Abstract
A rapid and quantitative DNA-binding assay was developed based on the translational fusion of a DNA-binding protein (DBP) with a Neocallimastix patriciarum β-1,4-d-glucanase, CelD. CelD releases a fluorescent 4-methylumbelliferyl product from 4-methylumbelliferyl cellobioside. This hydrolysis activity was used to quantify the amount of DBP–CelD bound to an immobilised biotin-labelled target sequence. The DNA-binding assay can be performed in a 96-well plate format for high- throughput analysis of putative DBPs. This method was applied to analysis of the binding properties and sequence selectivity of a cold-inducible transcription factor HvCBF1 from barley containing an AP2 DNA-binding domain. A base-scanning approach using degenerate oligonucleotide probes was employed for rapid identification of the conserved core motif of the HvCBF1 binding site. Quantitative analysis of the binding site of HvCBF1 using systematic base substitution revealed that a (G/a) (C/t)CGAC sequence was sufficient to constitute a functional motif, where the lower-case letters represent less efficient bases. The method enables us to provide accurate and quantitative data on a comprehensive DNA-binding profile for a cold-inducible AP2 transcription factor as well as information on environmental parameters potentially influencing the DNA-binding activity. The accurate binding sequence data facilitate identification of candidate genes regulated by HvCBF1 from genome sequence databases.
INTRODUCTION
Plant response to cold stress involves alterations in expression of a large number of genes that are mediated by both abscisic acid (ABA)-dependent and independent signal transduction pathways (1,2). In Arabidopsis, regulation of cold-responsive genes is mainly controlled by a group of transcriptional activators, called C-repeat (CRT) binding factors (CBFs) or dehydration-responsive element (DRE) binding proteins (DREBs) through an ABA-independent pathway (3–5). These transcriptional activators contain an AP2 DNA-binding domain, which interacts with a CCGAC core motif (3–5). Recently, a large amount of DNA sequence data has been generated through high-throughput sequencing projects. These data permit searching for genes encoding putative DNA-binding proteins (DBPs) containing an AP2 domain. AP2 proteins in plants form a superfamily, which consists of over 140 genes in Arabidopsis (6). A large number of gene sequences encoding AP2 proteins from other plant species have also been deposited in sequence databases and some of these AP2 proteins share high homology with Arabidopsis CBF/DREB proteins in their DNA-binding domains. However, characterisation of the DNA-binding sites of CBF/DREB-like proteins from plant species other than Arabidopsis has not been reported. Furthermore, many DBPs bind not only to a defined target sequence, but are also able to interact with degenerate sequences that have one or two bases differing from a known consensus sequence. This would require accurate and quantitative analysis of the DNA-binding affinity and sequence selectivity in order to define the DNA-binding sequence preference. Detailed sequence information of the DNA-binding sites of CBF/DREB proteins is essential for designing computer prediction tools using a weighted matrix (7) or a generalised profile (8) for identification of candidate cold-responsive genes from genome sequence databases.
To detect the DNA-binding activity of proteins and analyse their sequence-specific interaction, the electrophoretic mobility shift assay originally developed by Fried and Crothers (9) and Garner and Revzin (10) is the most widely used technique (11). In this technique, a labelled probe with a specific nucleotide sequence is mixed with a DBP. The binding reaction mixture is then loaded on a non-denaturing polyacrylamide gel. The binding of protein to DNA usually leads to a reduction in the electrophoretic mobility of a DNA probe. Radioactive 32P-labelled probes are most commonly used. Purification of a cloned DBP is often required in order to remove native DBPs from a host organism, which may lead to a false positive result. It is difficult to adapt the electrophoresis-based technique to high-throughput analyses. Although this technique has been used to provide both qualitative and quantitative analyses, it is not easy to obtain accurate quantification of protein-bound probes due to high experimental variations using the gel electrophoresis technique. The electrophoretic mobility shift assay is also inconvenient for optimising conditions for stabilising the DNA–protein complexes, as the stability of some DNA– protein complexes can be severely affected by electrophoresis conditions (11).
Some of the physical and chemical parameters that influence the DNA binding and subsequent stability of DNA– protein complexes may have physiological importance. For example, tightness of lacI repression on the Escherichia coli lacZ or tac promoter was influenced by temperatures (12) and the λ cI857 repressor is temperature sensitive (13). In plants, physical and chemical environments in cells are affected by climate (e.g. temperature and drought) and soil (concentrations of salt and other mineral elements) conditions. It is possible that the biological function of some DBPs may depend on these environmental conditions.
In this study, a rapid, quantitative and high-throughput method for studying the DNA-binding profile of recombinant proteins was developed. The method involves translational fusion of a putative DBP to the catalytic domain of a β-1,4-d-glucanase (also called cellulase) CelD from Neocallimastix patriciarum (14) and biotin-labelled oligonucleotide probes which interact with streptavidin immobilised on a solid support. The crude extract of a DBP–CelD fusion protein produced from E.coli was directly used in the DNA-binding assay. The catalytic activity of cellulase in the DBP–CelD fusion protein was used for quantification of the amount of fusion protein bound to immobilised biotin probes. The time-consuming steps in protein purification and gel electrophoresis are no longer required in this method. Furthermore, the present method provides a rapid, quantitative and high-throughput analysis of DNA–protein interaction with high accuracy. This method was applied to perform a systematic study on the binding properties and sequence selectivity of a barley transcription factor, HvCBF1 (GenBank accession no. AF418204). HvCBF1 contains an AP2 DNA-binding domain, highly homologous to those of cold-inducible Arabidopsis CBF/DREB1 proteins (3–5). A rapid approach to identify the conserved core motif interacting with HvCBF1 is also described.
MATERIALS AND METHODS
Materials
Oligonucleotides with or without a 5′-biotin molecule were custom synthesised by Invitrogen. Dynabeads M-280 Streptavidin was obtained from Dynal. StreptaWell High Bind (streptavidin-coated 96-well microtitre plates) was purchased from Roche. Restriction enzymes and DNA modification enzymes were from Promega. Escherichia coli strain XL1-Blue used as a cloning host was obtained from Stratagene. The Expand High Fidelity PCR system used for PCR amplification was obtained from Roche. All chemicals were purchased from Sigma Chemical Co., unless otherwise stated.
Construction of plasmids and gene expression cassettes
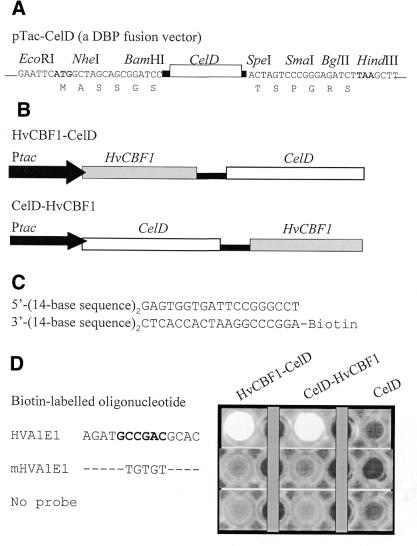
pTac–CelD, a DBP–CelD fusion vector, was constructed by the use of a 1077-bp fragment encoding the second catalytic domain of N.patriciarum celD (nucleotide positions from 1185 to 2261, GenBank accession no. AF053363) plus linker sequences encoding ASSGSGTPETTNPEEPTGN at the N-terminus and PTTSSTSPGRS at the C-terminus of the CelD (see Fig. 2A). This DNA fragment was inserted into the EcoRI and HindIII sites of pBtac2 (Roche). The linker sequences also contain several cloning sites for translational fusion of a DBP gene. Expression of celD is under the control of a strong tac promoter in the pBtac2 vector.
Figure 2.
Sequence-specific DNA-binding and cellulase activity of CelD-fused HvCBF1 proteins. (A) pTac–CelD was used as a DBP–CelD fusion vector. Expression of celD in E.coli is controlled by the tac promoter which is located upstream of the EcoRI site in the pBtac2 vector. A DBP coding sequence can be inserted either at the N-terminal or C-terminal cloning sites of CelD to create translational fusion to CelD. Black bars represent linker sequences. (B) A schematic presentation of HvCBF1–celD and celD– HvCBF1 fusion constructs driven by the tac promoter. (C) A biotin-labelled double-stranded oligonucleotide containing two copies (in direct repeats) of a 14-base sequence (HVA1E1 or its mutants) used as a probe for analysis of the binding activity of CelD-fused HvCBF1. (D) Detection of the sequence-specific binding activity of CelD-fused HvCBF1. Only a 14-bp sequence of each biotinylated oligonucleotide shown in (B) is specified here. Fusion proteins bound to the target sequence of a biotinylated oligonucleotide probe were detected as bright Mu fluorescent spots in the wells of a microtitre plate. The fluorescence is released from MUC by the cellulase activity of the target sequence bound CelD-fused HvCBF1 proteins.
pHvCBF1–CelD fusion plasmid was constructed by PCR amplification of the entire coding region sequence of HvCBF1 (GenBank accession no. AF418204) with primers containing adaptors (NheI site in the sense primer and BamHI in the anti-sense primer). The amplified DNA fragment was then inserted at the N-terminus of CelD using NheI and BamHI cloning sites to create in-frame fusion (see Fig. 2). The C-terminal HvCBF1 fusion plasmid (pCelD–HvCBF1) was constructed by inserting the NheI–BamHI fragment of HvCBF1 from pHvCBF1– CelD at the SpeI and BglII sites of pTac–CelD.
A semi-synthetic minimal promoter, PminiDhn6, was derived from a 107-bp fragment downstream of the TATA box of barley Dhn6 promoter (15; GenBank accession no. AF043091). A synthetic sequence 5′-GAGTGGTGATTCCGGGCCTATAAAT was added at the 5′ end, where TATAAAT is the consensus TATA-box. A synthetic 5′-untranslated region sequence 5′-GGATCCTAAAGCAAGCAAGAGCAATAAACAATG was added at the 3′ end of the promoter, where ATG serves as a translation start codon of a reporter. The PminiDhn6 promoter was inserted upstream of the xylanase (N.patriciarum XynA) reporter to obtain PminiDhn6XynR constructed in pSP72 vector (see Fig. 7) (16).
Figure 7.
A schematic representation of the pDhn8sHvCBF1 effector gene and oligonucleotide-linked PminiDhn6XynR xylanase reporter genes. 5′-UTR, 5′-untranslated region.
Synthetic oligonucleotides consisted of three direct repeats of a 14-base sequence containing a CRT/DRE motif or its mutants were placed upstream of PminiDhn6 to drive xylanase reporter expression. The 14-base sequences are shown in Table 2. Expression cassettes were assembled using PCR with a sense primer, 5′(14-base sequence)3GAGTGGTGATTCCGGGCCT, and an antisense primer, 5′-GGTGACACTATAGAACTCGAG (corresponding to the sequence at the XhoI site region of the pSP72 vector) with PminiDhn6XynR as a template DNA. The PCR-amplified gene expression cassettes were used for transient expression assays.
Table 2. Relationship between in vitro DNA-binding activity and in vivo transactivation activity of HvCBF1 interacting with various motifs.
Values are mean ± SD (n = 3). The transactivation activity is the expression level of the HVA1E1 or its mutant-driven xylanase (XynA) reporter gene in the presence of the HvCBF1 effector gene. The activity is expressed as the ratio of the XynA to GUS (internal control) activity relative to that of the HVA1E1-driven reporter gene (arbitrarily assigned as 1). No xylanase activity of these reporter genes was detected without co-introduction of the HvCBF1 effector gene. The DNA-binding activity is expressed as percentage relative to HVA1E1.
pDhn8sHvCBF1 (Dhn8s:HvCBF1:rice rbcS 3′) was constructed by replacing the gfp and rice GluB-1 3′ in pDhn8sGFPG (Dhn8s:gfp:rice GluB-1 3′) (16) with a HvCBF1 open-reading frame and rice rbcS 3′.
The nucleotide sequences of the constructed gene expression cassettes were determined using a BigDye terminator cycle sequencing kit (Applied Biosystems).
Screening of CelD-fusion constructs
Cellulase activity of recombinant E.coli XL1-Blue colonies carrying a DBP–CelD construct was screened by plating transformed cells on Luria broth (LB)/ampicillin plates containing 0.2% (w/v) carboxymethyl-cellulose (CM- cellulose). After overnight incubation at 37°C, the plates were stained with 0.5% (w/v) Congo red solution for 10 min at room temperature and destained by washing twice with 1 M NaCl. Cellulase-producing colonies were visualised as ones in the centre of CM-cellulose clearing zones on the plates. This method provides a rapid means for identification of transformed colonies containing a functional CelD enzyme in the fusion construct. An alternative method for screening cellulase activity was to include 0.1 mM 4-methylumbelliferyl β-d-cellobioside (MUC) in LB/ampicillin broth during culture growth. Cellulase activity was indicated by release of a fluorescent 4-methylumbelliferyl product from MUC.
Synthesis of double-stranded oligonucleotide probes and competitors
Biotin-labelled double-stranded oligonucleotide probes (see Fig. 2C) were synthesised by filling in partially double-stranded oligonucleotides using Klenow enzyme as follows. A 47-base sense oligonucleotide (4.7 µg) containing two direct repeats of a 14-base fragment, 5′-(14-base sequence in HVA1E1 or its mutants)2GAGTGGTGATTCCGGGCCT, was annealed with a 5′-biotin-labelled antisense oligonucleotide (2 µg), 5′-biotin-AGGCCCGGAATCAACCACTC, in a 50-µl reaction buffer [50 mM Tris–Cl, pH 7.5, 10 mM MgCl2 and 1 mM dithiothreitol (DTT)]. The annealing was carried out at 50°C for 30 min, 45°C for 30 min and then 37°C for 5 min after an initial 2-min heating at 94°C. The filling-in reaction was initiated by adding 5 U Klenow enzyme and dNTP at a final concentration of 0.5 mM and incubated at 23°C for 1 h. The filled-in product was purified using a Qiaex II kit (Qiagen).
Double-stranded oligonucleotide competitors were synthesised and purified as above except that a non-labelled anti-sense oligonucleotide was used during synthesis.
Preparation of DBP–CelD fusion protein extracts
Cultures of E.coli XL1-Blue carrying pHvCBF1–CelD or pCelD–HvCBF1 were grown in LB containing 1% glycerol and 100 µg/ml ampicillin at 32°C overnight. The overnight cultures were diluted with 3 vol of LB containing 1% glycerol, 100 µg/ml ampicillin and 1 mM isopropyl-β-d-thiogalactopyranoside for induction of fusion protein expression and grown at 32°C for 6 h. Cells were harvested by centrifugation and suspended in 0.15 vol (based on the culture volume) of cell lysis buffer (10 mM Tris–Cl, pH 8.0, 1 mM EDTA and 1 mg/ml lysozyme). The cell suspension was incubated in ice for 1 h. Cells were lysed by freeze-and-thaw twice. One volume of HEPES buffer [25 mM HEPES (pH 7.0), 50 mM KCl, 1 mM DTT and 1 mM EDTA] was added to the lysed cells. The cell debris was removed by centrifugation at 10 000 g and 4°C for 10 min. Supernatant (crude DBP–CelD extract) was stored at –20°C until analysis.
DNA-binding assays
In a standard DNA-binding assay using Dynabeads Streptavidin, 6 pmol of biotin-labelled double-stranded oligonucleotides were immobilised on 100 µg of Dynabeads Streptavidin in a final volume of 40 µl in an immobilisation buffer (10 mM Tris–Cl, pH 7.5, 1 mM EDTA and 1 M NaCl). The immobilisation was performed at room temperature for 30 min with gentle agitation. After probe immobilisation, 100 µl of a binding/washing buffer (25 mM HEPES/KOH, pH 7.0, 50 mM KCl, 1 mM EDTA, 0.5 mM DTT and 0.1 mg/ml bovine serum albumin) was added. The beads were then washed twice with 100 µl of the binding/washing buffer. DNA-binding reactions were carried out at room temperature for 40 min with gentle agitation in the above binding/washing buffer containing 0.4 µg/µl poly(dI–dC), 0.3 mg/ml bovine serum albumin, 10% glycerol, 0.05% Nonidet P-40 and crude HvCBF1–CelD protein extract (6.8 µg protein) in a final volume of 20 µl. Unbound proteins were removed by washing three times with 150–200 µl of the binding/washing buffer.
The cellulase activity of the HvCBF1-fusion protein captured on Dynabeads Streptavidin was assayed by suspending the washed beads in 100 µl of 1 mM MUC in 50 mM Na-citrate buffer, pH 6.0, and 1 mM DTT. Resuspended beads were transferred into a new tube to reduce background activity (this step is optional and is necessary only for assays of very low affinity motifs). The reaction was incubated at 40°C for 1–5 h with gentle agitation and terminated by adding 25 µl of 1 M Na2CO3. The fluorescence of the 4-methylumbelliferyl group released from MUC was measured in a fluorometer at excitation and emission wavelengths of 365 and 455 nm, respectively. DNA-binding assays without a biotin probe or a biotin-labelled oligonucleotide without a target motif were used as a control of background activity.
Variations from the above standard procedure, such as buffer compositions, pH, temperature, amounts of probe and HvCBF1–CelD, will be indicated in the legends to the respective tables and figures.
For binding assays conducted in StreptaWell High Bind (streptavidin-coated 96-well plate), immobilisation of biotin probes was carried out in 50 µl of the immobilisation buffer for 40 min. The binding reaction was performed for 1–2 h in a 50-µl binding/washing buffer containing 0.15 µg/µl poly(dI–dC) or 0.3 µg/µl yeast total RNA, 0.3 mg/ml bovine serum albumin, 10% glycerol and 0.05% Nonidet P-40. The volume of the washing buffer increased to 0.2–0.3 ml. Other conditions remained the same as in the assays using Dynabeads Streptavidin. For increasing the sensitivity of the assay, 24 pmol of a biotin-labelled probe was immobilised in 100 µl of the immobilisation buffer and 55 µg of crude HvCBF1–CelD protein from the supernatant of E.coli lysate was used in a 100-µl binding reaction.
Transactivation assays
Barley (Hordeum vulgare L. cv. Tallon) plants were grown at 16°C. Newly expanded leaves were freshly excised and placed in the centre of a Petri dish on two layers of Whatman No. 1 filter paper submerged in a solution of 100 mM sucrose and 5 mM sodium phosphate buffer, pH 7.0.
To avoid the possible interference of the vector sequence in the expression plasmids with functional analysis of the promoter sequences, all reporter gene expression cassettes were amplified by PCR. The products of PCR-amplified gene cassettes were purified using a Wizard PCR purification kit (Promega) and used for transactivation assays.
In transactivation assays the effector gene pDhn8sHvCBF1 (see Fig. 7) was co-introduced into barley leaves with a xylanase reporter gene at a molar ratio of effector:reporter at 1:15. A β-glucuronidase (GUS) control plasmid, pAAI1GUSR (rice Act1:Act1 intron 1: E.coli uidA:rice rbcS 3′), was also co-bombarded for normalisation of transformation effici ency between assays (16). An Act1 promoter-driven green fluorescent protein (GFP) plasmid, pAAI1GFPR (rice Act1 promoter:Act1intron 1:gfp:rice rbcS 3′) (17), was also included (100 ng of pAAI1GFPR per bombardment) for monitoring transformation frequency of leaf cells. The particle inflow gun was used for acceleration of DNA-coated gold particles (particle diameter of 1 µm) at a pressure of 2100 kPa and a vacuum of 28 mmHg (18). Barley leaves were bombarded twice with DNA-coated particles to increase the number of transformed cells in leaf tissues. The bombarded leaves were kept at 20°C for 24 h. GFP foci (transformed cells expressing GFP) developed in bombarded leaves were examined under a fluorescence microscope. The leaf sections with a relatively high number of GFP foci were selected and stored at –20°C until enzymatic assay of xylanase and GUS activities.
The leaf tissues were homogenised in 2 vol of an extraction buffer (25 mM sodium phosphate buffer, pH 7.4, 5 mM EDTA and 1 mM DTT). The supernatant obtained through centrifugation at 10 000 g in an Eppendorf microfuge for 5 min was used to assay for xylanase activity according to Patel et al. (18) and for fluorometric measurement of GUS activity according to Jefferson (19). Leaf tissues bombarded with vector (pSP72)-coated gold particles were used as a control of background activity.
RESULTS
Rationale of the enzymatic DNA-binding assay
The CelD enzyme is a highly active cellulase from N.patriciarum and is capable of hydrolysing a number of cellulosic compounds, such as cellulose, CM-cellulose and MUC (14). In particular, one of the hydrolysis products of MUC is a fluorescent 4-methylumbelliferyl product, which provides a sensitive and convenient assay system for measuring enzyme activity (Fig. 1). The CelD catalytic domain exhibits a modular structure and its enzymic activity is not usually affected by fusion to other proteins (G.-P. Xue, unpublished results). As E.coli does not produce enzymes capable of hydrolysing CM-cellulose or MUC (14), this makes CelD a particularly good reporter gene for analysis of DBPs cloned in this organism. The functionality of CelD in transformed cells carrying a DBP–CelD fusion construct can be rapidly screened either in LB plates containing CM-cellulose or in LB broth containing MUC. CelD also possesses a strong cellulose-binding activity (14) that can be used for purification of a CelD-fused protein.
Figure 1.
An illustration of general procedure for the enzymatic DNA- binding assay using a DBP–CelD fusion protein. Black ovals represent native proteins from E.coli. MU, 4-methylumbelliferyl.
Some of these properties of CelD were used to develop a rapid enzymatic assay for the quantitative measurement of DNA-binding activity of a protein by fusion to CelD. A DBP–CelD fusion vector, pTac–CelD, contained the second catalytic domain of N.patriciarum celD under the control of a strong tac promoter in the E.coli host (Fig. 2A). This vector can be used for cloning putative DBP genes to produce a translational fusion protein either at the N-terminus or C-terminus. A short linker sequence was placed at both sides of the celD sequence to reduce potential interference of biological activity due to protein fusion. A few restriction endonuclease sites are provided at both the N-terminus and C-terminus of the CelD. The NheI and BamHI cloning sites at the N-terminus of CelD produce compatible ends with those of SpeI and BglII sites at the C-terminus (Fig. 2A). This feature of the vector facilitates the cloning if one wishes to optimise the biological activity of a fusion protein by testing DBP fusion at both positions of CelD.
The general procedure of the enzymatic DNA-binding assay is illustrated in Figure 1. A double-stranded biotin-labelled oligonucleotide containing target binding sequences for a DBP (Fig. 2C) is immobilised on a streptavidin-coated solid support. A DBP–CelD protein from a crude extract (e.g. the supernatant of E.coli lysate) is applied to a DNA-binding reaction and captured by immobilised oligonucleotides. Unbound proteins are removed by washing. The bound DBP–CelD protein is then assayed for cellulase activity using MUC substrate. The amount of the fluorescent 4-methylumbelliferyl product released is in direct proportion with the amount of DBP–CelD protein bound to the immobilised target sequence. Purification of DBP–CelD fusion proteins produced from E.coli is not required for the DNA-binding assay. The whole process of the assay for a size of 10 samples can normally be completed within 3 h. For a large number of samples, assays can be performed in streptavidin-coated 96-well microtitre plates.
The sequence-specific binding activity of HvCBF1–CelD fusion proteins
A HvCBF1 cDNA (GenBank accession no. AF418204) isolated from barley encodes a transcriptional activator capable of activating cold-responsive genes such as HVA1s from barley (G.-P. Xue, unpublished results). HVA1s is a highly homologous gene to the late-embryogenesis-abundant protein HVA1 gene (16).
In this study, the entire coding region sequence of barley HvCBF1 was fused at either the N-terminus or C-terminus of CelD. As shown in Figure 2, both N-terminal and C-terminal fusion products were found to be active in interacting with the GCCGAC target sequence in HVA1E1 oligonucleotide derived from the HVA1s promoter. The mutation at the GCCGAC motif of HVA1E1 by replacing with GTGTGT (mHVA1E1) completely abolished the HvCBF1 binding activity. This suggests that fusion of HvCBF1 to CelD at either of the termini does not affect the sequence-specific binding of HvCBF1 to DNA. The CelD protein produced from the pTac–CelD vector did not bind to these oligonucleotides. The non-specific background binding of CelD and its fusion proteins was very low (see no probe control in Fig. 2D), indicating that these proteins do not interact with streptavidin.
Quantitative analysis of the DNA-binding activity of HvCBF1
To provide quantitative analysis of the DNA-binding activity, relationships between the DNA-binding activity and amounts of a biotin-labelled oligonucleotide probe or DBP–CelD protein were investigated (Fig. 3). The DNA-binding activity increased with amount (up to 6 pmol) of biotin-labelled HVA1E1 immobilised on 100 µg of Dynabeads Streptavidin (Fig. 3A). This is in close agreement with a specified range of the biotin-oligonucleotide binding capacity of the Dynabeads by the manufacturer. At the saturated amount of the immobilised biotin-HVA1E1, the bound cellulase activity also increased with amounts of HvCBF1–CelD in the protein– DNA binding reaction (Fig. 3B). However, the background activity (non-specific binding activity using mHVA1E1 as a control) did increase with increased amounts of HvCBF1– CelD. The background activity was generally <2% of that of HVA1E1 (fluorescence units per hour were 31 ± 7 for mHVA1E1 and 1908 ± 101 for HVA1E1, n = 3) under standard assay conditions. To see whether the relative binding activity between a high- and moderate-affinity sequence motif is affected by the amount of HvCBF1–CelD in the reaction, the relative binding affinity of HVA1E1 and its mutant (Mut7) was measured. As shown in Figure 3C, the HvCBF1–CelD levels in the range of 3.4–13.6 µg protein from the crude extract did not significantly alter the relative binding affinity between a high-affinity motif (HVA1E1) and a moderate-affinity motif (Mut7). But at the very low level (0.85 µg crude protein) of HvCBF1–CelD, the ratio of the binding activity of the moderate- to high-affinity motif was reduced. The linearity of cellulase assay of the HvCBF1–CelD protein bound to Dynabeads Streptavidin-immobilised HVA1E1 against incubation time is shown in Figure 3D. The amounts of the fluorescent 4-methylubiliferyl production linearly increased up to 5 h when a low level of the HvCBF1–CelD was used in the assay, indicating that bound HvCBF1–CelD was stable for at least 5 h under the assay conditions. At a higher level of HvCBF1–CelD, the presence of a high level of products or a significant reduction of the substrate concentration may become a factor limiting the linearity of enzyme reaction at longer incubation times. Although 1-h incubation is more than sufficient for enzyme assay of a DBP–CelD interacting with a high-affinity motif, longer incubation times are required for a low-affinity motif.
Figure 3.
Influence of amounts of probe and HvCBF1–CelD protein on the binding activity and linearity of the cellulase assay. Each point or bar is the mean value of duplicate assays using Dynabeads Streptavidin and is expressed as relative binding activity measured as fluorescence units generated by bound HvCBF1–CelD. (A) Varying amounts of the biotin-labelled HVA1E1 probe using 13.6 µg of crude HvCBF1–CelD protein. (B) Varying amounts of the HvCBF1–CelD protein using 6 pmol of the HVA1E1 probe. (C) Effects of HvCBF1–CelD levels on the relative binding activity between high (HVA1E1)- and moderate (Mut7)-affinity motifs (see Fig. 5 for the sequence of Mut7). The binding activity ratio of the Mut7 to HVA1E1 at each HvCBF1–CelD level is expressed as a percentage. (D) Linearity of cellulase assay on HvCBF1–CelD activity bound to HVA1E1 that was immobilised on Dynabeads Streptavidin against various incubation times. Aliquots of 0.85 or 6.8 µg of crude HvCBF1–CelD were used in these assays.
When the assays were performed in StreptaWell High Bind (streptavidin-coated 96-well microtitre plate), approximately four times more biotin-labelled probes can be loaded into each well. The relative background activity was <1% of that of HVA1E1 (fluorescence units per hour were 23 ± 2 for mHVA1E1 and 4071 ± 211 for HVA1E1, n = 3). This makes the assay more sensitive and easier than using Dynabeads Streptavidin when handling a large number of samples.
Parameters affecting the binding activity of HvCBF1
The binding activity of many DBPs may be affected by some physical and chemical parameters, such as non-ionic detergent, pH, salt and temperature. In electrophoretic mobility shift assays, assessing these parameters requires maintenance of assay conditions in both the DNA-binding reaction and subsequent gel electrophoresis. Some of these parameters are solely used to optimise the conditions for protein–DNA interaction and stability of protein–DNA complexes. Others may have physiological importance in determining the function of a transcriptional activator or repressor.
The potential influence of these parameters on the DNA-binding activity of HvCBF1 was investigated. Addition of Nonidet P-40 at 0.05% (v/v) in the DNA-binding buffer was found to double the binding activity of HvCBF1–CelD, compared with the Nonidet P-40 omitted buffer. It did not alter the relative binding affinity of HVA1E1 (high-affinity motif) and Mut7 (moderate-affinity motif) (data not shown). Therefore, Nonidet P-40 was included in the DNA-binding reaction to enhance the sensitivity of the assay.
The influence of pH in the binding activity of HvCBF1 is shown in Figure 4A. The binding activity was highest at pH 7.0 and reduced by either lowering or raising the pH of the binding and washing buffers. The binding activity at pH 8.0 and 8.5 was 18 and 8% of that at pH 7.0, respectively. The stability of the HvCBF1–DNA complex was similarly affected by higher pH values in washing buffer (a total of three 10-min washings were used for studying the complex stability). The activity of HvCBF1 bound to HVA1E1 at pH 8.5 and 8.0 of washing buffers was 8 and 20% of that at pH 7.0 (data not shown). The effect of the washing buffer pH on the stability of the HvCBF1–DNA complex was much more pronounced with moderate-affinity (Mut7) and low-affinity (Mut15) motifs (see Fig. 5 for the sequences of Mut7 and Mut15). At pH 8.5 the activity of HvCBF1 bound to the Mut7 and Mut15 probes was not detected.
Figure 4.
Effects of pH, NaCl and temperature on the binding of HvCBF1–CelD to HVA1E1. Each point is the mean value of duplicate assays (A) or the mean value ± SD from triplicate assays (B and C). The values are expressed as relative binding activity measured as fluorescence units. (A) Effects of pH in binding/washing buffer. (B) Effects of NaCl in binding/washing buffer. The standard binding/washing buffer containing 50 mM KCl used in the standard binding assay was also included for comparison. (C) Effects of temperature. Both binding and washing steps were performed at the indicated temperatures.
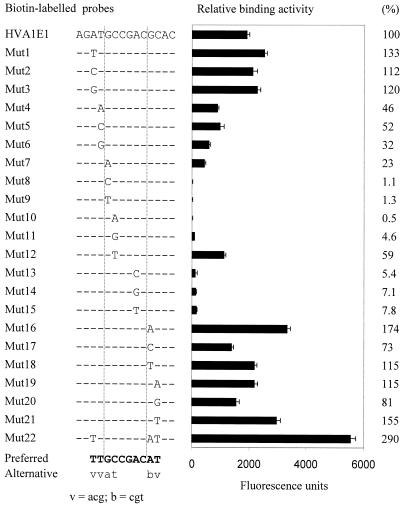
Figure 5.
Quantitative analysis of binding-sequence preference of HvCBF1. The sequences of biotin-labelled probes are shown at the left [only the mutated base(s) is shown in HVA1E1 mutants]. Relative binding activity of each probe is shown at the right and is expressed as fluorescence units and a percentage relative to HVA1E1. Values are the mean ± SD of triplicate assays. Preferred bases of the HvCBF1 binding site are in bold and alternative bases with less efficiency in lower-case letters.
Changes in NaCl concentrations also had a marked influence on the binding activity of HvCBF1 (Fig. 4B). HvCBF1 required salt (NaCl or KCl) for binding activity. The binding activity was highest at 50 mM and at this concentration KCl provided a higher binding activity than NaCl. Increase in the NaCl concentration dramatically reduced the binding activity. At 100 and 200 mM the binding activity was reduced to 41 and 7% of that at 50 mM, respectively. Virtually no activity was detected at 400 mM. Effects of temperature on the binding activity of HvCBF1 were also examined (Fig. 4C). The binding activity was highest at 20°C and a slight reduction was seen at 2 and 10°C. Raising temperature up to 35°C also resulted in a slight reduction in the binding activity. These results indicate that the binding activity of this transcription factor is not significantly affected by temperature within a climate range of barley growth.
A base-scanning approach for rapid identification of the conserved core-binding motif of HvCBF1
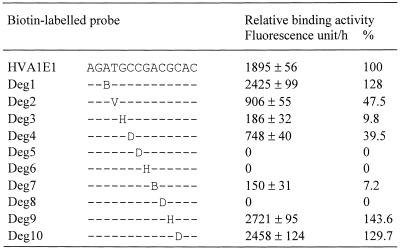
To identify the conserved core motif interacting with HvCBF1, a rapid base scanning at the ATGCCGACGC sequence in HVA1E1 was performed using degenerate oligonucleotides containing a base substitution mutation at each position with the other three alternative bases (Table 1). If the binding activity is detected in a degenerate oligonucleotide, it indicates that at least one of the other three alternative bases can be used to form a functional DNA-binding motif at the targeted base position. On the other hand, little or no binding activity of a degenerate oligonucleotide suggests that the mutated base position is highly conserved and cannot be replaced with any other bases. This approach would significantly reduce the number of oligonucleotides required for identification of the conserved core motif. This is particularly useful for DBPs that interact with long sequence motifs.
Table 1. Use of degenerate oligonucleotide probes to scan the conserved bases of the binding motif in HVA1E1 interacting with HvCBF1.
Values are mean ± SD (n = 3) and are expressed as relative binding activity measured in fluorescence units generated by bound HvCBF1–CelD or percentages relative to HVA1E1. In degenerate oligonucleotide probes only degenerate bases are shown. V, AGC; H, ACT; B, CGT; D, AGT.
Table 1 shows that binding activity of degenerate oligonucleotides at the C3, G4 or C6 position of the A–2T–1G1C2C3G4A5C6G7C8 motif (labelling for convenience of description) completely abolished the binding activity, indicating that these three base positions are strictly conserved. A >10-fold reduction in the binding activity was observed in degenerate oligonucleotides containing base mutation at the G1 or A5 position. A 40–50% reduction was also seen in degenerate oligonucleotides at the T–1 and C2 positions. In contrast, there was a significant increase seen in degenerate oligonuceotides at the A–2, G7 and C8 positions. These results indicate that this rapid base-scanning approach is effective in identification of the conserved core motif. The quantitative data also provide indicative information for further investigation on base positions surrounding the conserved core motif that may influence the binding affinity.
Analysis of the DNA-binding sequence preference of HvCBF1
To investigate the base preference at the position where strict base conservation was not observed in the above rapid base-scanning study, a detailed base substitution study at these positions was performed (Fig. 5). The base substitution analysis revealed that the preferred base at the G1 position is G with an alternative base A. However, the binding affinity of the A was approximately five times lower than the G at this position. The substitution with a pyrimidine base C or T at the G1 position resulted in a reduction in the binding affinity by 50-fold or more. In contrast, at the C2 position the pyrimidine bases C and T exhibited 10–20 times higher binding activity than purine bases. The base C was the preferred base and T was an alternative base, being 59% of the binding activity of the base C at this position. At the A5 position, A was a conserved base, although low binding activity (<8%) was observed in mutants substituted with one of other three bases. These results indicate that the C3G4A5C6 sequence is the conserved core motif and together with the semi-conserved (G/a)1(C/t)2 sequence to form a functional motif for HvCBF1. Base preference at the outside of the (G/a)1(C/t)2C3G4A5C6 motif was also observed. The bases providing a significantly higher binding activity than other bases at the A–2, T–1, G7 and C8 were T, T, A and T, respectively. Therefore, the preferred sequence of the HvCBF1 binding site was TTGCCGACAT (Fig. 5).
Quantitative analysis of binding sequence selectivity leads to the design of a high-affinity motif for constructing efficient promoters for a given transcription factor and its closely related factors. Use of a combination of the preferred binding bases identified through base substitution analysis to design an ideal motif (Mut22) resulted in three times higher binding affinity than the original HVA1E1 motif (Fig. 5).
Sequence competition analysis for comparison of the binding affinity of different sequence motifs
Sequence competition analysis is often associated with the electrophoretic mobility shift assay to eliminate the possibility of observed mobility shift due to non-specific DBPs in samples. This is because the binding activity is measured from retarded probe bands. In this method non-specific DNA binding from E.coli proteins does not confuse the binding activity of a DBP–CelD, as the cellulase activity of the probe-bound fusion protein was used for the measurement of the binding activity. However, the competition assay provides another means to compare the binding affinity of different sequence motifs. Three oligonucleotides containing the following motifs, GCCGAC (HVA1E1), ACCGAC (Mut7) and GTCGAC (Mut12), were used in the competition assay (Fig. 6). The GCCGAC motif in HVA1E1 was the most efficient motif for reduction of the binding of HvCBF1 to the biotin-labelled HVA1E1 and Mut7 probes, followed by the GTCGAC motif. The ACCGAC motif in Mut7 was the lowest affinity motif. These results are in good agreement with data using simple DNA-binding assays shown in Figure 5.
Figure 6.
Competition assays. Each bar is the mean value of duplicate assays and is expressed as relative binding activity measured in fluorescence units. An unlabelled HVA1E1, Mut12 or Mut7 competitor was added at 2- or 10-fold of the amount of an immobilised biotin-labelled HVA1E1 or Mut7 probe (3 pmol) to the binding reaction with 3.4 µg of the crude HvCBF1–CelD protein. The binding reaction without a competitor was used as control.
Correlation between the DNA-binding affinity and transactivation activity of HvCBF1
To evaluate whether the data on in vitro DNA-binding affinity correlate with transactivation activity of HvCBF1 in plant cells, HVA1E1 and some of its mutants were linked to a minimal promoter to drive the expression of a xylanase reporter gene (Fig. 7). HvCBF1 cDNA was under the control of a constitutive promoter (Fig. 7) and was co-introduced into barley with a reporter gene. HvCBF1 was capable of activating expression of HVA1E1 and many of its mutant-driven reporter genes (Table 2). Transactivation activity of these oligonucleotide-driven reporter genes was positively correlated with the binding affinity (Table 2) with a correlation coefficient of 0.99. However, it appears that low, but detectable, transactivation activity was observed in the HVA1E1 mutants which had no DNA-binding activity, indicating that the DNA-binding assay is more stringent than the transactivation assay. This is likely due to the multiple washing step involved in the DNA-binding method, which removes HvCBF1 from its weak interaction with very low-affinity motifs.
DISCUSSION
A novel enzymatic method was developed for rapid, quantitative and high-throughput analysis of the binding activity of putative DBPs and their binding sites. The method employed the translational fusion of a DBP to a highly active CelD enzyme capable of hydrolysing MUC to release a fluorescent 4-methylumbiliferyl product for sensitive measurement of DNA-binding activity. The catalytic domain of CelD exhibited a modular structure and fusion to a DBP at either the N-terminal or C-terminal location did not interfere with its enzyme activity. The DNA-binding domain also has a modular structure and the fusion of barley HvCBF1 to CelD at either location does not interfere with its capability to bind to its target DNA sequence. Recently, I have made another CelD fusion construct with a low-temperature-activated transcription factor isolated from barley (GenBank accession no. AF442489). Both the CelD activity and the DNA-binding capability of this fusion protein were maintained (G.-P. Xue, unpublished results). However, it is possible that some DBPs may fail to be produced in E.coli in an active form; this problem to some extent may be overcome by use of a yeast expression system.
In comparison with the most widely used electrophoretic mobility shift assay (11), the present method has the following advantages: it is more rapid, can be performed in a high-throughput 96-well plate format, has no requirement for purification of DBP, has no involvement of radioactivity, gives quantification of binding activity with high accuracy, allows easy optimisation of conditions for the stability of DNA–protein complexes, and provides a convenient means to study effects of environmental parameters on the binding properties of DBPs.
For analysis of the DNA-binding activity of heteromeric DBPs, only one subunit of a heteromeric protein is required to fuse with CelD. Dimerisation or multimerisation of subunits may be achieved in vitro by mixing individual subunit preparations. In the case of a dimeric protein, effects of heterodimerisation on the binding affinity of a target motif can be observed by analysis of the DNA-binding activity of a CelD-fused subunit with and without addition of an equimolar (or over) amount of another subunit under the oligonucleotide probe excess conditions, if a significant proportion of the CelD-fused subunit transforms from a monomer or homodimer state to a heterodimer. The heteromeric DBP can also be prepared by co-expression of two subunits in the same host cell if the proper heteromerisation of two subunits in vitro is not achieved. In addition, formation of heteromeric complexes using the CelD-fusion system can be conveniently verified by comparison of zymographic patterns in a native polyacrylamide gel. Protein bands in the gel containing CelD activity after completion of electrophoresis and washing in the CelD activity assay buffer can be easily detected by placing the gel on a 1.2% agarose gel containing its substrate, such as lichenan (0.1% w/v), CM-cellulose (0.2% w/v) or MUC (1 mM). It is most likely that the addition of another subunit to the CelD-fused subunit would result in formation of an extra protein band containing CelD activity. If dimerisation of two subunits requires the presence of a target motif, the motif-containing oligonucleotide can be added to samples before electrophoresis.
The present method could be easily adapted for automated analysis in a 96-well plate format using an automated robotic system. Similar to the electrophoretic mobility shift assay, this method can, in principle, be adapted to analysis of the binding activity of RNA-binding proteins using a biotinylated RNA probe or the study of proteins that interact with DBPs. In the latter case, the associating proteins, not the DBPs, will be fused to CelD. Another potential extension of this method is to determine the consensus binding sequence for a DBP of which the binding motif is completely unknown. This can be done by using the cellulose-binding capability of the DBP–CelD fusion protein for enrichment of a DBP–CelD bound target sequence from a highly degenerate biotin-labelled oligonucleotide. The cellulose-bound DBP–CelD carrying the target motifs can be eluted in a buffer containing cellobiose at 40°C (G.-P. Xue, unpublished results). It is also likely that the elution of the target motifs from protein–DNA complexes can be conveniently achieved by use of the biotin-probe immobilisation buffer that contains 1 M NaCl, as no detectable binding of HvCBF1–CelD was observed at 400 mM NaCl. The enriched target motifs in the eluate are then captured by streptavidin on a solid support as described in this method and amplified by PCR.
This method provides a rapid means to define the binding sequence for a given DBP with accurate and quantitative information. The accurate data on binding sequence are essential for reliable identification of candidate genes in a given signal transduction pathway from genome sequence databases. A base-scanning approach using degenerate oligonucleotides described herein significantly simplified the identification of core-binding motifs. Besides the identification of the core motif, quantitative data generated through the base scanning also enabled the identification of the surrounding bases that influence the binding affinity. This indicative information is useful for subsequent analysis of base preference of the binding motif. The rapid, high-throughput, accurate and quantitative features of this method would also expect to promote analysis of amino acid residues of a DBP involved in sequence-specific binding and facilitate the selection for potent mutant DBPs with an improved binding affinity through mutagenesis at the DNA-binding domain.
In this study, this new method was applied to a systematic analysis of the DNA-binding profile of barley HvCBF1 that contains an AP2 DNA-binding domain. HvCBF1 shares 48.7 and 51.3% of an overall amino acid sequence identity with that of Arabidopsis CBF1 and DREB1A, respectively (3–5). However, the AP2 domain of HvCBF1 is highly homologous to that of Arabidopsis CBF/DREB1 proteins (81 and 85% amino acid sequence identity with Arabidopsis CBF1 and DREB1A, respectively).
This study decoded the binding sequence of HvCBF1. The highly conserved core motif for HvCBF1 revealed in the base-scanning study was CGAC. The GC bases immediately upstream of this CGAC core motif in HVA1E1 were semi-conserved and can be considered as a part of the core motif. Thus, the extended core motif is (G/a)(C/t)CGAC, where the lower-case letters represent less efficient bases. The relative binding affinity of alternative bases at the (G/a) and (C/t) positions was confirmed by competition analysis. This six-base motif is sufficient to constitute a functional motif with a significant binding affinity for HvCBF1. However, the bases surrounding this core motif also have a significant influence on the binding affinity, indicating possible involvement in interaction with HvCBF1. The preferred base composition of a 10-bp motif is TTGCCGACAT. The binding affinity of this preferred motif was approximately three times higher than that of the wild-type HVA1E1. This indicates that each base in this 10-bp motif additively contributes to the binding affinity. The in vitro DNA-binding affinity data appear to correlate well with relative transactivation activity of HVA1E1 and its mutant motifs analysed in vivo.
After the completion of this work, a study on the DNA-binding specificity of Arabidopsis DBRB1A using the electrophoretic mobility shift assay was published (20). HvCBF1 and DREB1A share 85% amino acid identity in their DNA-binding domain. The sequence preference of the HvCBF1 binding in the CGAC positions of the HVA1E1 GCCGAC motif is similar to that of DREB1A. However, quantitative differences in some other base positions exist. DREB1A exhibits an equal binding affinity for purine bases (A and G) at the first position of the (g/a)(c/t)CGAC motif and a significant binding activity on pyrimidine bases (C and T), being ∼50% activity of the purine bases. At the second position the base T exhibits a much lower binding affinity for DREB1A than that for HvCBF1. Thus, the core binding motif for Arabidopsis DREB1A is CCGAC. There also exist quantitative differences between HvCBF1 and DREB1A in base preference surrounding the core binding motif. For example, the base composition immediately upstream of the (g/a)(c/t)CGAC motif does not affect the binding affinity of DREB1A, whereas for HvCBF1 the base T exhibited 2–3-fold higher binding activity than the other bases. These quantitative differences in the binding sequence specificity between HvCBF1 and DREB1A may be attributed to some sequence differences in their AP2 domain. However, it is also likely that some differences are due to the different methods used. Quantitative values for the binding sequence specificity of DREB1A appear to be derived from a single electrophoretic mobility shift assay (20), which is due to the low throughput of the method.
Another important aspect of this enzymatic method is its flexible assay system for investigating effects of environmental stress on the DNA-binding activity. In order to predict the potential impact of the stress on gene expression controlled by HvCBF1, effects of temperature and salt on HvCBF1 binding to its target motif were examined in this study. The DNA-binding activity of HvCBF1 was not significantly affected by temperature within a climate range of barley growth. However, NaCl concentrations of 100 mM and above led to a marked reduction in the DNA-binding activity of HvCBF1. NaCl levels in the leaf cells of barley can exceed 200 mM in plants grown under moderate and severe saline soil conditions (21,22), although the nuclear NaCl concentration of these salt-stressed cells is unknown. It is possible that the efficiency of HvCBF1 in activation of cold-responsive gene expression under saline conditions might be significantly impaired. The method presented herein offers flexible assay conditions to accommodate various physical and chemical parameters for determining the potential influence of environmental factors on the function of DBPs including temperature-sensitive microbial repressors.
One major concern of the enzymatic method is its sensitivity in detection of low-affinity DNA–DBP complexes. It is known that the electrophoretic mobility shift assay is highly sensitive and capable of detection of DNA–DBP complexes in the gel at the level of less than a femtomole amount if a high specific activity of a 32P-labelled probe is used. The sensitivity of the enzymatic method is lower than that of the mobility shift assay in this respect. However, the assay sensitivity in terms of the detection range of relative binding affinity demonstrated in this method was three orders of magnitude (Fig. 5). For a DBP with its DNA-binding affinity higher than that of HvCBF1, the detection range would be even greater. Furthermore, the sensitivity of the enzymatic method can further increase through improvement of the specific activity of the reporter enzyme in the future. In examination of the DNA-binding data of Arabidopsis DREB1A, which is highly homologous to HvCBF1 in their DNA-binding domain, obtained with the electrophoretic mobility shift assay using 32P-labelled probes (20), it showed that little or no binding of Arabidopsis DREB1A to many of the single-base substitution mutants at the CGAC positions of the CCGAC motif was detected. This indicates that in the case of HvCBF1 the sensitivity of this enzymatic method is almost comparable with that of the electrophoretic mobility shift assay in terms of detection of low-affinity binding. Furthermore, data from a microarray study of 1300 genes in transgenic Arabidopsis carrying a CaMV 35S promoter-driven DREB1A showed that genes responsive to constitutive over-expression of DREB1A in the transgenic plant all have at least one CCGAC motif in their promoters, based on the promoter sequence analysis of 11 DREB1A-induced genes (23). This indicates that the single-base substitution mutant HVA1E1 motifs with little or no detectable binding activity for HvCBF1 are unlikely to have physiological significance in driving HvCBF1-mediated expression in plants. Hence, the sensitivity level of this enzymatic method is likely to be sufficient for functional analysis of transcription factors. Observation of low transactivation activity in mutant HVA1E1 constructs with low-affinity motifs is likely due to over-expression of HvCBF1 driven by the strong Dhn8s promoter and introduction of a very high copy number of the transgene in the transient expression system.
Acknowledgments
ACKNOWLEDGEMENTS
The author is grateful to Dr Frank Smith and Dr John Manners for their valuable suggestions and to Jennifer Johnson for her technical assistance.
DDBJ/EMBL/GenBank accession no. AF418204
REFERENCES
- 1.Thomashow M.F. (1999) Plant cold acclimation: freezing tolerance genes and regulatory mechanisms. Annu. Rev. Plant Physiol. Plant Mol. Biol., 50, 571–599. [DOI] [PubMed] [Google Scholar]
- 2.Shinozaki K. and Yamaguchi-Shinozaki,K. (2000) Molecular responses to dehydration and low temperature: differences and cross-talk between two stress signaling pathways. Curr. Opin. Plant Biol., 3, 217–223. [PubMed] [Google Scholar]
- 3.Stockinger E.J., Gilmour,S.J. and Thomashow,M.F. (1997) Arabidopsis thaliana CBF1 encodes an AP2 domain-containing transcriptional activator that binds to the C-repeat/DRE, a cis-acting DNA regulatory element that stimulates transcription in response to low temperature and water deficit. Proc. Natl Acad. Sci. USA, 94, 1035–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu Q., Kasuga,M., Sakuma,Y., Abe,H., Miura,S., Yamaguchi-Shinozaki,K. and Shinozaki,K. (1998) Two transcription factors, DREB1 and DREB2, with an EREBP/AP2 DNA binding domain separate two cellular signal transduction pathways in drought- and low-temperature-responsive gene expression, respectively, in Arabidopsis. Plant Cell, 10, 1391–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gilmour S.J., Zarka,D.G., Stockinger,E.J., Salazar,M.P., Houghton,J.M. and Thomashow,M.F. (1998) Low temperature regulation of the Arabidopsis CBF family of AP2 transcriptional activators as an early step in cold-induced COR gene expression. Plant J., 16, 433–442. [DOI] [PubMed] [Google Scholar]
- 6.Riechmann J.L. and Ratcliffe,O.J. (2000) A genomic perspective on plant transcription factors. Curr. Opin. Plant Biol., 3, 423–434. [DOI] [PubMed] [Google Scholar]
- 7.Tronche F., Ringeisen,F., Blumenfeld,M., Yaniv,M. and Pontoglio,M. (1997) Analysis of the distribution of binding sites for a tissue-specific transcription factor in the vertebrate genome. J. Mol. Biol., 266, 231–245. [DOI] [PubMed] [Google Scholar]
- 8.Roulet E., Bucher,P., Schneider,R., Wingender,E., Dusserre,Y., Werner,T. and Mermod,N. (2000) Experimental analysis and computer prediction of CTF/NFI transcription factor DNA binding sites. J. Mol. Biol., 297, 833–848. [DOI] [PubMed] [Google Scholar]
- 9.Fried M. and Crothers,D.M. (1981) Equilibria and kinetics of lac repressor–operator interactions by polyacrylamide gel electrophoresis. Nucleic Acids Res., 9, 6505–6525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garner M.M. and Revzin,A. (1981) A gel electrophoresis method for quantifying the binding of proteins to specific DNA regions: application to components of the Escherichia coli lactose operon regulatory system. Nucleic Acids Res., 9, 3047–3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roder K. and Schweizer,M. (2001) Running-buffer composition influences DNA–protein and protein–protein complexes detected by electrophoretic mobility-shift assay (EMSA). Biotechnol. Appl. Biochem., 33, 209–214. [DOI] [PubMed] [Google Scholar]
- 12.Xue G.P., Johnson,J.S., Smyth,D.J., Dierens,L.M., Wong,X., Simpson,G.D., Gobius,K.S. and Aylward,J.H. (1996) Temperature-regulated expression of the tac/lacI system for overproduction of a fungal xylanase in Escherichia coli. Appl. Microbiol. Biotechnol., 45, 120–126. [DOI] [PubMed] [Google Scholar]
- 13.Rosenberg M., Ho,Y.-S. and Shatzman,A. (1983) The use of pKC30 and its derivative for controlled expression of genes. Methods Enzymol., 101, 123–155. [DOI] [PubMed] [Google Scholar]
- 14.Xue G.P., Gobius,K.S. and Orpin,C.G. (1992) A novel polysaccharide hydrolase cDNA (celD) from Neocallimastix patriciarum encoding three multi-functional catalytic domains with high endoglucanase, cellobiohydrolase and xylanase activities. J. Gen. Microbiol., 138, 2397–2403. [DOI] [PubMed] [Google Scholar]
- 15.Choi D.-W., Zhu,B. and Close,T.J. (1999) The barley (Hordeum vulgare L.) dehydrin multigene family: sequences, allele types, chromosome assignments, and expression characteristics of 11 Dhn genes of cv Dicktoo. Theor. Appl. Genet., 98, 1234–1247. [Google Scholar]
- 16.Xiao F.H. and Xue,G.P. (2001) Analysis of promoter activity of late embryogenesis abundant protein genes in barley seedlings under conditions of water deficit. Plant Cell Rep., 20, 667–673. [Google Scholar]
- 17.Xue G.P., Patel,M., Johnson,J.S., Elliott,A.R. and Ealing,P.M. (1998) Development of a gene expression system for the efficient production of recombinant proteins in barley endosperm during maturation. In Proceedings of the 4th Asia-Pacific Conference on Agricultural Biotechnology. UTC Publishing, Canberra, Australia, pp. 240–242.
- 18.Patel M., Johnson,J.S., Brettell,R.I.S., Jacobsen,J. and Xue,G.P. (2000) Transgenic barley expressing a fungal xylanase gene in the endosperm of the developing grains. Mol. Breeding, 6, 113–123. [Google Scholar]
- 19.Jefferson R.A. (1987) Assaying chimeric genes in plants: the Gus gene fusion system. Plant Mol. Biol. Rep., 5, 387–405. [Google Scholar]
- 20.Sakuma Y., Liu,Q., Dubouzet,J.G., Abe,H., Shinozaki,K. and Yamaguchi-Shinozaki,K. (2002) DNA-binding specificity of the ERF/AP2 domain of Arabidopsis DREBs, transcription factors involved in dehydration- and cold-inducible gene expression. Biochem. Biophys. Res. Commun., 290, 998–1009. [DOI] [PubMed] [Google Scholar]
- 21.Fricke W., Leigh,R.A. and Tomos,A.D. (1996) The intercellular distribution of vacuolar solutes in the epidermis and mesophyll of barley leaves changes in response to NaCl. J. Exp. Botany, 47, 1413–1426. [Google Scholar]
- 22.Flowers T.J. and Hajibagheri,M.A. (2001) Salinity tolerance in Hordeum vulgare: ion concentrations in root cells of cultivars differing in salt tolerance. Plant Soil, 231, 1–9. [Google Scholar]
- 23.Seki M., Narusaka,M., Abe,H., Kasuga,M., Yamaguchi-Shinozaki,K., Carninci,P., Hayashizaki,Y. and Shinozaki,K. (2001) Monitoring the expression pattern of 1300 Arabidopsis genes under drought and cold stresses by using a full-length cDNA microarray. Plant Cell, 13, 61–72. [DOI] [PMC free article] [PubMed] [Google Scholar]