Abstract
Kluyveromyces lactis killer strains secrete a heterotrimeric toxin (zymocin), which causes an irreversible growth arrest of sensitive yeast cells. Despite many efforts, the target(s) of the cytotoxic γ-subunit of zymocin has remained elusive. Here we show that three tRNA species tRNAGlumcm5s2UUC, tRNALysmcm5s2UUU, and tRNAGlnmcm5s2UUG are the targets of γ-toxin. The toxin inhibits growth by cleaving these tRNAs at the 3′ side of the modified wobble nucleoside 5-methoxycarbonylmethyl-2-thiouridine (mcm5s2U). Transfer RNA lacking a part of or the entire mcm5 group is inefficiently cleaved by γ-toxin, explaining the γ-toxin resistance of the modification-deficient trm9, elp1-elp6, and kti11-kti13 mutants. The K. lactis γ-toxin is the first eukaryotic toxin shown to target tRNA.
Keywords: K. lactis γ-toxin, tRNA endonuclease, ELP3, TRM9, 5-methoxycarbonylmethyl-2-thiouridine
INTRODUCTION
Many microorganisms produce toxic substances to gain a competitive growth advantage. Killer strains of the dairy yeast Kluyveromyces lactis secrete a heterotrimeric toxin (zymocin), which causes an irreversible arrest of sensitive yeast cells, such as Saccharomyces cerevisiae in the unbudded (G1) phase of the cell cycle (Gunge et al. 1981; Sugisaki et al. 1983; White et al. 1989; Schaffrath and Meinhardt 2005). Zymocin consists of three subunits, α, β, and γ, that are encoded by a linear plasmid (Gunge et al. 1981; Stark et al. 1990). Upon secretion, the α- and β-subunits dock the zymocin to the cell wall of susceptible yeasts and facilitate transfer of the γ-subunit (Schaffrath and Meinhardt 2005). Cytotoxicity resides within the γ-subunit, since intracellular expression of the γ-toxin mimics the action of exogenous zymocin (Tokunaga et al. 1989; Butler et al. 1991a).
Two classes of S. cerevisiae mutants resistant to zymocin have been described (Butler et al. 1991a, 1994), but the mechanism of γ-toxin–induced growth arrest is not clear (Sugisaki et al. 1983; White et al. 1989; Schaffrath and Meinhardt 2005). Class I resistant mutants are defective in binding and/or uptake of zymocin but are sensitive to endogenous expression of the γ-toxin. Class II mutants are believed to be target site mutants as they are resistant to both exogenous zymocin and endogenous γ-toxin. Strains with mutations in any of the six Elongator protein subunit genes (ELP1–ELP6) or the three killer toxin insensitivity genes (KTI11–KTI13) are class II mutants (Butler et al. 1994; Frohloff et al. 2001; Jablonowski et al. 2001). The Elongator complex has been implicated in elongation of RNA polymerase II (Pol II) transcription (Otero et al. 1999) and in regulation of exocytosis (Rahl et al. 2005). A model was proposed where the γ-toxin is targeted to Pol II by the Elongator complex, which would lead to a transcriptional inactivation of genes important for G1 exit (Schaffrath and Meinhardt 2005). However, this model is not easily reconciled with the evidence that the Elp1-Elp6 and Kti11-Kti13 proteins are all required for formation of 5-methoxycarbonylmethyl (mcm5) and 5-carbamoylmethyl (ncm5) groups on uridines at the wobble position in tRNA (Huang et al. 2005). These data rather suggest that phenotypes induced by mutations in the ELP1–ELP6 and KTI11–KTI13 genes could be a consequence of hypomodified tRNAs (Huang et al. 2005).
In this report we show that three tRNA species— tRNAGlumcm5s2UUC, tRNALysmcm5s2UUU, and tRNAGlnmcm5s2UUG— are cleaved by γ-toxin at the 3′ side of the modified wobble nucleoside 5-methoxycarbonylmethyl-2-thiouridine (mcm5s2U). The mcm5 side-chain is important for efficient cleavage of the target tRNAs, explaining the γ-toxin resistance of mutants defective in synthesis of this group.
RESULTS AND DISCUSSION
We previously showed that the class II zymocin-resistant elp1-elp6 and kti11-kti13 mutants are deficient in formation of mcm5 (Fig. 1A) and ncm5 side-chains at wobble uridines in tRNA (Huang et al. 2005). Interestingly, a strain deleted for the TRM9 gene encoding a methyltransferase responsible for the last step in the synthesis of the wobble mcm5 side-chain (Fig. 1A; Kalhor and Clarke 2003) is also a class II resistant mutant (Figs. 1B, 2E). Although the zymocin resistance is comparable between elp3 and trm9 mutants (Figs. 1B, 2E), there are clear differences in growth rates and phenotypes (data not shown). Lack of the entire mcm5 group at the wobble position prevents an ochre suppressor tRNA from reading ochre stop codons (Huang et al. 2005). In contrast, a deletion of the TRM9 gene does not abolish the ability of the suppressor tRNA to read ochre stop codons (data not shown). Thus, class II zymocin resistance correlates with a defect in synthesis of mcm5 side-chain but not with a general translational defect, indicating that tRNA(s) containing this side-chain could be the target(s) of γ-toxin. Interestingly, high dosage of a gene encoding tRNAGlumcm5s2UUC suppresses the zymocin sensitivity of a wild-type S. cerevisiae strain (Butler et al. 1994), suggesting that tRNAGlumcm5s2UUC is a potential target of γ-toxin (Huang et al. 2005).
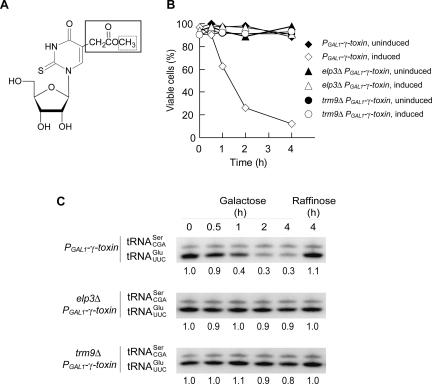
FIGURE 1.
Intracellular γ-toxin expression in S. cerevisiae reduces cell viability and the level of tRNAGlumcm5s2UUC. (A) Structure of mcm5s2U. An elp3Δ mutant lacks the entire mcm5 side-chain (box with solid lines) (Huang et al. 2005), whereas a trm9Δ strain lacks the indicated methyl group (box with dotted lines) (Kalhor and Clarke 2003). The formation of the 2-thio group appears not to be affected in the elp3 and trm9 mutants (Huang et al. 2005; data not shown). (B) Wild-type, elp3Δ, and trm9Δ strains with an integrated PGAL1-γ-toxin construct were shifted from raffinose (uninduced) to galactose (induced) containing medium. The ratio of viable to total cells was determined at indicated time points. (C) Northern blot analysis of total RNA isolated from the induced or uninduced cultures in B. The tRNAGlumcm5s2UUC signal was quantified and normalized to the tRNASerCGA signal. Below each lane is the normalized value expressed relative to the corresponding value at time point 0, which is set to 1.
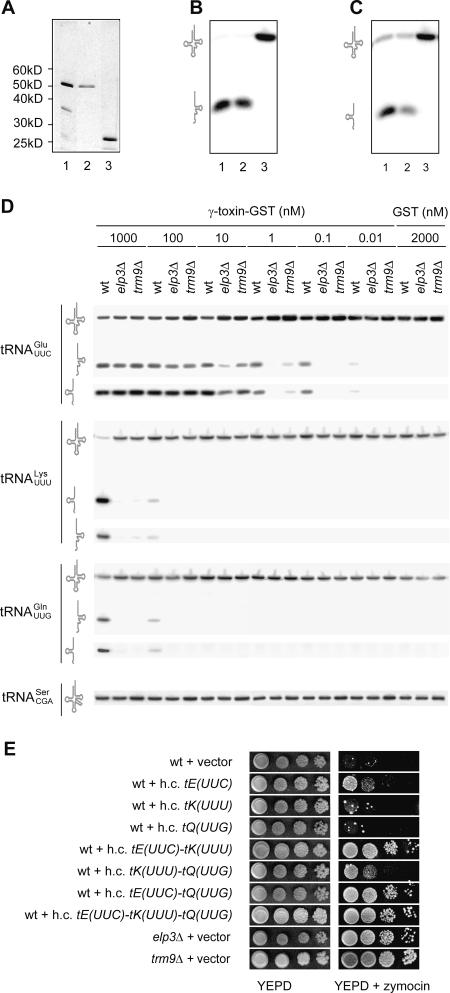
FIGURE 2.
The γ-toxin is an endonuclease that cleaves tRNAGlumcm5s2UUC, tRNALysmcm5s2UUU, and tRNAGlnmcm5s2UUG. (A) SDS-PAGE analysis of γ-toxin-GST fraction (lane 1), further purified 53-kD γ-toxin- GST (lane 2), and GST (lane 3). Proteins were visualized by silver staining. (B,C) Northern blot analysis of wild-type S. cerevisiae tRNA (5 μg) incubated with 1 μg of γ-toxin-GST fraction (lane 1), 53-kDa γ-toxin-GST (lane 2), or GST (lane 3) for 10 min at 30°C. The filter was probed by using an oligonucleotide complementary to the 3′- (B) or the 5′-part (C) of tRNAGlumcm5s2UUC. (D) Reactions containing the indicated concentration of γ-toxin-GST fraction or GST, and 5 μg of wild-type, elp3Δ, or trm9Δ total tRNA was incubated for 10 min at 30°C. Samples were analyzed by Northern blots; the identity of each signal is indicated on the left. (E) A wild-type S. cerevisiae strain CY4029 (W303-1A SSD1-v1) carrying the indicated high copy (h.c.) plasmid was serially diluted, spotted onto a YEPD plate or a YEPD plate supplemented with crude zymocin, and incubated for 3 d at 25°C. The elp3Δ and trm9Δ strains carrying the empty h.c. vector were included on the plates.
To investigate the hypothesis that tRNAGlumcm5s2UUC is a target of the γ-toxin, we analyzed the levels of this tRNA in wild-type, elp3Δ, and trm9Δ cells after induction of an integrated γ-toxin gene placed under the control of the PGAL1 promoter. Similar to exogenous zymocin treatment (White et al. 1989), the viability of wild-type cells dropped after induction of γ-toxin expression (Fig. 1B). Northern blot analysis showed that the amount of tRNAGlumcm5s2UUC decreased with increased induction time (Fig. 1C). Since these phenotypes were not a general consequence of growth in galactose media (data not shown) and not observed in the uninduced control (Fig. 1B,C), they are a result of γ-toxin expression. Furthermore, a decrease in the levels of tRNAGlumcm5s2UUC was observed in wild-type cells treated with exogenous zymocin, showing that intracellular γ-toxin expression mimics zymocin treatment (data not shown). Interestingly, no reduction in cell viability or tRNAGlu levels was observed upon γ-toxin induction in the elp3Δ and trm9Δ mutants (Fig. 1B,C), supporting the idea that the mcm5 side-chain in tRNAGlumcm5s2UUC is required for the cytotoxicity of γ-toxin. In addition to tRNAGlumcm5s2UUC, a mcm5 side-chain can be found in tRNALysmcm5s2UUU, tRNAGlnmcm5s2UUG, tRNAGly mcm5UCC, and tRNAArg mcm5UCU (Johansson and Byström 2005; data not shown). No obvious reduction in the levels of tRNALysmcm5s2UUU, tRNAGlnmcm5s2UUG, tRNAGly mcm5UCC, or tRNAArg mcm5UCU was observed after 4 h of γ-toxin induction in wild-type cells (data not shown).
The reduction of tRNAGlumcm5s2UUC in vivo could be a direct effect of γ-toxin or a secondary effect of growth arrest. To investigate if γ-toxin acts directly on tRNA, we purified a recombinant glutathione-S-transferase (GST) tagged γ-toxin from Escherichia coli (Fig. 2A, lane 1). The 53-kDa γ-toxin-GST fusion protein was further purified (Fig. 2A, lane 2) by using a gel filtration column. The γ-toxin-GST protein fraction, 53-kDa γ-toxin-GST, or GST (Fig. 2A) was incubated with total tRNA prepared from wild-type S. cerevisiae cells. Northern blot analysis showed that the amount of full length tRNAGlumcm5s2UUC decreased upon treatment with γ-toxin-GST fraction or 53-kDa γ-toxin-GST but not upon treatment with GST (Fig. 2B,C). Interestingly, γ-toxin–dependent signals at the approximate sizes of 34 (using a 5′ probe) and 41 (using a 3′ probe) nucleotides were detected (Fig. 2B,C), suggesting that tRNAGlumcm5s2UUC is cleaved by the γ-toxin. The tRNAGlumcm5s2UUC halves were not detected upon γ-toxin induction in vivo, suggesting that the cleavage products are rapidly turned over.
To investigate the influence of the wobble mcm5 side-chain on tRNA cleavage in vitro, the γ-toxin-GST fraction was serially diluted and incubated with total tRNA prepared from wild-type, elp3Δ, or trm9Δ cells. The hypomodified tRNAGlu was cleaved at high γ-toxin concentrations in vitro, but based on the dilution series at a lower efficiency than the fully modified counterpart (Fig. 2D). Interestingly, at high γ-toxin concentrations, we also observed cleavage of the fully modified tRNALysmcm5s2UUU and tRNAGlnmcm5s2UUG (Fig. 2D). Similar to tRNAGlu, hypomodified tRNALys and tRNAGln were inefficiently cleaved. Although tRNALysmcm5s2UUU and tRNAGlnmcm5s2UUG are cleaved by γ-toxin, tRNAGlumcm5s2UUC appears to be the best substrate since cleavage was detected at significantly lower γ-toxin concentrations. No in vitro cleavage of tRNAGlymcm5UCC or tRNAArgmcm5UCU was observed even at the highest γ-toxin concentration (data not shown), suggesting that features other than the mcm5 side-chain contribute to the substrate specificity. In the anticodon region tRNAGlumcm5s2UUC, tRNALysmcm5s2UUU, and tRNAGlnmcm5s2UUG share the sequence U33mcm5s2U34U35 (Fig. 3C). The tRNAGlymcm5UCC and tRNAArgmcm5UCU species lack the 2-thio group at position 34 and contain a C at position 35, implying that not only the mcm5 side-chain but also the 2-thio group and the primary sequence of the anticodon region could be important for recognition and/or cleavage by γ-toxin.
FIGURE 3.
K. lactis γ-toxin cleaves tRNAGlumcm5s2UUC, tRNALysmcm5s2UUU, and tRNAGlnmcm5s2UUG between position 34 and 35. (A) γ-toxin-GST– treated (+) or mock-treated (−) wild-type total tRNA was reverse transcribed by using 32P-labeled oligonucleotides complementary to the 3′-end of tRNAGlumcm5s2UUC, tRNALysmcm5s2UUU, or tRNAGlnmcm5s2UUG. The sequence ladder was derived from a gene coding for tRNAGlumcm5s2UUC. Arrowheads indicate reverse transcripts induced by γ-toxin treatment. (B) The purified 5′-half of tRNAGlumcm5s2UUC, tRNALysmcm5s2UUU, or tRNAGlnmcm5s2UUG was treated with the indicated enzyme(s) before ligation and RT-PCR amplification. (C) The sequence shared between tRNAGlumcm5s2UUC, tRNALysmcm5s2UUU, and tRNAGlnmcm5s2UUG in the anticodon region, and the site of γ-toxin cleavage.
Consistent with the different γ-toxin reactivity of the substrate tRNAs in vitro, elevated levels of tRNAGlumcm5s2UUC, but not tRNALysmcm5s2UUU or tRNAGlnmcm5s2UUG, rendered a wild-type strain resistant to exogenous zymocin (Fig. 2E). Simultaneous overexpression of tRNALysmcm5s2UUU and tRNAGlnmcm5s2UUG generated a resistance comparable to that observed for increased dosage of the tRNAGlumcm5s2UUC gene. Moreover, overexpression of tRNAGlumcm5s2UUC in combination with tRNALysmcm5s2UUU or tRNAGlnmcm5s2UUG or all three tRNA species together generated higher zymocin resistance than did elevated expression of tRNAGlumcm5s2UUC alone (Fig. 2E). These data indicate that not only tRNAGlumcm5s2UUC but also tRNALysmcm5s2UUU and tRNAGlnmcm5s2UUG are γ-toxin substrates in vivo.
To determine the position of the cleavage, we performed primer extension analysis by using γ-toxin–treated total wild-type tRNA and an oligonucleotide complementary to the 3′ end of tRNAGlumcm5s2UUC, tRNALysmcm5s2UUU, or tRNAGlnmcm5s2UUG. The size of the major products showed that the 5′ nucleotide of the 3′-halves corresponds to position 35 in tRNAGlumcm5s2UUC, tRNALysmcm5s2UUU, and tRNAGlnmcm5s2UUG (Fig. 3A). Purified tRNAGlumcm5s2UUC, tRNALysmcm5s2UUU, and tRNAGlnmcm5s2UUG were treated with γ-toxin, and the 5′- and 3′- fragments of each tRNA were isolated from a denaturing polyacrylamide gel. The 3′ tRNA fragments could be directly labeled with [γ-32P] ATP by using T4 polynucleotide kinase (T4 PNK), suggesting the presence of a 5′ hydroxyl terminus (data not shown).
To determine the 3′ end of the 5′ tRNA fragments, a procedure involving ligation of an oligonucleotide to the 3′ end was used. This provides a priming site for reverse transcription and subsequent PCR (Morse and Bass 1997). We obtained PCR products if the tRNA fragments were treated with T4 PNK before ligation to the oligonucleotide (Fig. 3B). No PCR product was obtained if the fragments were treated with calf intestinal alkaline phosphatase (CIP), which has 5′ and 3′ phosphatase activity. This suggests that the γ-toxin generates a 2′,3′ cyclic phosphate, since T4 PNK has a 2′,3′ cyclic phosphodiesterase activity that is not present in CIP (Morse and Bass 1997). DNA sequencing of cloned PCR products revealed that the γ-toxin cleavage occurs 3′ of the wobble nucleotide (data not shown). Taken together, these data show that γ-toxin cleaves tRNAGlumcm5s2UUC, tRNALysmcm5s2UUU, and tRNAGlnmcm5s2UUG between position 34 and 35 (Fig. 3C), generating a 2′,3′ cyclic phosphate and a 5′ hydroxyl group.
The cleavage mechanism of γ-toxin resembles that of the tRNA splicing endonuclease (Peebles et al. 1983; Trotta et al. 1997), although the position of cleavage is different. While γ-toxin targets mature tRNAs, the splicing endonuclease acts on precursor tRNAs that contain an intervening sequence located one nucleotide 3′ of the anticodon. A related mechanism is also utilized by the bacterial tRNA endonucleases, PrrC, colicin D, and colicin E5 (Amitsur et al. 1987; Ogawa et al. 1999; Tomita et al. 2000), which targets specific subsets of bacterial tRNAs. The PrrC protein cleaves tRNALys 5′ of the wobble nucleotide (Amitsur et al. 1987). Similar to γ-toxin, the cleavage by PrrC protein is stimulated by presence of modified nucleosides in the anticodon region (Jiang et al. 2001, 2002). Colicin D cleaves all four tRNAArg iso-acceptors 3′ of position 38 (Tomita et al. 2000), whereas colicin E5 cleaves 3′ of the wobble nucleotide in tRNATyr, tRNAHis, tRNAAsn, and tRNAAsp (Ogawa et al. 1999). Even though the mechanism of cleavage by γ-toxin is similar to other tRNA endonucleases, γ-toxin shows no obvious amino acid sequence homology to any of these.
Cells exposed to zymocin arrest in the G1 phase of the cell cycle (Butler et al. 1991b). Inhibition of protein synthesis by shifting strains with conditional mutations in genes coding for translation initiation factors or aminoacyl-tRNA synthetases to the nonpermissive condition causes a G1 arrest (Unger and Hartwell 1976; Johnston et al. 1977; Hohmann and Thevelein 1992; Wrobel et al. 1999; Pyronnet and Sonenberg 2001). Thus, the zymocin induced G1 arrest is most likely a consequence of a translational defect caused by depletion/reduction of tRNAGlumcm5s2UUC, tRNALysmcm5s2UUU, and tRNAGlnmcm5s2UUG. The γ-subunit of zymocin is the first identified eukaryotic toxin that targets tRNA. Interestingly, the γ-toxin shows regions of similarity to a plasmid encoded protein in the toxin secreting yeast Pichia inositovora (Klassen and Meinhardt 2003). Since an elp3 mutant lacking 5-substituted wobble uridines is less sensitive to the P. inositovora toxin (Klassen and Meinhardt 2003), the target of this toxin is probably one or several U34 containing tRNA species. This suggests that secretion of toxins to deplete competing fungi of tRNA could be a wide spread strategy to gain a growth advantage.
MATERIALS AND METHODS
Yeast strains, media, and genetic procedures
Yeast transformation (Gietz et al. 1992), media, and genetic procedures have been described (Burke et al. 2000). Plates supplemented with K. lactis zymocin were prepared as described earlier (Butler et al. 1991b). All S. cerevisiae strains are derivatives of W303-1A (MATa ura3-1 leu2-3,112 trp1-1 his3-11,15 can1-100 ade2-1) or W303-1B (MATα ura3-1 leu2-3,112 trp1-1 his3-11,15 can1-100 ade2-1). The elp3::KanMX4 and trm9::KanMX4 alleles were PCR amplified from the corresponding null mutants in the yeast deletion collection (Research genetics). The PCR products were transformed into strain W303-1B, generating UMY2843 (MATα elp3::KanMX4) and UMY3297 (MATα trm9::KanMX4). These strains were confirmed by PCR and the lack of mcm5-modified uridines in tRNA. The LYS2 open reading frame in W303-1A or W303-1B was replaced with the KanMX6-PGAL1-γ-toxin construct, generating UMY3263 (MATa lys2::KanMX6-PGAL1-γ-toxin) and UMY13264 (MATα lys2::KanMX6-PGAL1-γ-toxin). Strains UMY3266 (MATα elp3::KanMX4 lys2::KanMX6-PGAL1-γ-toxin) and UMY3268 (MATα trm9::KanMX4 lys2::KanMX6-PGAL1-γ-toxin) were derived from crosses between UMY3263 (MATa lys2:: KanMX6-PGAL1-γ- toxin) and UMY2843 (MATα elp3::KanMX4) or UMY3297 (MATα trm9::KanMX4). Strains with an integrated PGAL1-γ-toxin construct were grown to OD600 ~0.1 in raffinose containing YEP medium. Each culture was divided into two, followed by addition of galactose (inducing condition) or raffinose (non-inducing condition).
Plasmid constructions
DNA manipulations, plasmid preparations, and bacterial transformations were performed according to standard protocols. The E. coli strain used for plasmid constructions was DH5α (Bethesda Research Laboratories). Genes were PCR amplified by using Pwo DNA polymerase (Roche Applied Science). To place the γ-toxin gene under the GAL1 promoter, the γ-toxin gene (omitting codons 1–19 encoding the signal peptide) was PCR amplified from K. lactis killer strain 2105-1D (Gunge and Sakaguchi 1981) by using oligonucleotides 5′- ATCAGGATCCATGGCAGCTACTACTGCGAGA-3′ and 5′-ACTT GAGCTCGTCATTTTATTATACACATTTTCC-3′. The PCR product was digested with BamHI/SacI and cloned into corresponding sites in pRS316-PGAL1 (Liu et al. 1992), generating plasmid pRS316- PGAL1-γ-toxin (pABY1472). Plasmid pRS316-KanMX6-PGAL1-γ- toxin (pABY1637) was obtained by transforming a yeast strain carrying pRS316-PGAL1-γ-toxin with a KanMX6-PGAL1 DNA fragment amplified from pFA6a-KanMX6-PGAL1-3HA (Longtine et al. 1998). One oligonucleotide introduced 40 nucleotides of homology to the vector sequence of pRS316. Plasmids isolated from G418R transformants were confirmed by restriction analysis and DNA sequencing. To construct plasmid pRS316-PGAL1-γ-toxin-GST-KanMX6 (pABY16 43), a GST-KanMX6 cassette was PCR amplified from pFA6a-GST-KanMX6 (Longtine et al. 1998) and transformed into a yeast strain carrying pRS316-PGAL1-γ-toxin. The oligonucleotides used in the PCR generated homology to sequences located 40 nucleotides upstream and downstream of the stop codon of the γ-toxin gene. Plasmids from G418R tranformants were isolated, and the generation of an in-frame γ-toxin-GST fusion was confirmed by DNA sequencing. Wild-type yeast cells carrying this plasmid failed to grow on a galactose plate, confirming the functionality of the construct. Plasmid pETM-13-γ-toxin-GST (pABY1644) was constructed by cloning an NcoI/BglII γ-toxin-GST fragment from pRS316-PGAL1-γ-toxin-GST-KanMX6 into the NcoI/BamHI sites of pETM-13. In this plasmid, the γ-toxin-GST fusion gene is under the T7 promoter. A control plasmid, pETM-13-GST (pAB-Y1650), was obtained by cloning an NcoI/BglII GST fragment, PCR amplified from pRS316-PGAL1-γ-toxin-GST-KanMX6, into the NcoI/BamHI sites of pETM-13. The oligonucleotides used were 5′-ATGCCCATGGGGATCCCCGGGTTAATTAA-3′ and 5′-AATACGACTCACTATAG-3′. Plasmid pRS425-tE(UUC) (pABY1479) was constructed by cloning a HindIII/BamHI tE(UUC)M fragment, PCR amplified from W303-1A, into the corresponding sites of the high copy vector pRS425 (Christianson et al. 1992). A BamHI/EagI tK(UUU)L fragment, amplified from W303-1A, was cloned into pRS425, generating pRS425-tK(UUU) (pABY1604). The tQ(UUG)L gene was amplified by PCR from W303-1A, followed by addition of an A overhang using Taq DNA polymerase (Roche Applied Science), and cloned into the pGEM-T Easy Vector (Promega), generating pGEM-T Easy-tQ(UUG) (pABY1474). An ApaI/SacI tQ(UUG)L fragment from pGEM-T Easy-tQ(UUG) was cloned into the corresponding sites of pRS425, generating pRS425-tQ(UUG) (pABY1499). The oligonucleotides used were 5′-TTTTAAGCTTGAGACGTCAAGTTTC TCGTTG-3′ and 5′-TTTTGGATCCGGTGGCGTTTTTAACTTCT TC-3′ (tE(UUC)M), 5′-CGTAGGAT CCGGTAGAGTCTCTTCT TGGTC-3′ and 5′-GGTTCGGCCGACCTACTAGGTACTTTAGG-3′ (tK(UUU)L), or 5′-ATTAGGATCCGTTATTGTGTTTCCCGA GAGG-3′ and 5′-AATACTCGAGAATACGCGAAGGGGAATC-3′ (tQ(UUG)L). A BamHI/EagI tK(UUU) fragment from pRS425- tK(UUU) was cloned into the corresponding sites in pRS425- tE(UUC), generating pRS425-tE(UUC)-tK(UUU) (pABY1649). Plasmid pRS425-tK(UUU)-tQ(UUG) (pABY1707) was constructed by cloning a SacI/SacII tQ(UUG) fragment from pGEM-T Easy-tQ(UUG) into corresponding sites of pRS425-tK(UUU). A SacI/SacII tQ(UUG) fragment from pGEM-T Easy-tQ(UUG) was cloned into the corresponding sites of pRS425-tE(UUC), generating pRS425-tE(UUC)-tQ(UUG) (pABY1661). The tQ(UUG) gene was cloned from pGEM-T Easy-tQ(UUG) as a SacI/SacII fragment into the corresponding sites in pRS425-tE(UUC)-tK(UUU), generating pRS425-tE(UUC)-tK(UUU)-tQ(UUG) (pABY1653).
RNA preparation and Northern blot analysis
Total tRNA for in vitro γ-toxin treatment was isolated from exponentially growing cultures of W303-1B, UMY2843, or UMY3294 (Huang et al. 2005). Single tRNA species were isolated from W303-1A total tRNA as described previously (Huang et al. 2005). Total RNA was prepared by using hot phenol (Ausubel et al. 2001). Approximately 5 μg of total tRNA or RNA were separated on 8% polyacrylamide, 8 M urea gels, and transferred to Zeta-Probe membranes (Bio-Rad). Oligonucleotides used to detect tRNAs were 5′-GCCCAAGAGATTTCGAGTCTCT-3′ (tRNASerCGA), 5′- CTCCGCTACGGGGAGTCGAAC-3′ (tRNAGlumcm5s2UUC 3′ probe), 5′-AGCCGTTACACTATATCGGA-3′ (tRNAGlumcm5s2UUC 5′ probe), 5′- CTCCTCATAGGGGGCTC-3′ (tRNALysmcm5s2UUU 3′ probe), 5′-CAACT GAGCTAACAAGGA-3′ (tRNALysmcm5s2UUU 5′ probe), 5′-AGGTCC TACCCGGATTC-3′ (tRNAGlnmcm5s2UUG 3′ probe), and 5′-CCAC TACACTATAGGACC-3′ (tRNAGlnmcm5s2UUG 5′ probe). Oligonucleotides were labeled by using adenosine [γ32P]-triphosphate (6000 Ci/mmol, Amersham Biosciences) and polynucleotide kinase (Roche Applied Science). Northern blots were visualized and quantified by Phosphor-Imager analysis.
Purification of the γ-toxin-GST fusion protein
A total volume of 20 mL of LB medium containing 50 μg/mL of kanamycin was inoculated with E. coli strain BL21 (DE3) (Novagen) carrying pETM-13-γ-toxin-GST or pETM-13-GST and grown to OD600 ~2.0 at 30°C. The γ-toxin-GST and GST proteins were purified from cell extracts by using Glutathione–Sepharose 4B (Amersham Biosciences) according to manufacturer’s instructions. The purified proteins were applied onto a Sephacryl S-200HR XK26 column (Amersham Biosciences) and fractionated by using a buffer containing 20 mM Hepes (pH 7.3), 0.2 mM EDTA, 150 mM NaCl, and protease inhibitors (Roche Applied Science). Collected fractions were monitored by using Coomassie Plus protein assay reagent (Pierce) and silver staining of SDS-PAGE –separated aliquots.
Characterization of tRNAs treated with γ-toxin in vitro
Purified γ-toxin-GST protein was mixed with total or purified tRNA in 10 mM Tris-HCl, 10 mM MgCl2, 50 mM NaCl, and 1 mM dithiothreitol (pH 7.5) and incubated for 10 min at 30°C. The samples were precipitated and the pellets dissolved in water. The 5′-end of the 3′- halves of tRNAGlumcm5s2UUC, tRNALysmcm5s2UUU, and tRNAGlnmcm5s2UUG were determined by primer extension analysis. Appropriate tRNA samples (1μg) were mixed with 5 pmol of 32P-kinased oligonucleotides, 5′-CTCCGCTACGGGGAGTCGAAC-3′ (tRNAGlumcm5s2UUC), 5′- CTCCTCATAGGGGGCTC-3′ (tRNALysmcm5s2UUU), or 5′-AGGTCC TACCCGGATTC-3′ (tRNAGlnmcm5s2UUG) in AMV reverse transcriptase buffer (Roche Applied Science). Primers were annealed to the templates by incubating for 3 min at 70°C and for 5 min at 37°C and thereafter were placed on ice. Extensions were performed for 5 min at 37°C in the presence of 20 μM dNTPs and 25 U of AMV reverse transcriptase (Roche Applied Science). Reactions were stopped by addition of 35 μL 0.3 M NaAc (pH 5.2) containing 0.1 mg/mL RNase A and were incubated 15 min at 37°C. The samples were precipitated and applied next to a sequencing ladder on a denaturing 6% polyacrylamide gel. The ladder was obtained by sequencing a tRNAGlumcm5s2UUC gene using the oligonucleotide 5′- CTCCGCTACGGGGAGTCGAAC-3′.
The purified 5′ tRNA fragments were incubated in the presence or absence of 10 U of T4 PNK (USB) as described previously (Amitsur et al. 1987). After this, the samples were treated with 20 U of CIP (Roche Applied Science) according to manufacturer’s instructions. A kinased oligonucleotide 5′-GACATACGTACGAC GAGTACTGACCAGCTACGATGCATGAGCGCCTGddA-3′ was ligated to the dephosphorylated tRNA fragments as earlier described (Morse and Bass 1997), except the reaction contained 1 mM ATP. Following reverse transcription as described above, the cDNA was PCR amplified by using Taq DNA polymerase (Roche Applied Science). The oligonucleotides were 5′- CAGGCGCTCATGCAT-3′ (RT and PCR), 5′-TCCGATATAGTG TAACG-3′ (PCR for tRNAGlumcm5s2UUC), 5′-TCCTTGTTAGCT CAGTT-3′ (PCR for tRNALysmcm5s2UUU), and 5′-GGTCCTATAGTG TAGTG-3′ (PCR for tRNAGlnmcm5s2UUG). PCR products from two independent reactions for each tRNA were cloned by using the TOPO TA Cloning Kit (Invitrogen) and the DNA sequenced by using DYEnamic ET Dye Terminator Cycle Sequencing Kit.
Acknowledgments
We thank Drs. L. Symington and K. Arndt for S. cerevisiae strains W303-1A, W303-1B, and CY4029. Dr. N. Gunge is acknowledged for K. lactis strain 2105-1D. Drs. G.R. Björk, H. Wolf-Watz, and S. Tuck are acknowledged for valuable discussions. This work was financially supported by the Swedish Research Council (621-2004-2563) and the Swedish Cancer Society (3516-B03-10XAB). M.J.O.J. was supported by grants from the Swedish Research Council (621-2004-1700) and the Swedish Cancer Society (0608-B04-33XAB).
Article and publication are at http://www.rnajournal.org/cgi/doi/10.1261/rna.2172105.
REFERENCES
- Amitsur, M., Levitz, R., and Kaufmann, G. 1987. Bacteriophage T4 anticodon nuclease, polynucleotide kinase and RNA ligase reprocess the host lysine tRNA. EMBO J. 6: 2499–2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ausubel, F.M., Brent, R., Kingston, R.E., Moore, D.D., Seidman, J.G., Smith, J.A., and Struhl, K. 2001. Current protocols in molecular biology. John Wiley and Sons, New York.
- Burke, D., Dawson, D., and Stearns, T. 2000. Methods in yeast genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York.
- Butler, A.R., Porter, M., and Stark, M.J. 1991a. Intracellular expression of Kluyveromyces lactis toxin γ subunit mimics treatment with exogenous toxin and distinguishes two classes of toxin-resistant mutant. Yeast 7: 617–625. [DOI] [PubMed] [Google Scholar]
- Butler, A.R., White, J.H., and Stark, M.J. 1991b. Analysis of the response of Saccharomyces cerevisiae cells to Kluyveromyces lactis toxin. J. Gen. Microbiol. 137: 1749–1757. [DOI] [PubMed] [Google Scholar]
- Butler, A.R., White, J.H., Folawiyo, Y., Edlin, A., Gardiner, D., and Stark, M.J. 1994. Two Saccharomyces cerevisiae genes which control sensitivity to G1 arrest induced by Kluyveromyces lactis toxin. Mol. Cell. Biol. 14: 6306–6316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christianson, T.W., Sikorski, R.S., Dante, M., Shero, J.H., and Hieter, P. 1992. Multifunctional yeast high-copy-number shuttle vectors. Gene 110: 119–122. [DOI] [PubMed] [Google Scholar]
- Frohloff, F., Fichtner, L., Jablonowski, D., Breunig, K.D., and Schaffrath, R. 2001. Saccharomyces cerevisiae Elongator mutations confer resistance to the Kluyveromyces lactis zymocin. EMBO J. 20: 1993–2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gietz, D., St. Jean, A., Woods, R.A., and Schiestl, R.H. 1992. Improved method for high efficiency transformation of intact yeast cells. Nucleic Acids Res. 20: 1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunge, N. and Sakaguchi, K. 1981. Intergeneric transfer of deoxyribonucleic acid killer plasmids, pGKl1 and pGKl2, from Kluyveromyces lactis into Saccharomyces cerevisiae by cell fusion. J. Bacteriol. 147: 155–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunge, N., Tamaru, A., Ozawa, F., and Sakaguchi, K. 1981. Isolation and characterization of linear deoxyribonucleic acid plasmids from Kluyveromyces lactis and the plasmid-associated killer character. J. Bacteriol. 145: 382–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohmann, S. and Thevelein, J.M. 1992. The cell division cycle gene CDC60 encodes cytosolic leucyl-tRNA synthetase in Saccharomyces cerevisiae. Gene 120: 43–49. [DOI] [PubMed] [Google Scholar]
- Huang, B., Johansson, M.J.O., and Byström, A.S. 2005. An early step in wobble uridine tRNA modification requires the Elongator complex. RNA 11: 424–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jablonowski, D., Frohloff, F., Fichtner, L., Stark, M.J., and Schaffrath, R. 2001. Kluyveromyces lactis zymocin mode of action is linked to RNA polymerase II function via Elongator. Mol. Microbiol. 42: 1095–1105. [DOI] [PubMed] [Google Scholar]
- Jiang, Y., Meidler, R., Amitsur, M., and Kaufmann, G. 2001. Specific interaction between anticodon nuclease and the tRNA(Lys) wobble base. J. Mol. Biol. 305: 377–388. [DOI] [PubMed] [Google Scholar]
- Jiang, Y., Blanga, S., Amitsur, M., Meidler, R., Krivosheyev, E., Sundaram, M., Bajji, A.C., Davis, D.R., and Kaufmann, G. 2002. Structural features of tRNALys favored by anticodon nuclease as inferred from reactivities of anticodon stem and loop substrate analogs. J. Biol. Chem. 277: 3836–3841. [DOI] [PubMed] [Google Scholar]
- Johansson, M.J.O. and Byström, A.S. 2005. Transfer RNA modifications and modifying enzymes in Saccharomyces cerevisiae. In Fine-tuning of RNA functions by modification and editing (ed. H. Grosjean), pp. 87–120. Springer-Verlag, New York.
- Johnston, G.C., Pringle, J.R., and Hartwell, L.H. 1977. Coordination of growth with cell division in the yeast Saccharomyces cerevisiae. Exp. Cell Res. 105: 79–98. [DOI] [PubMed] [Google Scholar]
- Kalhor, H.R. and Clarke, S. 2003. Novel methyltransferase for modified uridine residues at the wobble position of tRNA. Mol. Cell. Biol. 23: 9283–9292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klassen, R. and Meinhardt, F. 2003. Structural and functional analysis of the killer element pPin1-3 from Pichia inositovora. Mol. Genet. Genomics 270: 190–199. [DOI] [PubMed] [Google Scholar]
- Liu, H., Krizek, J., and Bretscher, A. 1992. Construction of a GAL1-regulated yeast cDNA expression library and its application to the identification of genes whose overexpression causes lethality in yeast. Genetics 132: 665–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longtine, M.S., McKenzie III, A., Demarini, D.J., Shah, N.G., Wach, A., Brachat, A., Philippsen, P., and Pringle, J.R. 1998. Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast 14: 953–961. [DOI] [PubMed] [Google Scholar]
- Morse, D.P. and Bass, B.L. 1997. Detection of inosine in messenger RNA by inosine-specific cleavage. Biochemistry 36: 8429–8434. [DOI] [PubMed] [Google Scholar]
- Ogawa, T., Tomita, K., Ueda, T., Watanabe, K., Uozumi, T., and Masaki, H. 1999. A cytotoxic ribonuclease targeting specific transfer RNA anticodons. Science 283: 2097–2100. [DOI] [PubMed] [Google Scholar]
- Otero, G., Fellows, J., Li, Y., de Bizemont, T., Dirac, A.M., Gustafsson, C.M., Erdjument-Bromage, H., Tempst, P., and Svejstrup, J.Q. 1999. Elongator, a multisubunit component of a novel RNA polymerase II holoenzyme for transcriptional elongation. Mol. Cell 3: 109–118. [DOI] [PubMed] [Google Scholar]
- Peebles, C.L., Gegenheimer, P., and Abelson, J. 1983. Precise excision of intervening sequences from precursor tRNAs by a membrane-associated yeast endonuclease. Cell 32: 525–536. [DOI] [PubMed] [Google Scholar]
- Pyronnet, S. and Sonenberg, N. 2001. Cell-cycle–dependent translational control. Curr. Opin. Genet. Dev. 11: 13–18. [DOI] [PubMed] [Google Scholar]
- Rahl, P.B., Chen, C.Z., and Collins, R.N. 2005. Elp1p, the yeast homolog of the FD disease syndrome protein, negatively regulates exocytosis independently of transcriptional elongation. Mol. Cell 17: 841–853. [DOI] [PubMed] [Google Scholar]
- Schaffrath, R. and Meinhardt, F. 2005. Kluveromyces lactis zymocin and other plasmid-encoded yeast killer toxins. In Microbial protein toxins (eds. Schmitt M.J. and Schaffrath R.), pp. 133–155. Springer-Verlag, Berlin.
- Stark, M.J., Boyd, A., Mileham, A.J., and Romanos, M.A. 1990. The plasmid-encoded killer system of Kluyveromyces lactis: A review. Yeast 6: 1–29. [DOI] [PubMed] [Google Scholar]
- Sugisaki, Y., Gunge, N., Sakaguchi, K., Yamasaki, M., and Tamura, G. 1983. Kluyveromyces lactis killer toxin inhibits adenylate cyclase of sensitive yeast cells. Nature 304: 464–466. [DOI] [PubMed] [Google Scholar]
- Tokunaga, M., Kawamura, A., and Hishinuma, F. 1989. Expression of pGKL killer 28K subunit in Saccharomyces cerevisiae: Identification of 28K subunit as a killer protein. Nucleic Acids Res. 17: 3435–3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita, K., Ogawa, T., Uozumi, T., Watanabe, K., and Masaki, H. 2000. A cytotoxic ribonuclease which specifically cleaves four iso-accepting arginine tRNAs at their anticodon loops. Proc. Natl. Acad. Sci. 97: 8278–8283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trotta, C.R., Miao, F., Arn, E.A., Stevens, S.W., Ho, C.K., Rauhut, R., and Abelson, J.N. 1997. The yeast tRNA splicing endonuclease: A tetrameric enzyme with two active site subunits homologous to the archaeal tRNA endonucleases. Cell 89: 849–858. [DOI] [PubMed] [Google Scholar]
- Unger, M.W. and Hartwell, L.H. 1976. Control of cell division in Saccharomyces cerevisiae by methionyl-tRNA. Proc. Natl. Acad. Sci. 73: 1664–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White, J.H., Butler, A.R., and Stark, M.J.R. 1989. Kluyveromyces lactis toxin does not inhibit yeast adenylyl cyclase. Nature 341: 666–668. [Google Scholar]
- Wrobel, C., Schmidt, E.V., and Polymenis, M. 1999. CDC64 encodes cytoplasmic alanyl-tRNA synthetase, Ala1p, of Saccharomyces cerevisiae. J. Bacteriol. 181: 7618–7620. [DOI] [PMC free article] [PubMed] [Google Scholar]