Diabetic foot ulcers can be divided into two groups: those in neuropathic feet (so called neuropathic ulcers) and those in feet with ischaemia often associated with neuropathy (so called neuroischaemic ulcers). The neuropathic foot is warm and well perfused with palpable pulses; sweating is diminished, and the skin may be dry and prone to fissuring. The neuroischaemic foot is a cool, pulseless foot; the skin is thin, shiny, and without hair. There is also atrophy of the subcutaneous tissue, and intermittent claudication and rest pain may be absent because of neuropathy.
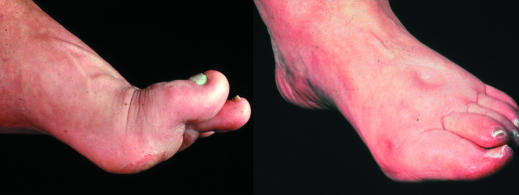
Figure 1.
Left: Neuropathic foot with prominent metatarsal heads and pressure points over the plantar forefoot. Right: Neuroischaemic foot showing pitting oedema secondary to cardiac failure, and hallux valgus and erythema from pressure from tight shoe on medial aspect of first metatarsophalangeal joint
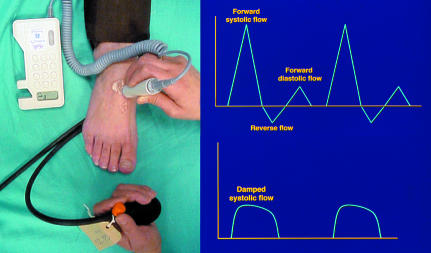
The crucial difference between the two types of feet is the absence or presence of ischaemia. The presence of ischaemia may be confirmed by a pressure index (ankle brachial pressure index < 1). As many diabetic patients have medial arterial calcification, giving an artificially raised ankle systolic pressure, it is also important to examine the Doppler arterial waveform. The normal waveform is pulsatile with a positive forward flow in systole followed by a short reverse flow and a further forward flow in diastole, but in the presence of arterial narrowing the waveform shows a reduced forward flow and is described as “damped.”
Figure 2.
Left: Hand held Doppler used with sphygmomanometer to measure ankle systolic pressure. Right: Doppler waveform from normal foot showing normal triphasic pattern (top) and from neuroischaemic foot showing damped pattern (bottom)
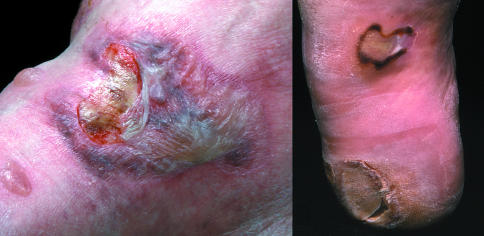
Neuropathic foot ulcer
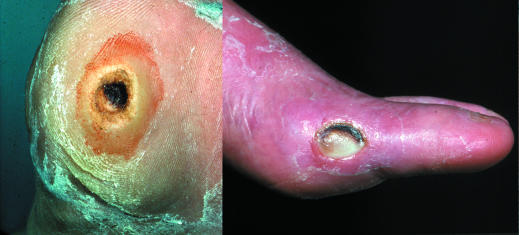
Figure 3.
Left: Neuropathic foot with plantar ulcer surrounded by callus. Right: Ulcer over medial aspect of first metatarsophalangeal joint of neuroischaemic foot
Neuropathic ulcers usually occur on the plantar aspect of the foot under the metatarsal heads or on the plantar aspects of the toes.
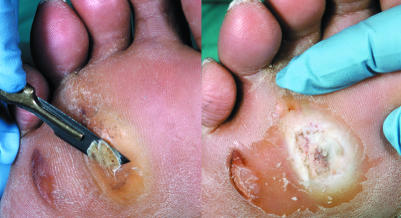
Figure 4.
Left: Callus removal by sharp debridement. Right: Whitish, macerated, moist tissue under surface of callus, indicating imminent ulceration
The most common cause of ulceration is repetitive mechanical forces of gait, which lead to callus, the most important preulcerative lesion in the neuropathic foot. If allowed to become too thick, the callus will press on the soft tissues underneath and cause ulceration. A layer of whitish, macerated, moist tissue found under the surface of the callus indicates that the foot is close to ulceration, and urgent removal of the callus is necessary. If the callus is not removed, inflammatory autolysis and haematomas develop under the callus. This leads to tissue necrosis, resulting in a small cavity filled with serous fluid giving the appearance of a blister under the callus. Removal of the callus reveals an ulcer.
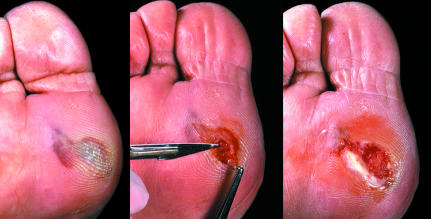
Figure 5.
Left: Blister under a callus over first metatarsal head. Centre: The roof of the blister is grasped in forceps and cut away, together with associated callus. Right: Ulcer is revealed underneath
This is the third in a series of 12 articles
A foot ulcer is a sign of systemic disease and should never be regarded as trivial
Neuroischaemic foot ulcer
Neuroischaemic ulcers are often seen on the margins of the foot, especially on the medial surface of the first metatarsophalangeal joint and over the lateral aspect of the fifth metatarsophalangeal joint. They also develop on the tips of the toes and beneath any toe nails if these become overly thick.
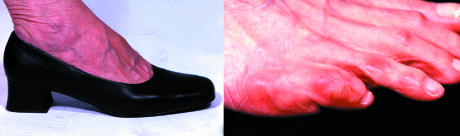
Figure 6.
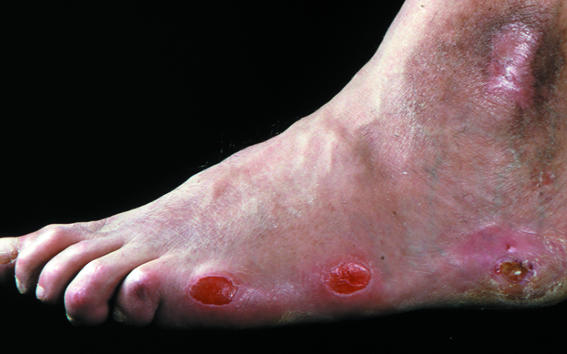
Top: Shoe with no proper fastening and with a narrow toe box (left); red marks on toes after wearing unsuitable shoes (right). Left: New ischaemic ulcers resulting from bullae on lateral margin of foot
The classic sign of preulceration in the neuroischaemic foot is a red mark on the skin, often precipitated by tight shoes or a slip-on shoe, leading to frictional forces on the vulnerable margins of the foot.
The first sign of ischaemic ulceration is a superficial blister, usually secondary to friction. It then develops into a shallow ulcer with a base of sparse pale granulation tissue or yellowish closely adherent slough.
Management
Wound control
In the neuropathic foot, all callus surrounding the ulcer is removed with a scalpel, together with slough and non-viable tissue. It is always important to probe the ulcer as this may reveal a sinus extending to bone (suggesting osteomyelitis) or undermining of the edges where the probe can be passed from the ulcer underneath surrounding intact skin.
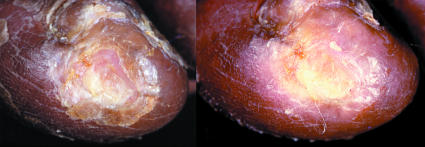
Figure 7.
Left: Ischaemic ulcer with halo of thin glassy callus. Right: The halo has been cut away without causing trauma
In the neuroischaemic foot, slough and dried necrotic material should be removed from the ulcer by sharp debridement. Debridement should be cautious if the foot is very ischaemic (pressure index < 0.5) as it is essential not to damage viable tissue.
Some ischaemic ulcers develop a halo of thin glassy callus that dries out, becomes hard, and curls up. These areas need to be smoothed off as they can catch on dressings and cause trauma to underlying tissue. If a subungual ulcer is suspected, the nail should be cut back very gently or layers of nail pared away, to expose and drain the ulcer. Maggot therapy is sometimes used in debridement, especially with neuroischaemic ulcers.
Vacuum assisted closure may be used to achieve closure of diabetic foot ulcers and wounds that have been debrided. This technique is increasingly used to treat postoperative wounds in a diabetic ischaemic foot, especially when revasularisation is not possible.
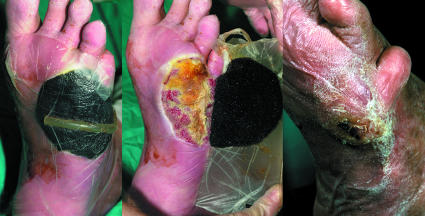
Figure 8.
Left: Vacuum assisted pump sponge attached to plantar aspect of foot. Centre: Pump sponge being removed from foot. Right: Healed wound
Mechanical control
In neuropathic feet the overall aim is to redistribute plantar pressures, wheareas in neuroischaemic feet it is to protect the vulnerable margins of the foot. Semicompressed adhesive felt padding may be used to divert pressure, especially from small ulcers in neuropathic feet. The most efficient way to redistribute plantar pressure is to use a total contact cast (treatment of choice for indolent neuropathic ulcers), a prefabricated cast such as Aircast, or a Scotchcast boot.
If casting techniques are not available, temporary shoes with a cushioning insole can be supplied. When the neuropathic ulcer has healed, the patient should be fitted with a cradled insole and bespoke shoes to prevent recurrence. Occasionally, extra-deep, “off the shelf” orthopaedic shoes with flat cushioning insoles may suffice in the absence of areas of very high pressure.
Figure 9.
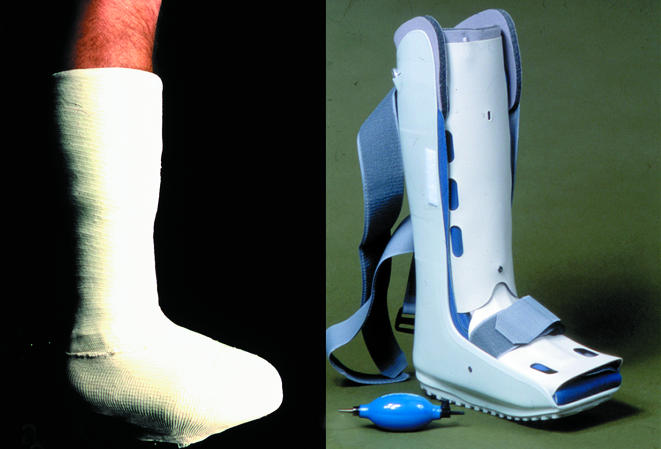
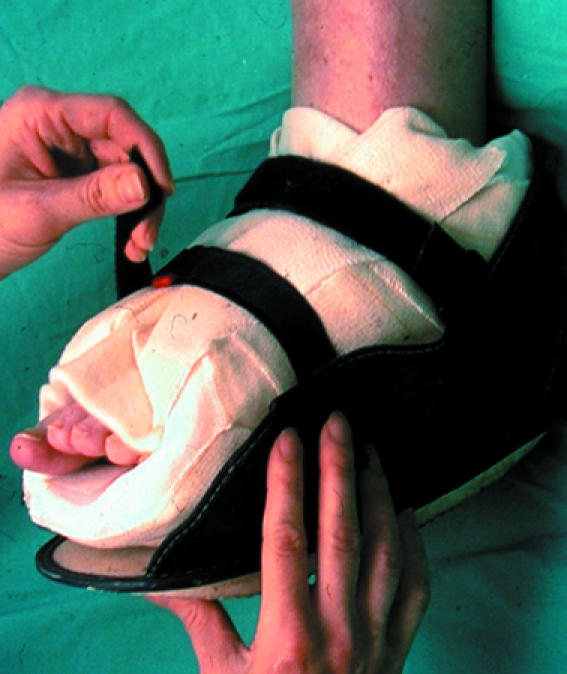

Top (left to right): Total contact cast; Aircast prefabricated cast; Scotchcast boot. Left: A suitable shoe bought in the high street may be sufficiently roomy to avoid pressure
As ulcers in neuroischaemic feet usually develop around the margins of the foot, a shoe bought from a high street shop may be adequate provided that the shoe is sufficiently long, broad, and deep and fastens with a lace or strap high on the foot. Alternatively, a Scotchcast boot or a wide-fitting, off the shelf shoe may be suitable.
The ulcer should be cleansed and dressed with an appropriate dressing (see ninth article in this series)
Pressure ulcer in the diabetic foot
All patients with neuropathic or neuroischaemic feet are at risk of pressure ulcers, especially of the heel. Pressure over heel ulcers can be off-loaded by “pressure relief ankle foot orthoses.” This orthosis is a ready-made device that has a washable fleece liner with an aluminium and polyproprylene adjustable frame and a non-slip, neoprene base for walking. It is used to relieve pressure over the posterior aspect of the heel and maintain the ankle joint in a suitable position, thus preventing pressure ulceration, aiding healing, and preventing deformity.
Figure 10.
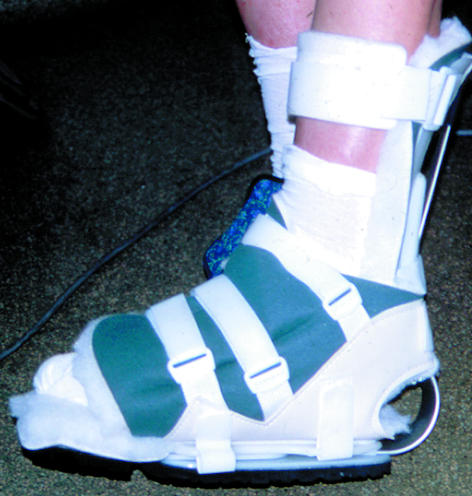
Pressure relief ankle/foot orthosis for use with heel ulcers
Vascular control
If an ischaemic ulcer has not shown progress in healing despite optimum treatment, then it may be possible to do duplex ultrasound and angiography. This should be done if any or all of the following are present:
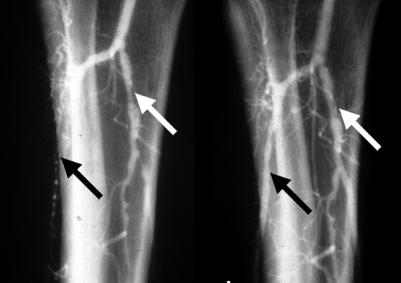
Figure 11.
Left: Angiogram showing occlusion of anterior tibial artery and stenosis of tibioperoneal trunk. Right: Post-angioplasty anterior tibial flow has been restored and tibioperoneal stenosis dilated
An ankle brachial pressure index of < 0.5 or a damped Doppler waveform
A transcutaneous oxygen (reflecting local arterial perfusion pressure) of < 30 mm Hg
A toe pressure of < 30 mm Hg.
Duplex ultrasound and angiography may show areas of stenoses or occlusions suitable for angioplasty. If lesions are too extensive for angioplasty, then arterial bypass may be considered.
Another manifestation of ischaemia is dry gangrene, particularly in a toe. Dry gangrene usually results from severe ischaemia secondary to poor tissue perfusion from atherosclerotic narrowing of the arteries of the leg. Ideally, the ischaemic foot should be revascularised and the digital necrosis be removed surgically, but if revascularisation is not possible, the gangrenous parts of the toes may be allowed to “autoamputate” (drop off naturally).
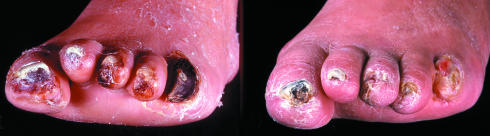
Figure 12.
Left: Necrotic fifth toe and necrotic apices of the first, third, and fourth toes undergoing podiatric debridement. Right: Autoamputation six weeks later, after regular debridement
Microbiological control
When an ulcer is present, there is a clear entrance for invading bacteria. Infection can range from local infection of the ulcer to wet gangrene. Only half of infection episodes show signs of infection. In the presence of neuropathy and ischaemia, the inflammatory response is impaired and early signs of infection may be subtle.
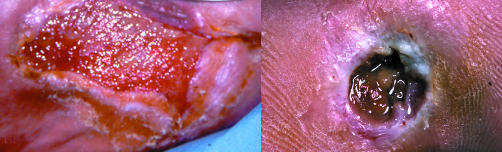
Figure 13.
Left: Increased friable granulation tissue. Right: Base of ulcer has areas of yellowish to grey tissue
Table 1.
Local signs of wound infection
| • Granulation tissue becomes increasingly friable |
| • Base of the ulcer becomes moist and changes from healthy pink granulations to yellowish or grey tissue |
| • Discharge changes from clear to purulent |
| • Unpleasant odour is present |
Deep swab and tissue samples (not surface callus) should be sent for culture without delay and wide spectrum antibiotics given to cover Gram positive, Gram negative, and anaerobic bacteria. Urgent surgical intervention is needed in certain circumstances.
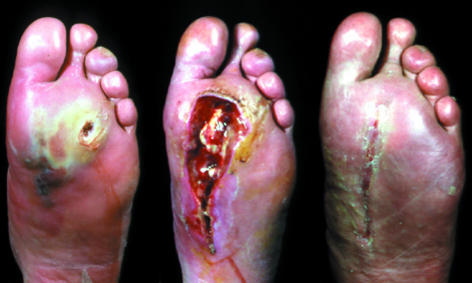
Figure 14.
Left: Deep ulcer with subcutaneous sloughing visible. Centre: Extent of debridement necessary to remove all necrotic tissue down to healthy bleeding tissue. Right: Wound has healed at 10 weeks
Table 2.
Indications for urgent surgical intervention
| • Large area of infected sloughy tissue |
| • Localised fluctuance and expression of pus |
| • Crepitus with gas in the soft tissues on x ray examination |
| • Purplish discoloration of the skin, indicating subcutaneous necrosis |
In neuropathic feet, gangrene is almost invariably wet and is caused by infection of a digital, metatarsal, or heel ulcer that leads to a septic vasculitis of the digital and small arteries of the foot. The walls of these arteries are infiltrated by polymorphs, leading to occlusion of the lumen by septic thrombus. Wet gangrene may need surgical intervention.
Figure 15.
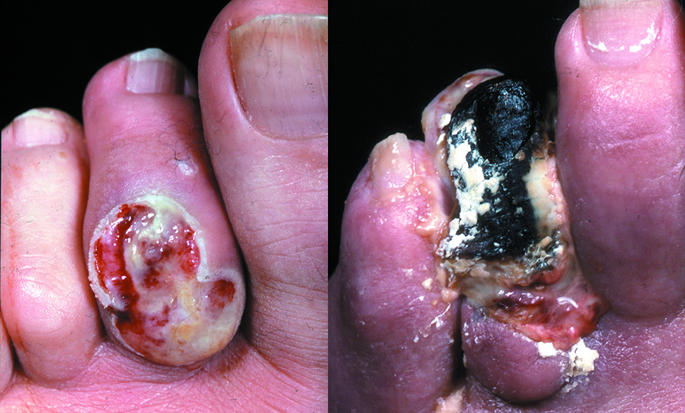
Left: Vein bypass seen passing across ankle to the dorsalis pedis artery. Centre: Infected ulcer with cellulitis. Right: Wet necrosis from infected toe ulcer
Wet gangrene caused by septic vasculitis can also occur in neuroischaemic feet, although reduced arterial perfusion due to atherosclerotic occlusive disease is an important predisposing factor. Gangrenous tissue should be surgically removed and the foot revascularised if possible.
Figure 16.
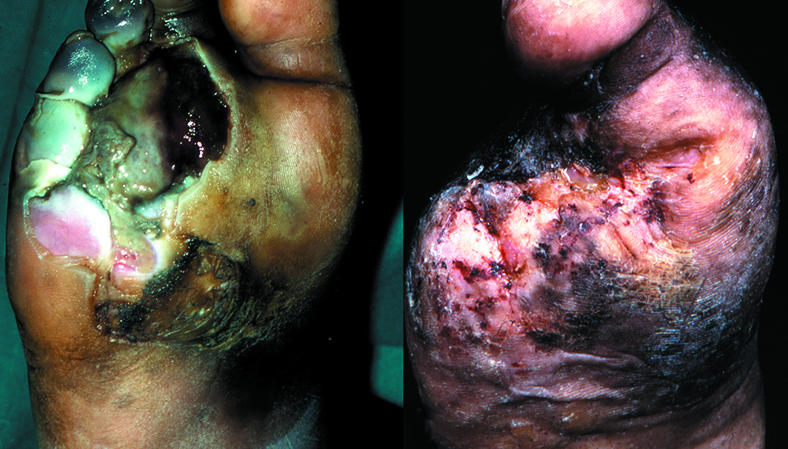
Left: Plantar view of infection after puncture wound that led to wet necrosis of the forefoot requiring amputation of four toes and their adjoining metatarsal heads. Right: Full healing of the large post-surgical tissue defect took six months
Metabolic control
Wound healing and neutrophil function is impaired by hyperglycaemia, so tight glycaemic control is essential. Patients with type 2 diabetes suboptimally controlled with oral hypoglycaemic drugs should be prescribed insulin. Hyperlipidaemia and hypertension should be treated. Patients should stop smoking. Those with neuroischaemic ulcers should take statins and antiplatelets. Diabetic patients with peripheral vascular disease may also benefit from an angiotensin converting enzyme inhibitor to prevent further vascular episodes.
Figure 17.
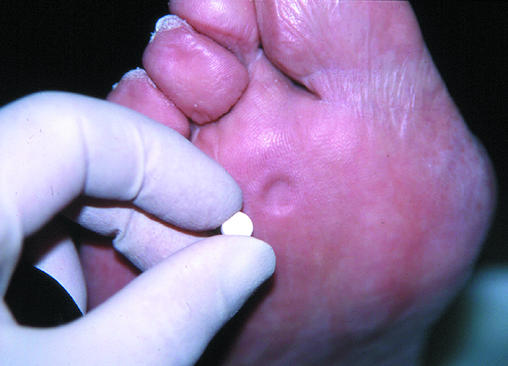
Oral hypoglycaemic agent found within the patient's shoe at annual review
Table 3.
Members of the multidisciplinary team
| • Physician | • Dietitian |
| • Podiatrist | • Radiologist |
| • Specialist nurse | • Vascular surgeon |
| • Orthotist | • Orthopaedic surgeon |
Education
Patients who have lost protective pain sensation need advice on how to protect their feet from mechanical, thermal, and chemical trauma. Patients should be instructed on the principles of ulcer care with emphasis on the importance of rest, footwear, regular dressings, and frequent observation for signs of infection. They should be taught the four danger signs: swelling, pain, colour change, and breaks in the skin.
Figure 18.
Left: Thermal trauma from convection heater. Right: Ulceration after use of foot spa
Successful management of diabetic feet requires the expertise of a multidisciplinary team that provides integrated care, rapid access clinics, early diagnosis, and prompt treatment. Patients will need close follow-up for the rest of their lives
The ABC of wound healing is edited by Joseph E Grey (jeg@petravore.freeserve.co.uk), consultant physician at the University Hospital of Wales, Cardiff, and honorary consultant in wound healing at the Wound Healing Research Unit, Cardiff University; and by Keith G Harding, director of the Wound Healing Research Unit, Cardiff University, and professor of rehabilitation medicine (wound healing) at Cardiff and Vale NHS Trust. The series will be published as a book in summer 2006.
Competing interests: For series editors' competing interests, see the first article in this series.
Further reading and resources
- • Edmonds M, Foster AVM, Sanders L. A practical manual of diabetic foot care. Oxford: Blackwell Science, 2004.
- • Bowker JH, Pfeifer MA, eds. Levin and O'Neal's the diabetic foot.6th ed. St Louis: Mosby, 2001.
- • Boulton AJM, Connor H, Cavanagh PR, eds. The foot in diabetes.3rd ed. Chichester: Wiley, 2000.
- • The International Working Group on the Diabetic Foot. International consensus on the diabetic foot. 2003 (www.iwgdf.org/concensus/introduction.htm)
- • Veves A, Giurini JM, Logerfo FW, eds. The diabetic foot. Medical and surgical management. Totowa, NJ: Humana Press, 2002.
- • National Institute for Clinical Excellence. Type 2 diabetes. Prevention and management of foot problems. London: NICE, 2004. (www.nice.org.uk/pdf/CG010NICEguideline.pdf)