Abstract
Although previous studies have identified several key transcription factors in the generation process of the vertebrate nervous system, the intracellular signalling pathways that function in this process have remained unclear. Here we identify the evolutionarily conserved mitogen-activated protein kinase kinase 5 (MEK5)–extracellular signal-regulated kinase 5 (ERK5) pathway as an essential regulator in neural differentiation. Knockdown of Xenopus ERK5 or Xenopus MEK5 with antisense morpholino oligonucleotides results in the reduced head structure and inhibition of neural differentiation. Moreover, forced activation of the MEK5–ERK5 module on its own induces neural differentiation. In addition, we show that the MEK5–ERK5 pathway is necessary for the neuralizing activity of SoxD, a regulator of neural differentiation, and is sufficient for the expression of Xngnr1, a proneural gene. These results show that the MEK5–ERK5 pathway has an essential role in the regulation of neural differentiation downstream of SoxD and upstream of Xngnr1.
Keywords: MAP kinase, neural development, signal transduction, Xenopus laevis
Introduction
During vertebrate embryogenesis, the neuroectoderm arises from uncommitted ectoderm. In Xenopus laevis, signals from the Spemann organizer induce neural tissues on the dorsal side of the ectoderm. The factors include Noggin, Chordin and Follistatin, which bind to bone morphogenetic protein-4 (BMP-4) in the extracellular space and inactivate BMP signalling (Lamb et al, 1993; Hemmati-Brivanlou et al, 1994; Sasai et al, 1995; Piccolo et al, 1996; Fainsod et al, 1997). As BMP signalling promotes epidermal differentiation and inhibits neural differentiation, the inhibition of BMP signalling by neural inducers promotes neural differentiation (Munozsanjuan & Brivanlou, 2002; De Robertis & Kuroda, 2004). Recent studies identified the factors that were regulated by neural inducers, including several transcription factors, such as basic helix–loop–helix (bHLH), Zic and Sox factors (Sasai, 1998). SoxD was identified as a gene induced by Chordin (Mizuseki et al, 1998). It is expressed widely in the ectoderm at the late blastula stages, and, after mid-gastrula stages, its expression is limited at the neuroectoderm. SoxD has a neuralizing activity, and its expression is enhanced by Zicr1, another mediator of neural differentiation. One of the genes functioning downstream of SoxD is Xngnr1, which is a member of the bHLH family. It is one of the earliest proneural genes and its transcript is detected in the neural plate at mid-gastrula stages (Ma et al, 1996). Several transcription factors have been identified, but the intracellular signalling pathways that regulate early neural development have not been fully defined.
The mitogen-activated protein (MAP) kinase pathways are among the conserved intracellular signalling pathways. So far, at least four independent MAP kinase pathways have been identified—the extracellular signal-regulated kinase (ERK) 1/2 pathway, the c-Jun amino-terminal kinase (JNK) pathway, the p38 pathway and the ERK5 pathway (Sturgill & Wu, 1991; Nishida & Gotoh, 1993; Robinson & Cobb, 1997; Davis, 2000; Kyriakis & Avruch, 2001). The ERK5 pathway is shown to be activated by oxidative stress, hyperosmolarity and growth factors, such as the nerve growth factor and epidermal growth factor (Abe et al, 1996; Kato et al, 1997, 1998; Kamakura et al, 1999). Mice that lack ERK5 or MAP kinase kinase 5 (MEK5), which is a direct activator of ERK5, are embryonic lethal and show cardiovascular defects (Regan et al, 2002; Sohn et al, 2002; Yan et al, 2003; Hayashi et al, 2004; Wang et al, 2005). However, the precise role of the MEK5–ERK5 module in embryonic developmental processes such as neural differentiation has not been fully understood.
In this study, we have identified the MEK5–ERK5 pathway as a novel signalling pathway that regulates neural differentiation. Our results show that activation of the MEK5–ERK5 pathway is necessary, and is sufficient, for neural differentiation in Xenopus early embryonic development. In addition, the MEK5–ERK5 pathway is required for SoxD-induced neural differentiation and is capable of inducing the expression of Xngnr1, suggesting that the MEK5–ERK5 pathway regulates neural differentiation downstream of SoxD and upstream of Xngnr1.
Results and Discussion
ERK5 and MEK5 are essential for neural differentiation
We isolated complementary DNAs encoding Xenopus orthologues of ERK5 (xERK5) and MEK5 (xMEK5) from the Xenopus gastrula cDNA library (supplementary Fig 1 online). The DDBJ/EMBL/GenBank accession numbers for xERK5 and xMEK5 are AB213560 and AB213561, respectively. While this work was in progress, Klein et al isolated cDNAs (unpublished; GenBank accession numbers BC077412 and AAH68926) that were almost identical to the cDNA clones that we had isolated. Coexpression of ERK5 and MEK5 in animal caps showed that xERK5 is a downstream target of xMEK5 and that the MEK5–ERK5 pathway is conserved among vertebrates (supplementary Fig 2 online). xERK5 and xMEK5 were expressed maternally and throughout early embryogenesis (supplementary Fig 3 online). Whole-mount in situ hybridization showed that xERK5 was expressed widely in the presumptive mesoderm and ectoderm during the blastula and gastrula stages, and in the head and dorsal structures during the neurula and tailbud stages (supplementary Fig 4 online). Reverse transcription–PCR (RT–PCR) analyses with microsectioning showed essentially the same results (data not shown). xMEK5 showed an expression pattern similar to that of xERK5 (data not shown).
To determine the loss-of-function phenotypes, we designed antisense morpholino oligonucleotides against xERK5 (ERK5 MO) or xMEK5 (MEK5 MO). The co-injection of MOs with Myc-tagged xERK5 or xMEK5 messenger RNA showed that ERK5 MO and MEK5 MO specifically reduced the protein level of xERK5 and xMEK5, respectively (Fig 1A). Then, we injected ERK5 MO, MEK5 MO or control MO into the dorsal marginal zones of four-cellstage embryos. Embryos injected with ERK5 MO or MEK5 MO were normal at the blastula and gastrula stages (data not shown). At the tailbud stages, embryos injected with ERK5 MO or MEK5 MO showed defects in the head region: a reduction or a complete loss of eyes and, in severe cases, loss of a cement gland. Embryos injected with control MO were normal (Fig 1B,C). We confirmed the specificity of ERK5 MO by rescue experiments with a mouse homologue of ERK5, mERK5, which was not downregulated by ERK5 MO (Fig 1A,D). In embryos injected with ERK5 MO, expression of a neural marker, N-tubulin, was reduced, especially in the head region (Fig 1E), whereas expression of a notochord gene, PA26 (Hikasa & Taira, 2001), was unaffected (Fig 1F). The result suggests that the phenotypes shown in Fig 1B,C are caused by defects in neural tissues. As previous studies have shown that ERK5 is important for cell survival in murine neural tissues (Liu et al, 2003; Yan et al, 2003), we tested the levels of cell death by TdT-mediated dUTP nick end labelling (TUNEL) assays. Embryos injected with ERK5 MO or MEK5 MO (supplementary Fig 5 online) did not show an increase of TUNEL-positive cells. This suggests that the defects in embryos that are injected with ERK5 MO or MEK5 MO are not caused by increased apoptosis.
Figure 1.
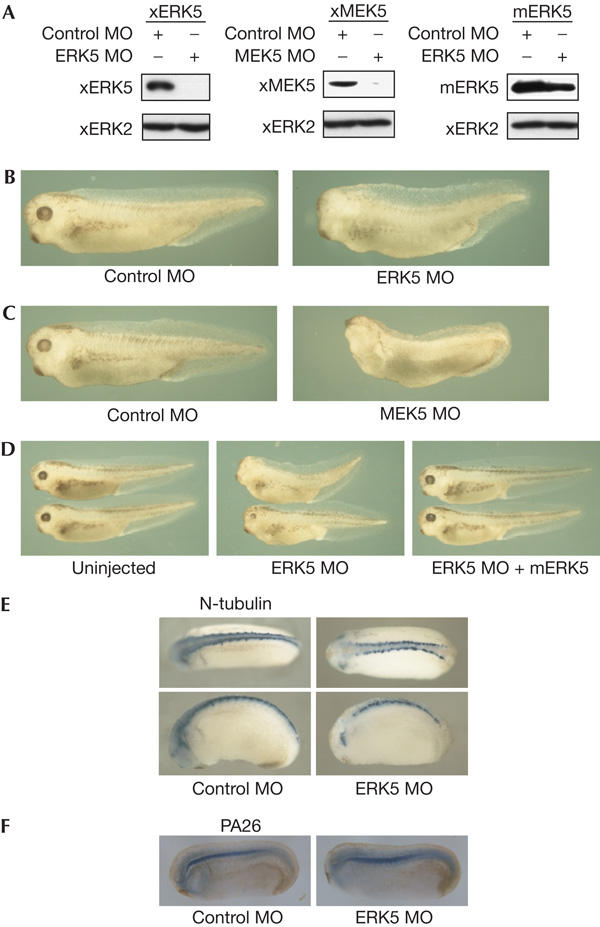
ERK5 and MEK5 are essential for neural development. (A) Indicated sets of morpholino oligonucleotides (MOs; 20 ng, left and right panels; 160 ng, middle panel) and messenger RNA (1.0 ng) were injected and the protein level was examined by immunoblotting with anti-Myc antibody (for Xenopus orthologues of ERK5 (xERK5; Myc–xERK5) and MEK5 (xMEK5; Myc–xMEK5)) or anti-ERK5 antibody (for mouse homologue of ERK5 (mERK5)). We used xERK2 as a loading control. (B) Control MO or ERK5 MO (20 ng) was injected into the dorsal marginal zone of four-cell-stage embryos. It was observed that 82% of embryos injected with ERK5 MO showed reduction or loss of eyes (n=60), whereas only 7% of embryos injected with control MO showed defects (n=85). (C) Control MO or MEK5 MO (200 ng) was injected as indicated in (B). It was observed that 72% of embryos injected with MEK5 MO showed reduction or loss of eyes (n=99), whereas only 12% of embryos injected with control MO showed defects (n=110). (D) mERK5 mRNA (0.7 ng) was co-injected with ERK5 MO (12 ng) for the rescue experiment. Only 11% of embryos that were injected with both ERK5 MO and mERK5 mRNA showed defects in the eyes (n=141), whereas 54% of embryos injected with ERK5 MO alone showed defects (n=81). (E,F) ERK5 MO or control MO (20 ng) was injected into the dorsal marginal zone and expression of N-tubulin or PA26 was examined by whole-mount in situ hybridization.
The MEK5–ERK5 pathway regulates neural differentiation
We then examined the role of the MEK5–ERK5 pathway in more detail. Animal caps treated with activin, a member of the transforming growth factor-β family, express various molecular markers. These include neural markers (N-tubulin, NCAM and NeuroD), mesodermal markers (MyoDa, muscle actin and collagen type 2) and an endodermal marker (endodermin; Fig 2A). ERK5 MO suppressed induction of neural markers caused by activin, whereas it had no effect on the expression of mesodermal or endodermal markers (Fig 2A). This result suggests that the MEK5–ERK5 pathway is required specifically for neural differentiation. However, activin is not a direct inducer of neural markers: it first induces mesoderm, and then neural inducers, which are expressed in the mesoderm, induce neural tissues. To confirm that ERK5 MO suppresses induction of neural markers with no effect on mesoderm induction, we used a dominant-negative form of BMP receptor (dnBMPR) instead of activin. dnBMPR inhibits BMP signalling and mimics the neuralizing activity of neural inducers. ERK5 MO or MEK5 MO suppressed the expression of neural markers induced by dnBMPR in animal caps (Fig 2B). In embryos, ERK5 MO suppressed the expression of neural markers, without affecting the expression of mesodermal markers. Thus, embryos injected with ERK5 MO on the right blastomeres were analysed by whole-mount in situ hybridization. Although N-tubulin, a neural marker, was expressed normally on the left side (Fig 2C, left panel, black arrowheads), its expression on the right side was suppressed (Fig 2C, left panel, white arrowheads). Nuclear localization signal–β-galactosidase was used as a tracer. The observation that the reduction of expression of N-tubulin was observed even in the region with no LacZ staining suggests that ERK5 MO might function in a non-cell-autonomous manner in some regions. ERK5 MO had no effect on the expression of MyoDa, a mesodermal marker (Fig 2C, right panel). Expression of an early neural marker gene, SoxD, was unchanged in the presence of ERK5 MO (Fig 2D), which is indicates that ERK5 is not required for neural induction. These results indicate that activation of the MEK5–ERK5 pathway is required for neural differentiation. Next, we examined whether activation of the MEK5–ERK5 pathway is sufficient for neural differentiation. Co-injection of xERK5 mRNA and constitutively active xMEK5 (xMEK5 D) mRNA caused the induction of neural markers such as N-tubulin, NCAM and NeuroD without mesoderm induction (Fig 2E,F). Forced activation of the MEK5–ERK5 pathway, however, was unable to induce expression of early neural markers, Sox2, Zicr1 and Zic2 (Fig 2G). Overexpression of mouse homologues of ERK5 and MEK5 D, mERK5 and mMEK5 D, had essentially the same effect on gene expression (Fig 2E,G). These results also indicate that the MEK5–ERK5 pathway is essential for neural differentiation, but not for neural induction. To examine whether the kinase activity of ERK5 is required for its neuralizing activity, we used a non-activatable mutant of ERK5 (mERK5 AEF and xERK5 AEF), in which the activating phosphorylation sites were mutated. Injection of mRNAs of mERK5 AEF with mMEK5 D, or of xERK5 AEF with xMEK5 D, did not induce expression of neural marker genes (Fig 2E,F), indicating a requirement of the kinase activity of ERK5. To examine whether the MEK5–ERK5 pathway is activated in neural differentiation, the effect of expression of dnBMPR on the kinase activity of expressed xERK5 was studied. Expression of dnBMPR was able to activate xERK5 significantly (Fig 2H), suggesting that the MEK5–ERK5 pathway lies downstream of neural inducers. As members of the myocyte enhancer factor-2 (MEF2) family are known to be direct targets of ERK5 (Kato et al, 1997), we examined the possible involvement of MEF2 in neural differentiation. We used dominant-negative MEF2C (MEF2C R24L), which has a mutation in the DNA-binding domain (Molkentin et al, 1996). Co-injection of MEF2C R24L inhibited the neural differentiation caused by activation of the MEK5–ERK5 pathway (Fig 2I), indicating that MEF2 may function downstream of ERK5 in this process.
Figure 2.
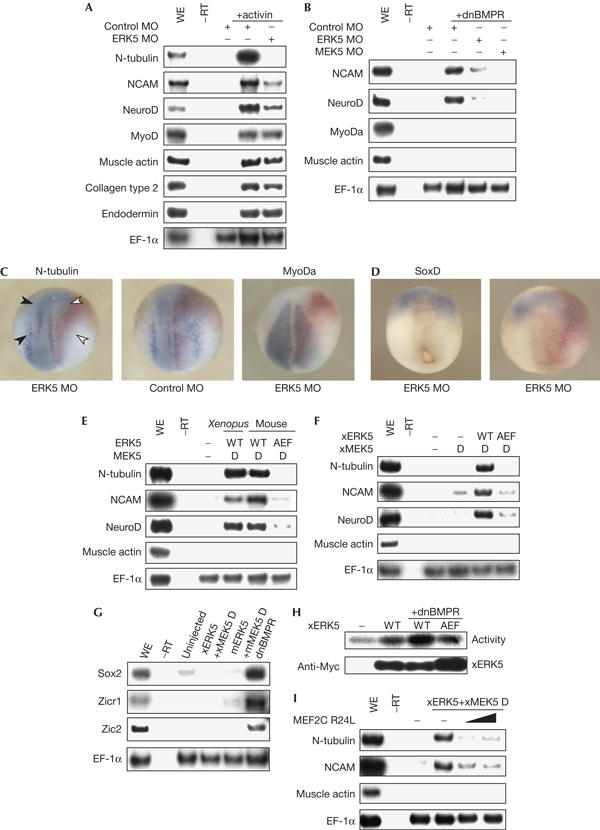
Involvement of the MEK5–ERK5 pathway in neural differentiation. (A) Control morpholino oligonucleotide (MO) or ERK5 MO (20 ng in total) was injected into each blastomere of four-cell-stage embryos. Animal caps were treated with 10 ng/ml activin and cultured to stage 20. The expression of marker genes was examined. Elongation factor-1α (EF-1α) served as a loading control; WE, whole embryo. (B) Dominant-negative bone morphogenetic protein receptor (dnBMPR) messenger RNA (0.4 ng) with control MO (160 ng), ERK5 MO (20 ng) or ERK5 MO (160 ng) was injected and animal caps from the embryos were analysed by reverse transcription–PCR (RT–PCR). (C,D) ERK5 MO or control MO (20 ng) was injected into the right blastomeres of four-cell-stage embryos. The expression pattern of N-tubulin or MyoDa (neurula stages) (C) or SoxD (gastrula stage) (D) was analysed by whole-mount in situ hybridization. Nuclear localization signal–β-galactosidase mRNA was co-injected and used as a tracer (red staining). Dorsal views of embryos are shown with the anterior side at the top. (E,F) Indicated sets of mRNAs (1.0 ng) were injected and animal caps from the embryos were analysed by RT–PCR; WT, wild type. (G) Indicated sets of mRNAs were injected. Animal caps were cultured to stage 12 and analysed by RT–PCR. (H) Myc–xERK5 (Xenopus orthologue of ERK5) or Myc–xERK5 AEF mRNA (1.0 ng) was injected with dnBMPR mRNA (0.4 ng) and animal caps were cultured to stage 14. Activity of xERK5 was measured by kinase assays. The amounts of immunoprecipitated xERK5 were detected by anti-Myc antibody. (I) xERK5 and xMEK5 D (constitutively active xMEK5) mRNAs were injected with MEF2C R24L mRNA (1.0 or 2.0 ng) and animal caps were subjected to RT–PCR.
Relationship with other regulators
We next examined the relationship of the MEK5–ERK5 pathway with some of the other known regulators of neural differentiation. A previous study had shown that inhibition of BMP signalling induces a transcription factor, SoxD, and the forced expression of SoxD causes ectopic neural tissues (Mizuseki et al, 1998). The injection of ERK5 MO or MEK5 MO suppressed the induction of neural tissues by SoxD (Fig 3A,B). These results indicate that the MEK5–ERK5 pathway is required for the neuralizing activity of SoxD. The crucial genes lying downstream of SoxD include Xngnr1, which is a proneural gene (Ma et al, 1996). In contrast to SoxD, induction of neural markers by Xngnr1 was not inhibited by ERK5 MO (Fig 3C). Furthermore, activation of the MEK5–ERK5 pathway, as well as the inhibition of BMP signalling, was able to induce the expression of Xngnr1 (Fig 3D). We then considered the possibility that expression of growth factors such as fibroblast growth factor (FGF) is induced downstream of SoxD, as previous studies have shown that FGF induces neural tissues in animal caps and dominant-negative FGF receptor inhibits the expression of neural genes in Noggin-treated animal caps (Kengaku & Okamoto, 1995; Launay et al, 1996) and that FGF can activate the MEK5–ERK5 pathway in mouse embryonic fibroblasts (Kesavan et al, 2004). It has also been reported that insulin-like growth factors (IGFs) are essential for anterior neural induction (Pera et al, 2001, 2003; Richard-Parpaillon et al, 2002). Then, we examined the expression levels of several growth factors after forced expression of SoxD. Interestingly, the expression level of FGF13, as well as that of FGF9, was found to be increased significantly by the expression of SoxD (Fig 3E). On the basis of these results, we propose a hypothetical model in which the MEK5–ERK5 pathway regulates neural differentiation downstream of SoxD and upstream of Xngnr1 (Fig 3F). It is possible that FGF13 is a candidate molecule lying between SoxD and the MEK5–ERK5 pathway, as it has been reported that FGF13 is expressed in the neural tube in the chick (Munozsanjuan et al, 1999) and FGF13 induces the phosphorylation of ERK2 in rat hippocampal astrocytes (Greene et al, 1998). Although it has been shown that SoxD is not essential for neural induction in the trunk CNS (Mizuseki et al, 1998), our results indicate that the MEK5–ERK5 pathway is essential for both anterior and posterior neural differentiation (Figs 1E, 2C). We may speculate that unidentified molecules other than SoxD function upstream of the MEK5–ERK5 pathway in posterior neural differentiation.
Figure 3.
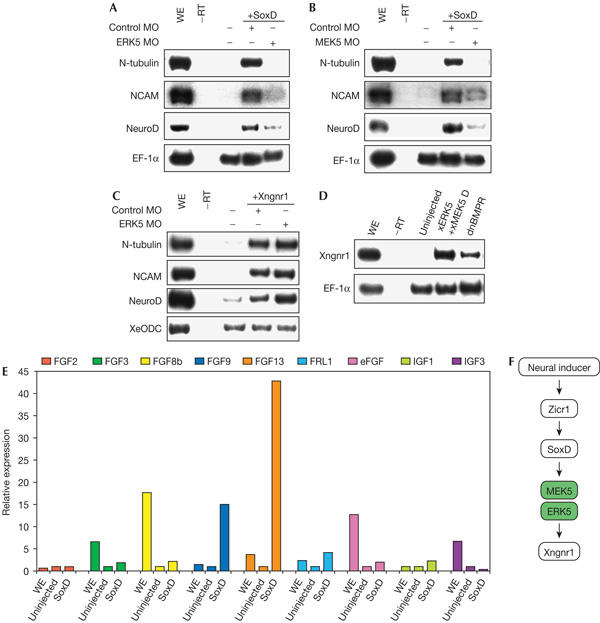
Relationship between the MEK5–ERK5 pathway and other regulators of neural differentiation. (A,B) SoxD messenger RNA (0.4 ng) was injected with ERK5 morpholino oligonucleotide (ERK5 MO; 20 ng) or MEK5 MO (160 ng) and animal caps were analysed by reverse transcription–PCR (RT–PCR) Elongation factor-1α (EF-1α); WE, whole embryo. (C) Xngnr1 mRNA (40 pg) with control MO or ERK5 MO (20 ng) was injected at the animal hemisphere and animal caps were analysed. (D) xERK5 (Xenopus orthologue of ERK5) mRNA plus xMEK5 D (constitutively active MEK5) mRNA or dominant-negative bone morphogenetic protein receptor (dnBMPR) mRNA was injected. Animal cap explants from the embryos were cultured to stage 12 and analysed by RT–PCR. (E) SoxD mRNA (0.4 ng) was injected and animal caps were cultured to stage 14. Expression of fibroblast growth factors (FGFs) and insulin-like growth factors (IGFs) was analysed by real-time RT–PCR. eFGF, embryonic FGF; FRL1, fibroblast growth factor receptor ligand 1. (F) A hypothetical model. The MEK5–ERK5 pathway regulates neural differentiation, probably downstream of SoxD and upstream of Xngnr1.
Conclusion
In this study, we have shown a novel function of the MEK5–ERK5 pathway in neural differentiation. Genetic studies with ERK5- or MEK5-deficient mice showed that the MEK5–ERK5 pathway has an important role in cardiovascular development (Regan et al, 2002; Sohn et al, 2002; Yan et al, 2003; Hayashi et al, 2004; Wang et al, 2005). They also indicated that the mutant embryos show increased apoptosis and defects in endothelial cells, and thus growth retardation. Our study suggests that the inhibition of neural differentiation may be one of the reasons for growth retardation in the head region, which is consistent with the finding that growth retardation is severe in the head region. This implies that the essential role of the MEK5–ERK5 pathway in neural development is evolutionarily conserved in vertebrates. As the method to regulate specifically the activation of the MAP kinase modules, such as the MEK5–ERK5 module, has been well developed, our finding of the involvement of the MEK5–ERK5 module in neural differentiation could facilitate in vitro regulation of neural differentiation as well as further mechanistic studies on neural development.
Methods
Embryonic manipulation, LacZ staining, whole-mount in situ hybridization and reverse transcription–PCR. In vitro fertilization, injection, LacZ staining, whole-mount in situ hybridization and RT–PCR were performed, as described (Kusakabe et al, 2001; Nitta et al, 2004). Recombinant human activin A was purchased from Genzyne-techne (MN, USA). For quantitative PCR analysis, we used 7300 Real Time PCR System (Applied Biosystems, CA, USA) with SYBR Green PCR Master Mix (Applied Biosystems). The sequences of primer pairs are described in the supplementary information online.
Morpholino oligos. Antisense MOs were obtained from Gene Tools Inc. (OR, USA). The morpholino oligo sequences were as follows: ERK5 MO, 5′-CTTGCTCCTTCCTTGGCTCCGCCAT-3′; MEK5 MO, 5′-CAGGCCGAAGCCAAATAACAGCATC-3′; a standard control oligo (control MO), 5′-CCTCTTACCTCAGTTACAATTTATA-3′. Sequences complementary to the predicted start codon are underlined.
Antibody. We used anti-Myc antibody (9E10; Santa Cruz, CA, USA) or anti-ERK5 antibody (Sigma, MO, USA) for immunoblotting.
Kinase assays. Animal caps were crushed in a buffer containing 20 mM Hepes pH 7.5, 25 mM β-glycerophosphate, 150 mM NaCl, 10% glycerol, 0.5% Triton X-100, 1.5 mM MgCl2, 2 mM EGTA, 50 mM NaF, 1 mM vanadate, 1 mM phenylmethylsulphonyl fluoride, 1% aprotinin and 2 mM dithiothreitol. Myc-tagged xERK5 was immunoprecipitated and incubated for 15 min at 37°C with 20 μg of myelin basic protein in a buffer (15 μl) containing 20 mM Tris–HCl (pH 7.5), 17 mM MgCl2 and 50 μM ATP (3 μCi of [γ-32P]ATP).
Supplementary information is available at EMBO reports online (http://www.emboreports.org).
Supplementary Material
Supplementary Information
Acknowledgments
We thank H. Hanafusa for a Xenopus gastrula library, S. Kamakura for her help in the cloning of xERK5 and H. Yamanaka and T. Yasunaga for technical advice. We also thank the members of our laboratory for helpful discussion. This work was supported by grants from the Ministry of Education, Culture, Sports, Science and Technology of Japan (to E.N.).
References
- Abe J, Kusuhara M, Ulevitch RJ, Berk BC, Lee JD (1996) Big mitogen-activated protein kinase 1 (BMK1) is a redoxsensitive kinase. J Biol Chem 271: 16586–16590 [DOI] [PubMed] [Google Scholar]
- Davis RJ (2000) Signal transduction by the JNK group of MAP kinases. Cell 103: 239–252 [DOI] [PubMed] [Google Scholar]
- De Robertis EM, Kuroda H (2004) Dorsal–ventral patterning and neural induction in Xenopus embryos. Annu Rev Cell Dev Biol 20: 285–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fainsod A, Deissler K, Yelin R, Marom K, Epstein M, Pillemer G, Steinbeisser H, Blum M (1997) The dorsalizing and neural inducing gene follistatin is an antagonist of BMP-4. Mech Dev 63: 39–50 [DOI] [PubMed] [Google Scholar]
- Greene JM et al. (1998) Identification and characterization of a novel member of the fibroblast growth factor family. Eur J Neurosci 10: 1911–1925 [DOI] [PubMed] [Google Scholar]
- Hayashi M, Kim SW, Imanaka-Yoshida K, Yoshida T, Abel ED, Eliceiri B, Yang Y, Ulevitch RJ, Lee JD (2004) Targeted deletion of BMK1/ERK5 in adult mice perturbs vascular integrity and leads to endothelial failure. J Clin Invest 113: 1138–1148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmati-Brivanlou A, Kelly OG, Melton DA (1994) Follistatin, an antagonist of activin, is expressed in the Spemann organizer and displays direct neuralizing activity. Cell 77: 283–295 [DOI] [PubMed] [Google Scholar]
- Hikasa H, Taira M (2001) A Xenopus homolog of a human p53-activated gene, PA26, is specifically expressed in the notochord. Mech Dev 100: 309–312 [DOI] [PubMed] [Google Scholar]
- Kamakura S, Moriguchi T, Nishida E (1999) Activation of the protein kinase ERK5/BMK1 by receptor tyrosine kinases. Identification and characterization of a signaling pathway to the nucleus. J Biol Chem 274: 26563–26571 [DOI] [PubMed] [Google Scholar]
- Kato Y, Kravchenko VV, Tapping RI, Han J, Ulevitch RJ, Lee JD (1997) BMK1/ERK5 regulates serum-induced early gene expression through transcription factor MEF2C. EMBO J 16: 7054–7066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato Y, Tapping RI, Huang S, Watson MH, Ulevitch RJ, Lee JD (1998) Bmk1/Erk5 is required for cell proliferation induced by epidermal growth factor. Nature 395: 713–716 [DOI] [PubMed] [Google Scholar]
- Kengaku M, Okamoto H (1995) bFGF as a possible morphogen for the anteroposterior axis of the central nervous system in Xenopus. Development 121: 3121–3130 [DOI] [PubMed] [Google Scholar]
- Kesavan K, Lobel-Rice K, Sun W, Lapadat R, Webb S, Johnson GL, Garrington TP (2004) MEKK2 regulates the coordinate activation of ERK5 and JNK in response to FGF-2 in fibroblasts. J Cell Physiol 199: 140–148 [DOI] [PubMed] [Google Scholar]
- Kusakabe M, Masuyama N, Hanafusa H, Nishida E (2001) Xenopus FRS2 is involved in early embryogenesis in cooperation with the Src family kinase Laloo. EMBO Rep 2: 727–735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyriakis JM, Avruch J (2001) Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol Rev 81: 807–869 [DOI] [PubMed] [Google Scholar]
- Lamb TM, Knecht AK, Smith WC, Stachel SE, Economides AN, Stahl N, Yancopolous GD, Harland RM (1993) Neural induction by the secreted polypeptide noggin. Science 262: 713–718 [DOI] [PubMed] [Google Scholar]
- Launay C, Fromentoux V, Shi DL, Boucaut JC (1996) A truncated FGF receptor blocks neural induction by endogenous Xenopus inducers. Development 122: 869–880 [DOI] [PubMed] [Google Scholar]
- Liu L, Cavanaugh JE, Wang Y, Sakagami H, Mao Z, Xia Z (2003) ERK5 activation of MEF2 mediated gene expression plays a critical role in BDNF promoted survival of developing but not mature cortical neurons. Proc Natl Acad Sci USA 100: 8532–8537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q, Kintner C, Anderson DJ (1996) Identification of neurogenin, a vertebrate neuronal determination gene. Cell 87: 43–52 [DOI] [PubMed] [Google Scholar]
- Mizuseki K, Kishi M, Shiota K, Nakanishi S, Sasai Y (1998) SoxD: an essential mediator of induction of anterior neural tissues in Xenopus embryos. Neuron 21: 77–85 [DOI] [PubMed] [Google Scholar]
- Molkentin JD, Black BL, Martin JF, Olson EN (1996) Mutational analysis of the DNA binding, dimerization, and transcriptional activation domains of MEF2C. Mol Cell Biol 16: 2627–2636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munozsanjuan I, Brivanlou AH (2002) Neural induction, the default model and embryonic stem cells. Nat Rev Neurosci 3: 271–280 [DOI] [PubMed] [Google Scholar]
- Munozsanjuan I, Simandl BK, Fallon JF, Nathans J (1999) Expression of chicken fibroblast growth factor homologous factor (FHF)-1 and of differentially spliced isoforms of FHF-2 during development and involvement of FHF-2 in chicken limb development. Development 126: 409–421 [DOI] [PubMed] [Google Scholar]
- Nishida E, Gotoh Y (1993) The MAP kinase cascade is essential for diverse signal transduction pathways. Trends Biochem Sci 18: 128–131 [DOI] [PubMed] [Google Scholar]
- Nitta KR, Tanegashima K, Takahashi S, Asashima M (2004) XSIP1 is essential for early neural gene expression and neural differentiation by suppression of BMP signaling. Dev Biol 275: 258–267 [DOI] [PubMed] [Google Scholar]
- Pera EM, Wessely O, Li SY, De Robertis EM (2001) Neural and head induction by insulin-like growth factor signals. Dev Cell 1: 655–665 [DOI] [PubMed] [Google Scholar]
- Pera EM, Ikeda A, Eivers E, De Robertis EM (2003) Integration of IGF, FGF, and anti-BMP signals via Smad1 phosphorylation in neural induction. Genes Dev 17: 3023–3028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccolo S, Sasai Y, Lu B, De Robertis EM (1996) Dorsoventral patterning in Xenopus: inhibition of ventral signals by direct binding of chordin to BMP-4. Cell 86: 589–598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regan CP, Li W, Boucher DM, Spatz S, Su MS, Kuida K (2002) Erk5 null mice display multiple extraembryonic vascular and embryonic cardiovascular defects. Proc Natl Acad Sci USA 99: 9248–9253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard-Parpaillon L, Heligon C, Chesnel F, Boujard D, Philpott A (2002) The IGF pathway regulates head formation by inhibiting Wnt signaling in Xenopus. Dev Biol 244: 407–417 [DOI] [PubMed] [Google Scholar]
- Robinson MJ, Cobb MH (1997) Mitogen-activated protein kinase pathways. Curr Opin Cell Biol 9: 180–186 [DOI] [PubMed] [Google Scholar]
- Sasai Y (1998) Identifying the missing links: genes that connect neural induction and primary neurogenesis in vertebrate embryos. Neuron 21: 455–458 [DOI] [PubMed] [Google Scholar]
- Sasai Y, Lu B, Steinbeisser H, De Robertis EM (1995) Regulation of neural induction by the Chd and Bmp-4 antagonistic patterning signals in Xenopus. Nature 376: 333–336 [DOI] [PubMed] [Google Scholar]
- Sohn SJ, Sarvis BK, Cado D, Winoto A (2002) ERK5 MAPK regulates embryonic angiogenesis and acts as a hypoxiasensitive repressor of vascular endothelial growth factor expression. J Biol Chem 277: 43344–43351 [DOI] [PubMed] [Google Scholar]
- Sturgill TW, Wu J (1991) Recent progress in characterization of protein kinase cascades for phosphorylation of ribosomal protein S6. Biochim Biophys Acta 1092: 350–357 [DOI] [PubMed] [Google Scholar]
- Wang X et al. (2005) Targeted deletion of mek5 causes early embryonic death and defects in the extracellular signal-regulated kinase 5/myocyte enhancer factor 2 cell survival pathway. Mol Cell Biol 25: 336–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan L, Carr J, Ashby PR, Murry-Tait V, Thompson C, Arthur JS (2003) Knockout of ERK5 causes multiple defects in placental and embryonic development. BMC Dev Biol 3: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information


