specific or RNA-specific polynucleotide ligases. However, when breakage occurs with the opposite polarity, resulting in 5′-OH and 3′-PO4 (or 2′,3′ cyclic PO4) termini, the broken ends must be “healed” before they can be sealed. Healing entails two steps: (1) hydrolysis of the 3′-PO4 (or 2′,3′ cyclic phosphate) to form a 3′-OH, and (2) phosphorylation of the 5′-OH to form a 5′-PO4 end. The bacteriophage T4 proteins polynucleotide kinase/phosphatase (Pnkp), RNA ligase 1 (Rnl1), and RNA ligase 2 (Rnl2) are the prototypal RNA healing and sealing enzymes (Richardson 1965; Novogrodsky and Hurwitz 1966; Novogrodsky et al. 1966; Cameron and Uhlenbeck, 1977; Silber et al. 1972; Ho and Shuman 2002) T4 Pnkp and Rnl1 function in vivo to repair a break in the anticodon loop of Escherichia coli tRNALys triggered by phage-activation of a host-encoded anticodon nuclease PrrC (Amitsur et al. 1987). Depletion of tRNALys by PrrC blocks phage protein synthesis and arrests the infection before it can spread. However, Pnkp and Rnl1 repair the broken tRNAs and thereby thwart the host defense mechanism.
Whereas bacteriophage tRNA restriction provided the initial insights to RNA repair biology, little attention has been paid to the issue of whether bacteria themselves (as opposed to phage) might specify enzymes that heal and seal broken RNAs. The recent discovery and characterization of an Rnl2-like RNA ligase encoded by Deinococcus radiodurans provided the first clue to the existence an RNA repair capacity in bacteria (Martins and Shuman 2004a). To date, there has been no description of an end-healing enzyme in bacteria analogous to T4 Pnkp, even though Pnkp-like enzymes have been identified and characterized from a variety of bacteriophages, eukarya, and a eukaryotic virus (Blondal et al. 2005; Martins and Shuman 2004b; Zhu et al. 2004; Bernstein et al. 2005).
Here we identify a polynucleotide end-healing enzyme from the bacterium Clostridium thermocellum, an anerobic thermophilic bacterium that has attracted biotechnology interest because of its capacity to convert cellulose to ethanol (Demain et al. 2005). The proteome of C. thermocellum strain ATCC 27405 (available at NCBI) includes an 870-amino-acid polypeptide containing an N-terminal module that resembles the kinase domain of T4 Pnkp, a central module that resembles a metal-dependent phosphoesterase, and a C-terminal module that resembles the nucleotidyl transferase domain of polynucleotide ligases (Fig. 1). We show here that the purified recombinant C. thermocellum protein has an intrinsic 5′-OH RNA/DNA kinase activity and a ribonucleotide 2′,3′ phosphatase activity. These results are consistent with a role in end-healing during RNA or DNA repair. Thus, we name the enzyme CthPnkp. We also show that CthPnkp reacts with ATP to form a covalent enzyme-adenylate adduct via a polynucleotide ligase-type mechanism. The presence of homologs of CthPnkp in other bacterial proteomes suggests that RNA repair might be relevant to bacterial physiology.
FIGURE 1.
A new family of bacterial Pnkp proteins. The amino acid sequence of C. thermocellum Pnkp (Cth; NCBI accession ZP_00312808) is aligned to homologous polypeptides encoded by Kineococcus radiotolerans (Kra; NCBI accession ZP_00354175), Nostoc sp. PCC 7120 (Nostoc; NCBI accession BAB75430), and Streptomyces coelicolor (Sco, NCBI accession NP_630089). Gaps in the alignment are indicated by dashes. Positions of amino acid side-chain identity or similarity in all four proteins are indicated by dots above the alignment. The polynucleotide kinase, phosphoesterase, and nucleotidyltransferase motifs are highlighted in shaded boxes. The translation start sites and stop sites of truncated versions of CthPnkp are indicated by arrows.
RESULTS
Identification of a C. thermocellum polynucleotide kinase with a novel domain structure
The amino acid sequence of the 870-aminoacid C. thermocellum polynucleotide kinase is shown in Figure 1, aligned to the sequences of homologous proteins of similar size encoded by Kineococcus radiotolerans, Nostoc sp. PCC 7120, and Streptomyces coelicolor. The N-terminal 150-aminoacid segment of the C. thermocellum polypeptide displays primary structure similarity to the polynucleotide kinase domains of coliphage, vibriophage, mycobacteriophage, and baculovirus Pnkp enzymes (Martins and Shuman 2004b; Zhu et al. 2004). In particular, CthPnkp and its bacterial homologs contain the three peptide motifs that comprise the active site of T4 Pnkp (Wang and Shuman, 2001, 2002; Galburt et al. 2002; Wang et al. 2002), which are highlighted in Figure 1. The kinase active site is composed of (1) a classical P-loop motif (GxxGxGKS) that coordinates the β phosphate of the NTP donor; (2) an essential distal Arg side-chain in the RxxxR motif, which also coordinates the NTP β phosphate; (3) an essential Arg side-chain in the DxxR motif, which coordinates the 3′ phosphate of the 5′-OH acceptor; plus (4) an essential Asp side-chain in the DxxR motif, which has been suggested to function as a general acid to activate the 5′-OH for direct nucleophilic attack on the NTP γ phosphate.
CthPnkp diverges from the viral Pnkp end-healing enzymes with respect to the domains flanking the kinase module. The phosphatase domains of viral Pnkp enzymes, which are situated downstream of the kinase domain, exemplify the DxDxT superfamily of metal-dependent phosphotransferases that act through a covalent aspartyl phosphate intermediate (Collet et al. 1998). CthPnkp bears no similarity to the acyl phosphatases; rather, its central segment contains the signature motifs of a different metal-dependent calineurin-like phosphoesterase superfamily (pfam00149 at NCBI) that includes phosphoprotein phosphatases and diadenosine tetraphosphatases (Goldberg et al. 1995; Voegtli et al. 2000). The phosphoesterase motifs GDIHG, GDLVDR, and GNHG are highlighted in Figure 1. A Blast search revealed extensive conservation of primary structure between CthPnkp-(180–400) and the 221-aminoacid phosphoprotein phosphatase of bacteriophage λ. CthPnkp contains the full constellation of conserved residues that bind a dinuclear metal cluster and coordinate the phosphate in the active site in the crystal structure of λ phosphatase (Voegtli et al. 2000).
Further sequence gazing revealed similarity of the C-terminal segment of CthPnkp to the nucleotidyl transferase domains of DNA ligases, RNA ligases, and mRNA capping enzymes, which comprise a superfamily of proteins that catalyze nucleotidyl transfer to polynucleotide 5′ ends via covalent enzyme-(lysyl-N)-NMP intermediates (Shuman and Lima 2004). We identified within the C-terminal segment of CthPnkp putative counterparts of nucleotidyl transferase motifs I (KxxGxR), III (VCLDCELM), IV (EGMVV), and V (AVKxR), which are conserved in the three other bacterial proteins aligned in Figure 1. It is noteworthy that the candidate motif I sequence of the bacterial Pnkp proteins (KHMGSR) deviates from the KxDGxR motif I sequence characteristic of most ATP-dependent polynucleotide ligases and GTP-dependent mRNA capping enzymes. The motif I lysine is the nucleophile that attacks the NTP substrate to form the enzyme-NMP intermediate. Also, whereas polynucleotide ligases and capping enzymes contain additional domains flanking the core nucleotidyltransferase module that are essential for ligation/capping activity (typically an OB-fold domain located distal to motif V) (Subramanya et al. 1996; Odell et al. 2000; Shuman and Lima 2004), we detected no such flanking elements in CthPnkp.
Polynucleotide kinase activity of recombinant CthPnkp
To address whether the C. thermocellum protein possesses any of the enzymatic functions suggested by these sequence comparisons, we produced the 870-amino-acid CthPnkp protein in E. coli as a His10-tagged fusion and purified it from a soluble bacterial extract by adsorption to Ni-agarose and elution with buffer containing imidazole. SDS-PAGE analysis showed that the protein preparation consisted principally of a ~97-kDa polypeptide corresponding to His10-CthPnkp (Fig. 2A). A ladder of minor polypeptides in the 45- to 70-kDa range likely corresponded to proteolytic fragments of CthPnkp. The polynucleotide kinase activity of CthPnkp was demonstrated by the transfer of 32Pi from [γ-32P]ATP to the 5′-OH terminus of an 36-mer oligodeoxyribonucleotide to form a 5′ 32P-labeled oligonucleotide product that was resolved from free ATP by polyacrylamide gel electrophoresis (Fig. 2C, WT).
FIGURE 2.
Kinase and adenylyltransferase activities of recombinant CthPnkp proteins. (A) Aliquots (5 μg) of the Ni-agarose preparations of recombinant full-length wild-type (WT) His10-CthPnkp; full-length mutant proteins K531A, H532A, and R536A; and truncated proteins CthPnkp-(1–425), CthPnkp-(433–870), and CthPnkp-(462–870) were analyzed by SDS-PAGE. The Coomassie blue–stained gel is shown. The positions and sizes (kDa) of marker polypeptides are indicated on the left. (B) Adenylyltransferae activity. Reaction mixtures (10 μL) containing 50 mM Tris-acetate (pH 7.0), 5 mM MgCl2, 5 mM DTT, 20 μM [α-32P]ATP, and 2 μg of the indicated protein preparation were incubated for 15 min at 45°C. The products were analyzed by SDS-PAGE and visualized by autoradiography. The positions and sizes (kDa) of marker polypeptides are indicated on the left. (C) Kinase activity. Reaction mixtures (10 μL) containing 50 mM Tris-acetate (pH 7.0), 5 mM DTT, 10 mM MgCl2, 100 pmol of a 36-mer 5′-OH oligodeoxyribonucleotide 5′-d(TGTAGTCACTATCG GAATAAGGGCGACACGGATATG), 100 μM [γ-32P]ATP, and 50 ng of the indicated protein preparation were incubated for 30 min at 45°C. The products were analyzed by PAGE and visualized by autoradiography.
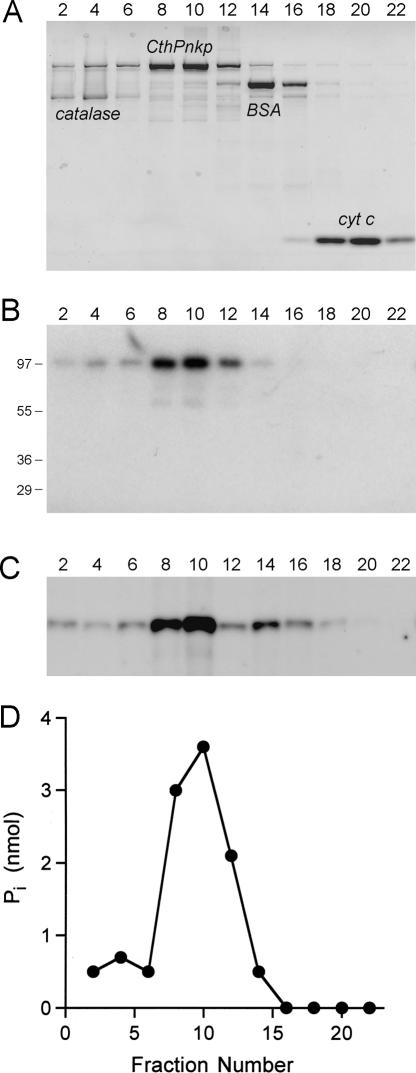
The native size of CthPnkp was gauged by sedimentation through a 15%–30% glycerol gradient. Marker proteins catalase (248 kDa), BSA (66 kDa), and cytochrome c (13 kDa) were included as internal standards. The majority of the applied CthPnkp sedimented as a discrete peak between BSA and catalase (Fig. 3A). A minor fraction was present in a more rapidly sedimenting shoulder. The peak of the kinase activity profile coincided with the CthPnkp protein peak in fractions 8–10 (Fig. 3C). A low level of kinase activity was seen in more rapidly sedimenting fractions that contained the 97-kDa CthPnkp polypeptide, and a second minor peak of activity was detected in fraction 14 that did not coincide with a peak of the 97-kDa recombinant polypeptide. We suspect that this activity resides within an N-terminal proteolytic fragment of CthPnkp that was resolved from the native enzyme during sedimentation. A plot of the S values of the three standards versus fraction number yielded a straight line (data not shown). An S value of 7.5 was determined for the peak component of CthPnkp by interpolation to the internal standard curve. As the apparent S value of CthPnkp was larger than expected for a 100-kDa globular monomer, we surmise that CthPnkp is probably an asymmetrically shaped homodimer.
FIGURE 3.
Glycerol gradient sedimentation of CthPnkp. Sedimentation was performed as described in Materials and Methods. (A) Aliquots (10 μL) of the even-numbered gradient fractions were analyzed by SDS-PAGE. The Coomassie blue–stained gel is shown. The positions of the recombinant Pnkp protein and the internal standards catalase, BSA, and cytochrome c are indicated. (B) Adenylyltransferase reaction mixtures (10 μL) containing 50 mM Tris-acetate (pH 7.0), 10 mM MgCl2, 5 mM DTT, 100 μM [α-32P]ATP, and 2 μL of the indicated glycerol gradient fractions were incubated for 15 min at 45°C. The products were analyzed by SDS-PAGE and visualized by autoradiography. The positions and sizes (kDa) of marker polypeptides are indicated on the left. (C) Kinase reaction mixtures (10 μL) containing 50 mM Tris-acetate (pH 7.0), 5 mM DTT, 10 mM MgCl2, 100 pmol of a 36-mer 5′-OH oligodeoxyribonucleotide, 100 μM [γ-32P]ATP, and 2 μL of a 1:20 dilution of the indicated glycerol gradient fractions were incubated for 30 min at 45°C. The products were analyzed by PAGE and visualized by autoradiography. (D) Phosphatase reactions mixtures (50 μL) containing 50 mM Tris-acetate (pH 7.0), 5 mM NiCl2, 5 mM 2′,3′ cAMP, and 4 μL of the even-numbered gradient fractions were incubated for 30 min at 45°C. Release of Pi was measured with the malachite green reagent.
Characterization of the kinase activity
The kinase reaction of CthPnkp was characterized initially by using a 36-mer 5′-OH DNA substrate. The extent of label transfer from [γ-32P]ATP to the oligonucleotide acceptor was proportional to input CthPnkp (Fig. 4A). From the slope of the titration curve, we calculated a turnover number of ~17 min−1. Activity was optimal from pH 5.5–8.0, declined to half of the peak value at pH 5.0, and was abolished at pH ≤4.0 (Fig. 4B). The enzyme required a divalent cation cofactor. Various metals were tested at 5 mM concentration for their ability to support kinase activity. Magnesium was most effective, manganese was about half as active as magnesium, cobalt about one-fourth as active, and calcium and nickel were even less effective (Fig. 5A). No activity was observed with 5 mM cadmium, copper, or zinc (Fig. 5A). Kinase activity increased with ATP concentration in the range of 0.25 to 30 μM and plateaued at ≥60 μM (Fig. 4D). From a double reciprocal plot, we calculated a Km of 16 μM ATP. CthPnkp catalyzed the transfer of 32Pi from [γ-32P]ATP to the5′-OH terminus of either a 18-mer DNA or 18-mer RNA oligonucleotide (Fig. 5B). RNAs with chain lengths of 18, 15, 12, or 9 nucleotides were phosphorylated to similar extents by CthPnkp (Fig. 5B). Shorter 5′-OH substrates were not tested.
FIGURE 4.
Characterization of the 5′ kinase reaction. (A) Enzyme titration. Reaction mixtures (10 mL) containing 50 mM Tris-acetate (pH 7.0), 5 mM DTT, 10 μM MgCl2, 100 μM [γ-32P]ATP, 100 pmol 36-mer 5′-OH DNA, and CthPnkp as specified were incubated for 30 min at 45°C. (B) pH dependence. Reaction mixtures (10 μL) containing 50 mM buffer (either Tris-formate at pH 3.5; Tris-acetate at pH 4.0, 4.5, 5.0, 5.5, 6.0, 6.5, or 7.0; or Tris-HCl at pH 7.5, 8.0, 8.5, 9.0 or 9.5), 5 mM DTT, 10 mM MgCl2, 100 μM [γ-32P]ATP, 100 pmol 36-mer 5′-OH DNA, and 8 ng CthPnkp were incubated for 30 min at 45°C. (C) ATP dependence. Reaction mixtures (10 μL) containing 50 mM Tris-acetate (pH 7.0), 5 mM DTT, 10 mM MgCl2, 100 pmol 36-mer 5′-OH DNA, 8 ng CthPnkp, and [γ-32P]ATP as specified were incubated for 30 min at 45°C.
FIGURE 5.
Metal specificity and RNA kinase activity. (A) Reaction mixtures (10 μL) containing 50 mM Tris-acetate (pH 7.0), 100 μM [γ-32P]ATP, 100 pmol 36-mer 5′-OH DNA, 8 ng CthPnkp, and either no divalent cation (lane −) or 5 mM MgCl2, MnCl2, CaCl2, CdCl2, CoCl2, NiCl2, or ZnCl2 as specified were incubated for 30 min at 45°C. The products were analyzed by PAGE and visualized by autoradiography. The relative kinase activities (normalized to the magnesium-containing reaction, defined as 100%) are indicated below the lanes. (B) RNA kinase activity. Reaction mixtures (10 μL) containing 50 mM Tris-acetate (pH 7.0), 5 mM DTT, 10 mM MgCl2, 100 μM [γ32P]ATP, 50 ng of CthPnkp, and 100 pmol of 5′-OH–terminated 18-mer, 15-mer, 12-mer, or 9-mer oligonucleotides as specified were incubated for 30 min at 45°C. The prodducts were analyzed by PAGE and visualized by autoradiography.
2′,3′ Phosphatase activity of recombinant CthPnkp
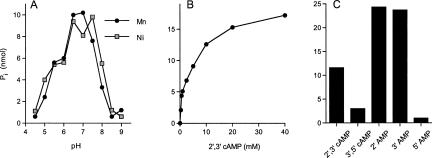
The 3′ end-healing phase of the phage tRNA restriction pathway entails the conversion of a 2′,3′ cyclic phosphate terminus to a 2′-OH, 3′-OH terminus that is suitable for ligation to a 5′-PO4 strand (Amitsur et al. 1987). To test whether CthPnkp might catalyze an analogous 3′ end-healing reaction, we assayed the recombinant protein for its ability to release phosphate from 2′,3′ cyclic adenosine monophosphate (2′,3′ cAMP). Phosphate release was measured colorimetrically by using the malachite green method (Lanzetta et al. 1979). CthPnkp hydrolyzed 2′,3′ cAMP to Pi in the presence of either nickel or manganese (Fig. 6A). Activity with either 5 mM nickel or manganese was optimal at pH 6.5 to 7.5 and declined sharply as the pH was raised to ≥8.5 or lowered to ≤4.5 (Fig. 6A). The Ni-dependent phosphatase activity cosedimented with the Cth1Pnkp polypeptide during glycerol gradient sedimentation (Fig. 3D).
FIGURE 6.
Characterization of the 2′,3′ phosphatase activity. (A) pH dependence. Reaction mixtures (50 μL) containing 50 mM buffer (either Tris-acetate at pH 4.5, 5.0, 5.5, 6.0, 6.5, or 7.0; or Tris-HCl at pH 7.5, 8.0, 8.5, or 9.0), 5 mM DTT, 5 mM 2′,3′ cAMP, and either 5 mM MnCl2 and 2 μg CthPnkp or 5 mM NiCl2 and 1 μg CthPnkp as specified were incubated at 30 min at 45°C. (B) cAMP dependence. Reactions mixtures (50 μL) containing 50 mM Tris-acetate (pH 7.0), 10 mM NiCl2, 1 μg CthPnkp, and 2′,3′ cAMP as specified were incubated for 30 min at 45°C. (C) Substrate specificity. Reactions mixtures (50 μL) containing 50 mM Tris-acetate (pH 7.0); 10 mM NiCl2; 1 μg CthPnkp; and 5 mM 2′,3′ cAMP, 3′,5′ cAMP, 2′ AMP, 3′ AMP, or 5′ AMP as specified were incubated for 30 min at 45°C.
Phosphate release from 5 mM 2′,3′ cAMP at pH 7.0 was optimal at 5–20 mM NiCl2 or MnCl2 (data not shown). Neither magnesium, calcium, copper, cobalt, cadmium, nor zinc was able to support 2′,3′ cAMP phosphatase activity (data not shown). CthPnkp protein titration experiments showed that phosphatase specific activity in the presence of nickel was threefold higher than activity in the presence of manganese (data not shown). The fastidious requirement of CthPnkp for either nickel or manganese is a property shared with bacteriophage λ phosphatase, an enzyme that is structurally homologous to the phosphoesterase domain of CthPnkp (Zhuo et al. 1993).
Pi release by CthPnkp displayed a hyperbolic dependence on the concentration of the 2′,3′ cAMP substrate (Fig. 6B). From a double reciprocal plot, we calculateda Km value of 4.4 mM and a kcat of ~60 min−1. Whereas Cth1Pnkp readily released Pi from 2′,3′ cAMP; it was fourfold less effective in releasing Pi from 3′,5′ cAMP (Fig. 6C). The release of Pi from 2′,3′ cAMP likely entails two enzymatic activities: a 2′,3′ cyclic phosphodiesterase and a phosphomonoesterase. CthPnkp displayed vigorous phosphomonoesterase activity with either 3′-AMP or 2′-AMP, but not 5′-AMP (Fig. 6C). Control experiments verified that the 2′,3′ cAMP substrate was refractory to hydrolysis by alkaline phosphatase, whereas the 3′-AMP and 2′-AMP substrates were sensitive to alkaline phosphatase (data not shown).
Covalent adenylyltransferase activity of recombinant CthPnkp
An adenylyltransferase activity of recombinant CthPnkp was evinced by label transfer from [α-32P]ATP to the CthPnkp polypeptide to form a covalent enzyme-adenylate adduct (Fig. 2B). The adenylyltransferase activity profile during glycerol gradient sedimentation paralleled the sedimentation profile of the 97-kDa CthPnkp polypeptide (Fig. 3B). CthPnkp required a divalent cation cofactor to form the covalent adduct. MgCl2 and MnCl2 supported approximately equivalent activity at the optimal concentrations of 2.5 mM and 0.6 mM, respectively (Fig. 7). Cobalt and calcium were less effective in supporting enzyme adenylylation at 5 mM concentration; little or no adenylylation was supported by 5 mM nickel, cadmium, or zinc (Fig. 7). CthPnkp adenylylation was optimal at pH 5.0–5.5 in Tris-acetate buffer (Fig. 8A). Activity fell precipitously as the pH was lowered to ≤4.5, but declined gradually as the pH was incrementally raised to 9.5 in Tris-HCl buffer (Fig. 8A). The yield of CthPnkp-AMP complex reached saturation at ≥ 16 μM ATP (Fig. 8B). Half-saturation was achieved at ~5 μM ATP. We calculated that ~25% of the CthPnkp protein preparation could be adenylated in vitro with 32P-AMP. CthPnkp was unreactive with [α-32P]GTP (data not shown).
FIGURE 7.
Divalent cation specificity and dependence of the adeny-lyltransferase reaction. (A) Divalent cation specificity. Reaction mixtures (10 μL) containing 50 mM Tris-acetate (pH 7.0); 100 μM [α-32P]ATP; 2 μg CthPnkp; and either no divalent cation (lane −) or 10 mM MgCl2, MnCl2, CaCl2, CdCl2, CoCl2, NiCl2, or ZnCl2 as specified were incubated for 15 min at 45°C. (B) Divalent cation dependence. Reaction mixtures (10 μL) containing 50 mM Tris-acetate (pH 7.0); 5 mM DTT; 100 μM [α-32P]ATP; 2 μg CthPnkp; and either 0, 0.63, 1.25, 2.5, 5, or 10 mM MgCl2 (•) or MnCl2 (○) were incubated for 15 min at 45°C. The yield of enzyme-AMP adduct (E-AMP) is plotted as a function of divalent cation concentration.
FIGURE 8.
pH- and ATP-dependence of the adenylyltransferase reaction. (A) Reaction mixtures (10 μL) containing 50 mM buffer (either Tris-formate at pH 3.5; Tris-acetate at pH 4.0, 4.5, 5.0, 5.5, 6.0, 6.5, or 7.0; or Tris-HCl at pH 7.5, 8.0, 8.5, 9.0, or 9.5), 5 mM DTT, 10 mM MgCl2, 100 μM [α-32P]ATP, and 2 μg CthPnkp were incubated at 45°C for 15 min. (B) Reaction mixtures (10 μL) containing 50 mM Tris-acetate (pH 7.0), 5 mM DTT, 10 mM MgCl2, 2 μg CthPnkp, and [α-32P]ATP as specified were incubated at 45°C for 15 min.
Lys531 is required for adenylyltransferase activity
The 531KHMGSR536 peptide of CthPnkp is a putative counterpart of the motif I of the polynucleotide ligase/capping enzyme superfamily, which is typically KxDGxR and contains the lysine nucleophile to which the NMP is covalently attached. The contribution of Lys531 to the activities of CthPnkp was surmised from the effects of a single alanine substitution K531A. The K531A mutant protein was produced in bacteria and purified from soluble lysates by nickel-affinity chromatography (Fig. 2A). K531A was apparently inert in enzyme-adenylate formation (Fig. 2B) but retained activity as a polynucleotide kinase (Fig. 2C) and a 2′,3′ cAMP phosphatase (data not shown). A parallel titration experiment comparing the extent of adenylylation of wild type versus K531A verified that its activity was undetectable at a level of sensitivity of ≤0.5% of the wild type. These findings are consistent with Lys531 being the site of covalent adenylation.
Additional alanine substitutions were introduced in place of flanking motif I residues His532 and Arg536. The H532A protein retained adenylyltransferase (Fig. 2B) and polynucleotide kinase (Fig. 2B) activities. A titration experiment showed that H532A was 40% as active as was wild-type CthPnkp (data not shown). The R536A mutant was impaired for adenylyltransferase activity (Fig. 2B), although it retained polynucleotide kinase activity (Fig. 2C). A protein titration indicated that R536A was 5% as active in adenylylation as wild-type CthPnkp. The corresponding motif I Arg side-chain of other members of the nucleotidyl transferase superfamily comprises part of the nucleotide binding pocket, where it typically makes contact with the ribose sugar of the NTP substrate (Subramanya et al. 1996; Odell et al. 2000; Shuman and Lima 2004).
Separable kinase and adenylyltransferase domains of CthPnkp
We produced recombinant His10-tagged versions of two N-terminal deletion mutants, CthPnkp-(433–870) and CthPnkp-(462–870), that contained the nucleotidyl transferase motifs but lacked the signature motifs of the kinase and phosphoesterase enzyme families. The translation start sites of the truncated proteins are indicated by arrows in Figure 1. The truncated proteins were purified from soluble E. coli extracts by Ni-agarose chromatography (Fig. 2A). Both proteins displayed autoadenylylation activity (Fig. 2B) but were in inert with respect to polynucleotide kinase (Fig. 2C) and 2′,3′ cAMP phosphatase (data not shown). We conclude that the C-terminal 409-amino-acid segment of CthPnkp comprises an autonomous adenylyltransferase domain.
An N-terminal His10-tagged fragment, CthPnkp-(1–425) was produced and purified in parallel (Fig. 2A). CthPnkp-(1–425) was had no detectable adenylyltransferase activity (Fig. 2B) but was active as a polynucleotide kinase (Fig. 2C). Thus, the N-terminal 425-amino-acid segment is an autonomous kinase domain. CthPnkp-(1–425) was 10% as active as was full-length CthPnkp in releasing Pi from 2′,3′ cAMP (data not shown).
DISCUSSION
Here we identified and characterized a new end-healing enzyme from C. thermocellum that catalyzes the phosphorylation of 5′-OH termini of DNA or RNA polynucleotides and the dephosphorylation of 2′,3′ cyclic phosphate, 2′-phosphate, and 3′-phosphate ribonucleotides. These activities suggest a role for CthPnkp in RNA or DNA repair. We show that CthPnkp also catalyzes an autoadenylylation reaction via a polynucleotide ligase-type mechanism. However, we have been unable to demonstrate a strand-joining activity of CthPnkp by using single-stranded or duplex substrates that are readily sealed by prototypal DNA and RNA ligases studied in this laboratory (data not shown). The substrates tested included the following: a nicked DNA duplex (with 3′-OH and 5-PO4 termini at the nick) composed of synthetic DNA oligonucleotides, a nicked RNA duplex composed of synthetic RNA oligonucleotides, a nicked RNA–DNA hybrid duplex formed by annealing 3′-OH and 5-PO4 RNA strands to a DNA template strand, and an 18-mer single-stranded 5′-PO4 RNA oligonucleotide.
Although the compaction of 5′ kinase, 2′,3′ cyclic phosphodiesterase or 3′ phosphatase, and covalent adenylyltransferase activities within a single polyfunctional protein has been described previously for yeast tRNA ligase (Apostol et al. 1991; Sawaya et al. 2003) and baculovirus RNA ligase (Martins and Shuman 2004b), the CthPnkp enzyme is novel with respect to its domain order and the structure and mechanism of its phosphoesterase component. Baculovirus RNA ligase consists of an N-terminal adenylyltransferase domain that resembles T4 RNA ligase 1 fused to a C-terminal end-healing domain that resembles T4 Pnkp. The baculovirus end-healing domain is composed of a proximal 5′ kinase module (similar to that of CthPnkp) and a distal 3′ phosphatase module that belongs to the acyl-phosphatase superfamily of phosphotransferases (Martins and Shuman 2004b). Yeast tRNA ligase consist of an N-terminal adenylyltransferase domain (again similar to T4 RNA ligase 1), a central T4-like kinase domain, and a C-terminal 2′,3′ cyclic phosphodiesterase domain that belongs to the 2H superfamily of phosphotransferases (Apostol et al. 1991; Mazumder et al. 2002; Sawaya et al. 2003). CthPnkp and its bacterial homologs are unique in that they have an adenylyltransferase domain appended at the C-terminus. Moreover, the adenylyltransferase domain of CthPnkp does not have the structural signatures upstream of motif I that are characteristic of the Rnl1-type subfamily of RNA ligases (Wang et al. 2003), which includes baculovirus RNA ligase and yeast tRNA ligase.
The most distinctive feature of CthPnkp vis-à-vis known RNA repair enzymes is that its 3′ end modification component belongs to the calcineurin-type phosphoesterase superfamily. It contains putative counterparts of the amino acids that form the dinuclear metal-binding site and the phosphate-binding site seen in the crystal structure of λ phoshatase (Voegtli et al. 2000). As with λ phosphatase (Zhuo et al. 1993), the 2′,3′ cAMP phosphatase activity of CthPnkp is specifically dependent on either nickel or manganese, to the exclusion of magnesium and various other divalent cations. A functionally analogous Ni-dependent 2′,3′ cAMP phosphatase activity was described recently for the E. coli tRNA nucleotidyltransferase (the CCA-adding enzyme) (Yakunin et al. 2004). However, the C-terminal domain of CCA-adding enzyme responsible for the 2′,3′ phosphatase activity belongs to the HD superfamily of metal-dependent phosphotransferases (Aravind and Koonin 1998; Yakunin et al. 2004), which are structurally unrelated to the CthPnkp-type phosphoesterase module. A manganese-dependent 2′,3′ cAMP phosphatase activity associated with bacteriophage RM378 Pnkp also belongs to the HD superfamily (Blondal et al. 2005).
We propose that CthPnkp exemplifies a new class of end-remodeling enzyme. In addition to the putative trifunctional homologs of CthPnkp encoded by K. radiotolerans, Nostoc, and S. coelicolor, we have identified putative trifunctional kinase-phosphatase-adenylyltransferase homologs in several other bacterial species, including Fusobacterium nucleatum, Bacillus licheniformis, and Helicobacter hepaticus (Fig. 9). We have also identified bifunctional Pnkp homologs in Deinococcus radiodurans and Thermobifida fusca that are composed only of the kinase and the phosphoesterase domain equivalents of CthPnkp, with no equivalent of the adenylyltransferase domain (data not shown). It appears that Pnkp enzymes are present in many bacteria, which hints at an as yet unexplored role for RNA repair in bacterial physiology.
FIGURE 9.
Bacterial Pnkp/adenylyltransferase homologs. The aligned amino acid sequences of putative trifunctional kinase-phosphatase-adenylyltransferases from Fusobacterium nucleatum (Fnu; NCBI accession EAA24876), Bacillus licheniformis (Bli; NCBI accession YP_079636), and Helicobacter hepaticus (Hhe; NCBI accession AAP78353) are shown. Gaps in the alignment are indicated by dashes. The polynucleotide kinase, calcineurin-type phosphoesterase, and nucleotidyltransferase motifs are highlighted in shaded boxes.
MATERIALS AND METHODS
Materials
Oligodeoxyribonucleotides were purchased from Biosource International. Oligoribonucleotides were purchased from Dharmacon and deprotected as instructed by the vendor. Concentrations of oligonucleotide stock solutions were determined by UV absorbance at 260 nM, using extinction coefficients calculated according to their base compositions. 2′,3′ cAMP, 2′AMP, 3′AMP, and 5′AMP were purchased from Sigma. Malachite green reagent was purchased from BIOMOL Research Laboratories. C. thermocellum genomic DNA was obtained from ATCC.
Recombinant CthPnkp
The C. thermocellum gene encoding an 870-amino-acid Pnkp homolog (NCBI accession ZP_00312808) was amplified by two-stage PCR from genomic DNA with Pfu DNA polymerase by using primers designed to eliminate internal NdeI and BamHI sites (by introducing translationally silent mutations) while introducing an NdeI restriction site at the start codon and a BamHI site 3′ of the stop codon. The PCR products were digested with NdeI and BamHI and inserted into pET16b to generate an expression plasmid encoding the CthPnkp polypeptide fused to an N-terminal His10 tag. Alanine substitution mutations were introduced by PCR using the two-stage overlap extension method. N-terminal truncation alleles CthPnkp-(433–870) and CthPnkp-(462–870) were generated by PCR amplification using sense primers that introduced an NdeI site at the Met433 or Met462 codons. The C-terminal truncation alleles CthPnkp-(1–425) was constructed by PCR amplification using an antisense primer that introduced a stop codon in lieu of the coding for Pro426 and a BamHI site immediately 3′ of the stop codon.
Wild-type and mutant pET-CthPnkp plasmids were transformed into E. coli BL21(DE3). Cultures (1 L) of E. coli BL21(DE3)/pET-CthPnkp were grown at 37°C in Luria-Bertani medium containing 0.1 mg/mL ampicillin until the A600 reached ~0.6. The cultures were chilled on ice for 30 min, adjusted to 0.1 mM isopropyl-D-thiogalactopyranoside and 2% ethanol, and then incubated at 17°C for 16 h with continuous shaking. Cells were harvested by centrifugation, and the pellet was stored at − 80°C. All subsequent procedures were performed at 4°C. Thawed bacteria were resuspended in 30 mL of buffer A (50 mM Tris-HCl at pH 7.5, 0.5 M NaCl, 10% sucrose). Lysozyme, PMSF, and Triton X-100 were added to final concentrations of 1 mg/mL, 1 mM, and 0.1%, respectively. The lysates were sonicated to reduce viscosity, and insoluble material was removed by centrifugation. The soluble extracts were applied to 1 mL columns of Ni-nitrilotriacetic acid-agarose (Qiagen) that had been equilibrated with buffer A. The columns were washed with 8 mL of the same buffer and then eluted stepwise with 4 mL aliquots of 25, 50, and 200 mM imidazole in buffer B (50 mM Tris HCl at pH 8.0, 0.5 M NaCl, 10% glycerol). The polypeptide compositions of the column fractions were monitored by SDS-PAGE. The His10-CthPnkp proteins adsorbed to the column and were recovered predominantly in the 200 mM imidazole eluates. Protein concentrations were determined by SDS-PAGE analysis of serial dilutions of the CthPnkp preparations in parallel with serial dilutions of a BSA standard. The gels were stained with Coomassie blue, and the staining intensities of the CthPnkp and BSA polypeptides were quantified using a Digital Imaging and Analysis System from Alpha Innotech Corporation. Approximately 8 mg of full-length wild-type CthPnkp was recovered from a 1-L bacterial culture.
Polynucleotide kinase assay
Reaction mixtures (10 μL) containing a 5′-OH oligodeoxynucleotide or oligoribonucleotide substrate, [γ-32P]ATP, and other components as specified in the figure legends were incubated at 45°C. The kinase reactions were initiated by adding CthPnkp and terminated with formamide/EDTA. The products were analyzed by electrophoresis through a 15-cm 18% polyacrylamide gel containing 7 M urea in 45 mM Tris-borate, 1 mM EDTA at 10 W constant power. The radiolabeled oligo-nucleotide products were visualized by autoradiography of the gel and quantified by scanning the gel with a Fujix BAS2500 imaging apparatus.
Adenylyltransferase assay
Reaction mixtures (10 μL) containing 50 mM Tris-acetate (pH 7.0), 5 mM DTT, 5 mM MgCl2, [α-32P]ATP, and CthPnkp as specified were incubated for 15 min at 45°C. The reactions were quenched with SDS, and the products were analyzed by SDS-PAGE (12% acrylamide). The protein-[32P]AMP adduct was visualized by autoradiography of the dried gel and quantified by scanning the gel.
Phosphatase assay
Reaction mixtures (50 μL) contained 50 mM Tris-acetate (pH 7.0), 5 mM 2′,3′ cyclic AMP, 5 or 10 mM NiCl2 or MnCl2, and other components as specified in the figure legends. The reactions were quenched by adding 1 mL of malachite green reagent. Release of phosphate was determined by measuring A620 and interpolating the value to a phosphate standard curve.
Glycerol gradient sedimentation
An aliquot (200 μg) of CthPnkp was mixed with catalase (100 μg), BSA (100 μg), and cytochrome c (100 μg). The mixture was applied to a 4.8 mL 15%–30% glycerol gradient containing 50 mM Tris-HCl (pH 8.0), 0.3 M NaCl, 2 mM EDTA, 2 mM DTT, and 0.1% Triton X-100. The gradient was centrifuged for 18 h at 4°C in a Beckman SW55Ti rotor at 50,000 rpm. Fractions (~0.2 mL) were collected form the bottom of the tube. Aliquots were analyzed by SDS-PAGE and assayed for kinase and phosphatase activity as specified in the figure legends.
Acknowledgments
This work was supported by NIH grant GM42498. S.S. is an American Cancer Society Research Professor.
Article published online ahead of print. Article and publication date are at http://www.rnajournal.org/cgi/doi/10.1261/rna.2690505.
REFERENCES
- Amitsur, M., Levitz, R., and Kaufman, G. 1987. Bacteriophage T4 anticodon nuclease, polynucleotide kinase, and RNA ligase reprocess the host lysine tRNA. EMBO J. 6: 2499–2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apostol, B.L., Westaway, S.K., Abelson, J., and Greer, C.L. 1991. Deletion analysis of a multifunctional yeast tRNA ligase polypeptide: Identification of essential and dispensable functional domains. J. Biol. Chem. 266: 7445–7455. [PubMed] [Google Scholar]
- Aravind, L. and Koonin, E.V. 1998. The HD domain defines a new superfamily of metal-dependent phosphohydrolases. Trends Biochem. Sci. 23: 469–472. [DOI] [PubMed] [Google Scholar]
- Bernstein, N.K., Williams, R.S., Rakovszky, M.L., Cui, D., Green, R., Karimi-Busheri, F., Mani, R.S., Galicia, S., Koch, C.A., Cass, C.E., et al. 2005. The molecular architecture of the mammalian DNA repair enzyme, polynucleotide kinase. Mol. Cell 17: 657–670. [DOI] [PubMed] [Google Scholar]
- Blondal, T., Hjorleifsdottir, S., Aevarsson, A., Fridjonsson, O.H., Skirnisdottir, S., Wheat, J.O., Hermannsdottir, A.G., Hreggvidsson, G.O., Smith, A.V., and Kristjansson, J.K. 2005. Characterization of a 5′-polynucleotide kinase/3′-phosphatase from bacteriophage RM378. J. Biol. Chem. 280: 5188–5194. [DOI] [PubMed] [Google Scholar]
- Cameron, V. and Uhlenbeck, O.C. 1977. 3′-Phosphatase activity in T4 polynucleotide kinase. Biochemistry 16: 5120–5126. [DOI] [PubMed] [Google Scholar]
- Collet, J.F., Stroobant, V., Pirard, M., Delpierre, G., and Van Schaftingen, E. 1998. A new class of phosphotransferases phosphorylated on an aspartate residue in an amino-terminal DXDX(T/V) motif. J. Biol. Chem. 273: 14107–14112. [DOI] [PubMed] [Google Scholar]
- Demain, A.L., Newcomb, M., and Wu, J.H.D. 2005. Cellulase, clostridia and ethanol. Microbiol. Mol. Biol. Rev. 69: 124–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galburt, E.A., Pelletier, J., Wilson, G., and Stoddard, B.L. 2002. Structure of a tRNA repair enzyme and molecular biology workhorse: T4 polynucleotide kinase. Structure 10: 1249– 1260. [DOI] [PubMed] [Google Scholar]
- Goldberg, J., Huang, H., Kwon, Y., Greengard, P., Nairn, A.C., and Kuriyan, J. 1995. Three-dimensional structure of the catalytic subunit of protein serine/threonine phosphatase-1. Nature 376: 745–753. [DOI] [PubMed] [Google Scholar]
- Ho, C.K. and Shuman, S. 2002. Bacteriophage T4 RNA ligase 2 (gp24.1) exemplifies a family of RNA ligases found in all phylogenetic domains. Proc. Natl. Acad. Sci. 99: 12709–12714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanzetta, P.A., Alvarez, L.J., Reinach, P.S., and Candia, O.A. 1979. An improved assay for nanomole amounts of inorganic phosphate. Anal. Biochem. 100: 95–97. [DOI] [PubMed] [Google Scholar]
- Martins, A. and Shuman, S. 2004a. An RNA ligase from Deinococcus radiodurans. J. Biol. Chem. 279: 50654–50661. [DOI] [PubMed] [Google Scholar]
- ———. 2004b. Characterization of a baculovirus enzyme with RNA ligase, polynucleotide 5′ kinase and polynucleotide 3′ phosphatase activities J. Biol. Chem. 279: 18220–18231. [DOI] [PubMed] [Google Scholar]
- Mazumder, R., Iyer, L., Vasudevan, S., and Aravind. L. 2002. Detection of novel members, structure-function analysis and evolutionary classification of the 2H phosphoesterase family. Nucleic Acids Res. 30: 5229–5243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novogrodsky, A. and Hurwitz, J. 1966. The enzymatic phosphorylation of ribonucleic acid and deoxyribonucleic acid: Phosphorylation at 5′-hydroxyl termini. J. Biol. Chem. 241: 2923–2932. [PubMed] [Google Scholar]
- Novogrodsky, A., Tal., M., Traub, A., and Hurwitz, J. 1966. The enzymatic phosphorylation of ribonucleic acid and deoxyribonucleic acid: Further properties of the 5′-hydroxyl polynucleotide kinase. J. Biol. Chem. 241: 2933–2943. [PubMed] [Google Scholar]
- Odell, M., Sriskanda, V., Shuman, S., and Nikolov, D. 2000. Crystal structure of eukaryotic DNA ligase-adenylate illuminates the mechanism of nick sensing and strand joining. Mol. Cell 6: 1183–1193. [DOI] [PubMed] [Google Scholar]
- Richardson, C.C. 1965. Phosphorylation of nucleic acid by an enzyme from T4 bacteriophage-infected Escherichia coli. Proc. Natl. Acad. Sci. 54: 158–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawaya, R., Schwer, B., and Shuman, S. 2003. Genetic and biochemical analysis of the functional domains of yeast tRNA ligase. J. Biol. Chem. 278: 43298–43398. [DOI] [PubMed] [Google Scholar]
- Shuman, S. and Lima, C.D. 2004. The polynucleotide ligase and RNA capping enzyme superfamily of covalent nucleotidyltransferases. Curr. Opin. Struct. Biol. 14: 757–764. [DOI] [PubMed] [Google Scholar]
- Silber, R., Malathi, V.G., and Hurwitz, J. 1972. Purification and properties of bacteriophage T4-induced RNA ligase. Proc. Natl. Acad. Sci. 69: 3009–3013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanya, H.S., Doherty, A.J., Ashford, S.R., and Wigley, D.B. 1996. Crystal structure of an ATP-dependent DNA ligase from bacteriophage T7. Cell 85: 607–615. [DOI] [PubMed] [Google Scholar]
- Voegtli, W.C., White, D.J., Reiter, N.J., Rusnak, F., and Rosenzweig, A.C. 2000. Structure of the bacteriophage lambda Ser/Thr protein phosphatase with sulfate ion bound in two coordination modes. Biochemistry 39: 15365–15374. [DOI] [PubMed] [Google Scholar]
- Wang, L.K. and Shuman, S. 2001. Domain structure and mutational analysis of T4 polynucleotide kinase. J. Biol. Chem. 276: 26868–26874. [DOI] [PubMed] [Google Scholar]
- ———. 2002. Mutational analysis defines the 5′-kinase and 3′-phosphatase active sites of T4 polynucleotide kinase. Nucleic Acids Res. 30: 1073–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, L.K., Lima, C.D., and Shuman, S. 2002. Structure and mechanism of T4 polynucleotide kinase: An RNA repair enzyme. EMBO J. 21: 3873–3880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, L.K., Ho, C.K., Pei, Y., and Shuman, S. 2003. Mutational analysis of bacteriophage T4 RNA ligase 1: Different functional groups are required for the nucleotidyl transfer and phosphodiester bond formation steps of the ligation reaction. J. Biol. Chem. 278: 29454–29462. [DOI] [PubMed] [Google Scholar]
- Yakunin, A.F., Proudfoot, M., Kuznetsova, E., Savchenko, A., Brown, G., Arrowsmith, C.H., and Edwards, A.M. 2004. The HD domain of the Escherichia coli tRNA nucleotidyltransferase has 2′,3′-cyclic phosphodiesterase, 2′-nucleotidase, and phosphatase activities. J. Biol. Chem. 279: 36819–36827. [DOI] [PubMed] [Google Scholar]
- Zhu, H., Yin, S., and Shuman, S. 2004. Characterization of polynucleotide kinase/phosphatase enzymes from mycobacteriophages Omega and Cjw1 and vibriophage KVP40. J. Biol. Chem. 279: 26358–26369. [DOI] [PubMed] [Google Scholar]
- Zhuo, S., Clemens, J.C., Hakes, D.J., Barford, D., and Dixon, J.E. 1993. Expression, purification, crystallization, and biochemical characterization of a recombinant protein phosphatase. J. Biol. Chem. 268: 17754–17761. [PubMed] [Google Scholar]