Abstract
The oligomeric c ring of the F-ATP synthase from the alkaliphilic cyanobacterium Spirulina platensis was isolated and characterized. Mass spectroscopy analysis indicated a mass of 8,210 Da, reflecting that of a c monomer. The mass increased by 206 Da after treatment with the c-subunit-specific inhibitor dicyclohexylcarbodiimide (DCCD), which indicated modification of the ion-binding carboxylate by DCCD. Atomic force microscopy topographs of c rings from S. platensis showed 15 symmetrically assembled subunits. The c15-mer reported here is the largest c ring that is isolated and does not show the classical c-ring mismatch to the three-fold symmetry of the F1 domain.
Keywords: atomic force microscopy, c-subunit oligomer, alkaliphiles, symmetry mismatch
Introduction
The conversion of electrochemical ion gradients into the universal energy currency adenosine triphosphate (ATP) presents a central process for all life forms. ATP synthesis from ADP and phosphate is coupled to the flux of protons or sodium ions across the membrane. The ATP-synthesizing machinery, F1F0 ATP synthase, consists of two rotary motors, F1 and F0, which are connected by a central and peripheral stalk to exchange energy. During ATP synthesis, the electrochemical ion gradient fuels the membrane-embedded F0 motor to drive the F1 motor to act as a generator for ATP synthesis (Capaldi & Aggeler, 2002; Dimroth et al, 2003).
The three-fold symmetry of the F1 motor, harbouring three catalytic nucleotide-binding sites at the β-subunits in the interface to the α-subunits, has been established by the crystal structure of this motor (Abrahams et al, 1994). Each of these sites is in a different conformation at any time and following rotation of the γsubunit, these sites interconvert sequentially to produce three molecules of ATP in a complete 360° turn (Boyer, 1993).
The overall shape of the ATP synthase and the assignment of individual subunits to the rotor and stator have been shown by combining electron microscopy with analyses by crosslinking and other biochemical methods (Capaldi et al, 2000; Mellwig & Böttcher, 2003). According to these data, the main part of the F0 motor consists of an oligomeric ring of c subunits, which is connected with the F1 rotor subunits γ and ɛ. The c ring is functionally and structurally coupled to the a and b2 subunits, which form the peripheral stalk that is connected at the top to the stator F1 subunits α and δ. Structurally, the most well-characterized part of the F0 motor is the c ring. First, NMR studies showed the structures of c monomers of Escherichia coli or Propionigenium modestum in solutions containing organic solvents or sodium dodecylsulphate (SDS), respectively (Girvin et al, 1998; Matthey et al, 1999). Later, medium-resolution structural information on entire c rings from the ATP synthase from yeast or Ilyobacter tartaricus was obtained by X-ray or electron crystallography, respectively (Stock et al, 1999; Vonck et al, 2002). Recently, the first high-resolution crystal structure of a c ring from I. tartaricus (Meier et al, 2005) and a corresponding structure from a bacterial V-ATPase (Murata et al, 2005) were characterized. These studies were complemented by electron crystallography and atomic force microscopy (AFM) of other c-ring samples. Altogether, they uncovered a remarkable flexibility in c-ring stoichiometry. Thus, 10, 11 and 14 subunits were found in c rings from yeast, I. tartaricus or P. modestum, and spinach chloroplasts, respectively (Stock et al, 1999; Seelert et al, 2000; Meier et al, 2003).
In this work, we use high-resolution AFM to show that the c ring from the alkaliphilic cyanobacterium Spirulina platensis consists of 15 protomers. This is the largest c ring that has been isolated until now and it is the first one to show no symmetry mismatch between the two rotary motors of the F1F0 ATP synthase. The functional and energetic implications of this finding for the ATP synthase function are discussed.
Results
Properties of the c ring from S. platensis ATP synthase
Isolated c rings from thylakoid membranes of the alkaliphilic cyanobacterium S. platensis were highly stable, that is, they did not denature on boiling in 20 mM Tris–HCl (pH 8.0) buffer for 5 min. Running SDS–polyacrylamide gel electrophoresis (SDS–PAGE) of all c-ring preparations from every single purification step showed that the rings always migrated at identical levels. This suggests that the individual preparation steps did not disturb the native oligomer structure. However, c rings from S. platensis migrated slower on SDS–PAGE than c14 rings from spinach chloroplasts, and significantly slower than c11 rings isolated from I. tartaricus ATP synthase (Fig 1). This indicates that c rings from S. platensis might consist of more than 14 subunits. For comparison, c rings were isolated from the freshwater cyanobacterium Synechocystis 6803 PCC. Their migration behaviour on SDS–PAGE was the same as that from spinach chloroplasts, which suggests a similar subunit stoichiometry (Fig 1). After treatment of the different c-ring samples with trichloroacetic acid or chloroform:methanol (1:1), the oligomeric rings disassembled into monomeric units. A c ring of size similar to that isolated from the thylakoid membranes of S. platensis was also isolated from the plasma membrane of this organism, supporting previous data on the presence of ATP synthase in both cyanobacterial membranes (Neisser et al, 1994; Huang et al, 2002). The abundance of c rings in the plasma membrane was about ten times lower than that in the thylakoid membrane.
Figure 1.
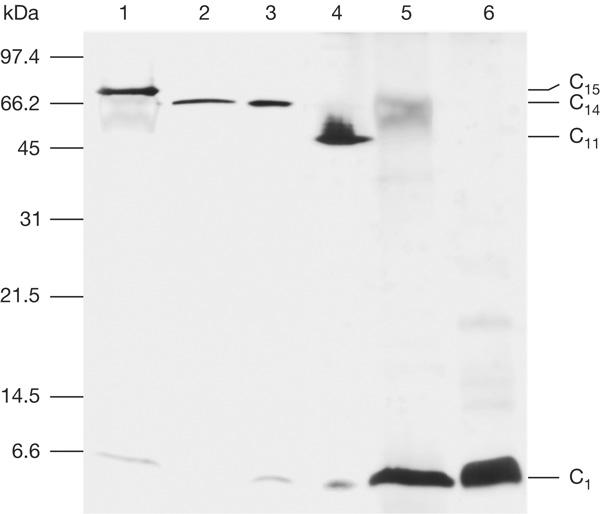
SDS–polyacrylamide gel electrophoresis of c rings that are isolated from thylakoid membranes of two cyanobacterial strains. Lane 1 shows the c ring from the alkaliphilic cyanobacterium Spirulina platensis and lane 2 shows the c ring from the freshwater cyanobacterium Synechocystis PCC 6803. Lanes 3 and 4 show the c14 and c11 rings from spinach chloroplasts and Ilyobacter tartaricus, respectively. Lanes 5 and 6 show c monomers of S. platensis and Synechocystis PCC 6803 obtained by trichloroacetic acid treatment of the rings. A standard gel marker is indicated on the left.
Analysis of the S. platensis c ring by mass spectroscopy
When isolated c rings of S. platensis were analysed by matrix-assisted laser desorption ionization time-of-flight mass spectroscopy, a single mass peak of 8,210±3 Da was observed, as was expected for a c monomer (Fig 2). The complete dissociation of the ring into monomeric units in the mass spectrometer was in agreement with previous measurements of the I. tartaricus c ring (Stahlberg et al, 2001). For further identification, the c ring was incubated sufficiently long with 50 μM dicyclohexylcarbodiimide (DCCD), which specifically modifies the ion-binding carboxylate of subunit c. This treatment resulted in an increase in the mass of the protein by 206 Da (Fig 2), which is to be expected if each monomer of the c ring is modified by a single DCCD molecule.
Figure 2.
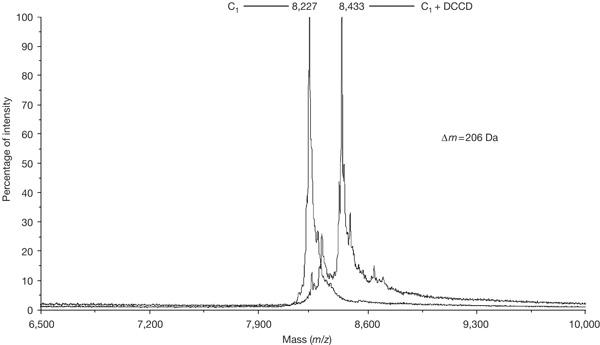
Overlay of mass spectra of subunit c of Spirulina platensis and its dicyclohexylcarbodiimide (DCCD)-modified derivative. For the modification, the c-ring sample (20 mM Tris–HCl (pH 7.0) and 1.5% octylglucoside) was incubated at 4°C with 50 μM DCCD for an extended time (24 h) to ensure the modification of a c-subunit majority. The modified c monomer was extracted with CHCl3:CH3OH (1:1) and subjected to matrix-assisted laser desorption ionization time-of-flight mass spectroscopy. Note that the samples analysed had been stored for 2 weeks at 4°C before measurement, which results in a mass increase of 16 Da with respect to fresh samples. This increase may be caused by the oxidation of one of the methionine residues of the protein.
High-resolution AFM imaging
Overview AFM topographs of the sample preparation showed membrane patches (Fig 3A) with small densely packed areas of proteins surrounded by lipid membranes (Fig 3B,C). Whereas the lipid bilayer showed a height of 4.2±0.3 nm (n=20), the proteins protruded 7.6±0.3 nm (n=20) from the supporting mica surface. At a lateral resolution of about 1 nm, the individual ringshaped c oligomers became visible (Fig 4A). At a higher magnification, individual subunits of the oligomers could be resolved (Fig 4B). On average, the wide oligomers protruded 1.5±0.3 nm (n=22) from the lipid bilayer and showed an outer diameter of 6.3±0.3 nm (n=18), measured at full-width half-maximum of their protruding height (Fig 4). Reference-free averages generated by translational and rotational alignment of single particles enhanced common structural features among the c oligomers (Fig 4D,E). It seemed that single particles and reference-free averages showed a stoichiometry of 15 subunits (Fig 4C), except for those of defect particles.
Figure 3.
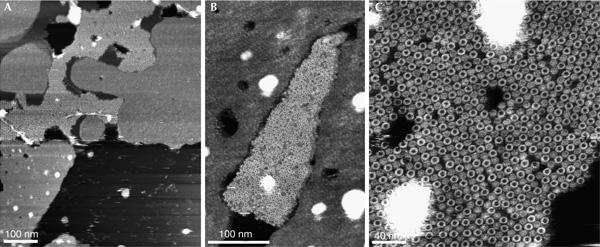
Overview atomic force microscopy topographs of reconstituted c-subunit oligomers of F1F0 ATP synthase from Spirulina platensis. (A) Densely distributed membrane patches adsorbed onto mica showing empty lipid membranes and densely packed c oligomers (bright corrugated region). A higher magnification shows a densely packed assembly of oligomeric rings (centre of images B,C). Full grey level ranges correspond to vertical dimensions of 15 nm (A) and 5 nm (B,C).
Figure 4.
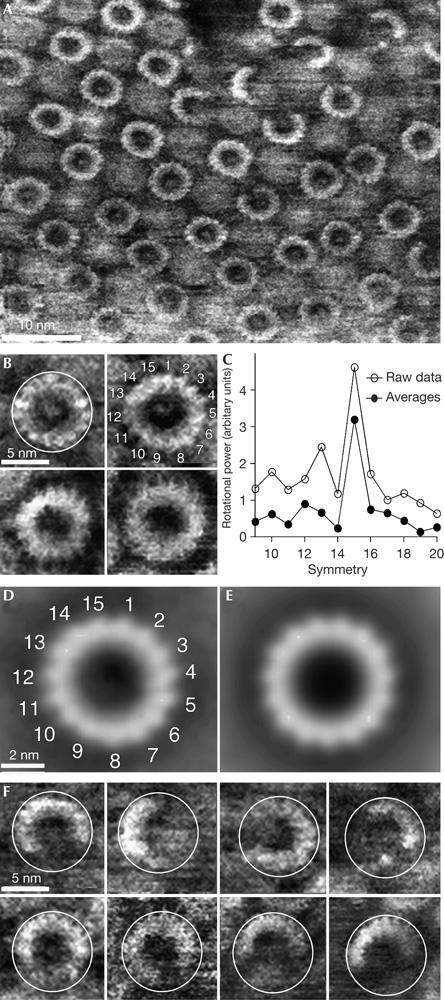
High-resolution atomic force microscopy topographs of reconstituted rotor rings. (A) Survey of an almost two-dimensional crystalline arrangement of c rings in the membrane. (B) At a higher magnification, the c subunits of single rotors could be directly observed. The c rings were selected from different topographs. (C) Rotational power spectra calculated from single rings with sufficiently high signal-to-noise ratio (such as those shown in B) and from the three major averages (as shown in D) found by reference-free averaging, show a clear symmetry. (D) Average, generated by reference-free translational and rotational alignment of 90 complete c rings. (E) A 15-fold symmetrized topography of average shown in (C). (F) Gallery of incomplete rotors showing the same diameter (white circles) as the complete rotors (B). Full grey level ranges correspond to vertical scales of 4 nm (A) and 3.5 nm (B,D–F).
Defect particles (Fig 4F) showed an outer diameter that was identical to that of the complete c rings (6.4±0.3 nm, n=34). This observation is in agreement with previous AFM topographs of chloroplast and I. tartaricus rings (Müller et al, 2001), and thus it may be concluded that the stoichiometry of the rotor ring is an intrinsic, structural property of the c subunit from F-ATP synthases and is not variable, such as that observed in other ring-like membrane-embedded oligomers (Czajkowsky et al, 2004).
The lower protruding c rings showed a central plug, which probably represented lipid headgroups such as that shown for two-dimensional crystalline c rings from I. tartaricus (Meier et al, 2001).
Discussion
Much interest has been focused on the number of c subunits assembled into the membrane-embedded rotor ring of F-type ATP synthases. On the basis of structural, biochemical and genetic studies, the c-ring stoichiometry is not fixed but varies among species. Rotor rings containing ten protomers seem to exist in the ATP synthases from yeast, E. coli or Bacillus sp PS 3 (Stock et al, 1999; Jiang et al, 2001; Mitome et al, 2004), and undecameric c rings were found in the I. tartaricus (Stahlberg et al, 2001) and P. modestum (Meier et al, 2003) ATP synthases. Furthermore, a 13-mer was deduced from the gene sequence of M. kandleri (Lolkema & Boekema, 2003) and a ring with 14 subunits was observed for the ATP synthase from spinach chloroplasts (Seelert et al, 2000). The number of c subunits is equal to the number of ion-binding sites, each contributing to the translocation of one H+ or Na+ across the membrane per 360° turn. As three molecules of ATP are produced in one revolution of the ring, these numbers have profound implications on the H+ (Na+) to ATP stoichiometries and thus on the energetic output of various ATP synthases. It is clear that, at a given proton or sodium motive force of sufficient magnitude, an ATP synthase with only ten c subunits, operating at a H+ (Na+) to ATP stoichiometry of 3.3, is a more economical energy converter than one with 14 subunits, operating at a H+ to ATP stoichiometry of 4.7. However, ATP synthases with a high H+ to ATP stoichiometry may be needed if the proton motive force drops to values that can only match the phosphorylation potential at these high ratios.
It is striking that the number of protomers of all c-subunit rings characterized so far cannot be divided by three. This implies that the H+ (Na+) to ATP stoichiometries are not integral and, simultaneously, that the symmetries between the c ring and the F1 motor do not match. It has been noted that non-matching symmetries between the F0 and F1 components may be an advantage for the operation of the enzyme, avoiding the machinery to move through deeper energy minima that would accompany matching symmetries (Stock et al, 1999; Murata et al, 2005). Furthermore, it has been suggested that the elastic power transmission between the F1 and F0 motors is important for operation of the enzyme under symmetry mismatch conditions (Junge et al, 2001).
In this report, we describe the rotor ring from the F-ATP synthase of the alkaliphilic cyanobacterium S. platensis, assembled from 15 c subunits. This is the largest c ring that has been isolated and the first one without a symmetry mismatch between the two motors of this enzyme. On the basis of this structural insight, the proton to ATP stoichiometry for the S. platensis ATP synthase is 5. This high number may reflect a low ion motive force across the thylakoid or plasma membrane of this alkaliphilic cyanobacterium. In vitro experiments seemed to indicate that ATP synthesis required a very low proton motive force (5 kJ/mol), which implied a H+ to ATP stoichiometry of at least 7 (Bakels et al, 1993). However, the experiments that were reported included energizing of the membrane by an acid–base transition with succinate as the acidic buffer, which has been shown to generate not only a ΔpH but also a membrane potential (Δψ) of significant size (Kaim & Dimroth, 1999). As the latter was not taken into account, these data need to be re-evaluated. The S. platensis c ring with 15 protomers shows that there are no stringent conditions for a symmetry mismatch in the ATP synthases, at least not if the c ring is of a large size. To generate the same torque, the increment of a single step in the c15 motor would be 67% of that of the c10 motor. This might imply the presence of lower energy wells between steps in the larger motor, reducing the necessity for a symmetry mismatch between the two motors, which may, conversely, be required in the smaller motors.
Methods
Strain and growth conditions. S. platensis strain C1 (Arthrospira sp PCC9438) cells were grown at 30°C in Zarrouk's medium (Pogoryelov et al, 2003). Cells were collected in the mid-log phase at a density of about 5 μg chlorophyll per millilitre culture and were frozen in liquid nitrogen for storage.
Isolation of thylakoid membranes of Spirulina platensis. Cells (1 g wet weight) were suspended in 2 ml buffer A (20 mM Tris–HCl (pH 8.0) and 5 mM EDTA) and disrupted by three passages through a French pressure cell at 1,000 bar. The suspension was centrifuged at 15,000g for 15 min to remove cell debris, and the supernatant was subjected to sucrose density gradient centrifugation, as described (Murata & Omata, 1988). Fractions containing the thylakoid membranes were combined and diluted ten times with buffer A. The membranes were subsequently collected by ultracentrifugation for 1 h at 200,000g at 4°C.
Preparation of c rings from thylakoid membranes of Spirulina platensis. Thylakoid membranes obtained from 1 g of cells were resuspended in 0.5 ml buffer A containing 1% N-lauroylsarcosine and incubated for 10 min at 65°C to solubilize the proteins. After ultracentrifugation at 25°C for 1 h, the pellet was discarded and (NH4)SO4 was added to the supernatant to yield 65% saturation. After incubation at 25°C overnight, the sample was centrifuged for 15 min at 39,000g and the filtrated supernatant containing the c ring was dialysed against 10 mM Tris–HCl buffer, pH 8.0. The c-ring sample was subsequently concentrated by ultrafiltration (10 kDa cutoff) to 0.5 ml and 1.5% β-octyl-glucoside was added as detergent.
Two-dimensional crystallization of the c ring from Spirulina platensis. The purified c-ring sample with a protein concentration of 1 mg/ml was mixed with digalactosyl diacylglycerol (DGDG) from wheat (Larodan Fine Chemicals, Sweden) at a lipid-to-protein ratio of 0.4 (w/w) to yield a β-octyl-glucoside concentration of 0.6%. We carried out microdialysis in bent glass capillaries for 5 days at 25°C against 25 ml buffer containing 10 mM Tris–HCl, pH 8.0, 200 mM NaCl and 3 mM NaN3 using dialysis membranes with a cutoff of 3.5 kDa.
Atomic force microscopy. An AFM equipped with a 100 μm X–Y piezo scanner was optimized for observing single molecules in the buffer solution (Nanoscope IIIa, DI-Veeco, USA). It was noted that 100-μm-long silicon nitride AFM cantilevers (Olympus, Tokyo, Japan) showed nominal spring constants of about 0.9 N/m. To adsorb the protein membranes, 20 μl of the sample buffer (10 mM Tris–HCl, 200 mM NaCl, 0.02% NaN3 and 10% glycerol, pH 7.8) was placed onto freshly cleaved mica for about 30 min. After this, the sample was rinsed with dialysis buffer to remove weakly attached material. Contact-mode AFM topographs were recorded in the above buffer solution at 25°C, a stylus loading force of <100 pN and a line frequency of 4–6 Hz. No differences between topographs recorded in the trace and in the retrace direction were observed, which indicated that the scanning process did not influence the appearance of the sample.
Image processing. Individual particles of the AFM topographs were selected manually and subjected to reference-free alignment and averaging using the SPIDER image processing system (Wadsworth Labs, New York, USA). To assess the rotor symmetry, the rotational power spectrum of the reference-free averages was calculated using the SEMPER image processing system (Synoptics Ltd, UK). Alternatively, the rotational power spectrum of single rotors was calculated. In the event of a of sufficiently high signal of the raw data, the spectrum showed a clear signal, which was then averaged with that from other particles.
Other methods. SDS gels were stained with silver (Nesterenko et al, 1994). The protein concentration of samples was determined according to the bicinchoninic acid method (Smith et al, 1985), with bovine serum albumin as a standard.
Acknowledgments
We thank G. Cook for discussion and Z. Gombos for providing strains of S. platensis and Synechocystis PCC 6803. This study was supported by the Eidgenössische Technische Hochschule research commission, the Deutsche Forschungsgemeinschaft, the free state of Saxony and the European Union.
References
- Abrahams JP, Leslie AG, Lutter R, Walker JE (1994) Structure at 2.8 Å resolution of F1-ATPase from bovine heart mitochondria. Nature 370: 621–628 [DOI] [PubMed] [Google Scholar]
- Bakels RH, van Walraven HS, Krab K, Scholts MJ, Kraayenhof R (1993) On the activation mechanism of the H+-ATP synthase and unusual thermodynamic properties in the alkalophilic cyanobacterium Spirulina platensis. Eur J Biochem 213: 957–964 [DOI] [PubMed] [Google Scholar]
- Boyer PD (1993) The binding change mechanism for ATP synthase—some probabilities and possibilities. Biochim Biophys Acta 1140: 215–250 [DOI] [PubMed] [Google Scholar]
- Capaldi RA, Aggeler R (2002) Mechanism of the F1F0-type ATP synthase, a biological rotary motor. Trends Biochem Sci 27: 154–160 [DOI] [PubMed] [Google Scholar]
- Capaldi RA, Schulenberg B, Murray J, Aggeler R (2000) Cross-linking and electron microscopy studies of the structure and functioning of the Escherichia coli ATP synthase. J Exp Biol 203: 29–33 [DOI] [PubMed] [Google Scholar]
- Czajkowsky DM, Hotze EM, Shao Z, Tweten RK (2004) Vertical collapse of a cytolysin prepore moves its transmembrane β-hairpins to the membrane. EMBO J 23: 3206–3215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimroth P, von Ballmoos C, Meier T, Kaim G (2003) Electrical power fuels rotary ATP synthase. Structure (Camb) 11: 1469–1473 [DOI] [PubMed] [Google Scholar]
- Girvin ME, Rastogi VK, Abildgaard F, Markley JL, Fillingame RH (1998) Solution structure of the transmembrane H+-transporting subunit c of the F1F0 ATP synthase. Biochemistry 37: 8817–8824 [DOI] [PubMed] [Google Scholar]
- Huang F, Parmryd I, Nilsson F, Persson AL, Pakrasi HB, Andersson B, Norling B (2002) Proteomics of Synechocystis sp. strain PCC 6803: identification of plasma membrane proteins. Mol Cell Proteomics 1: 956–966 [DOI] [PubMed] [Google Scholar]
- Jiang W, Hermolin J, Fillingame RH (2001) The preferred stoichiometry of c subunits in the rotary motor sector of Escherichia coli ATP synthase is 10. Proc Natl Acad Sci USA 98: 4966–4971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junge W, Pänke O, Cherepanov DA, Gumbiowski K, Müller M, Engelbrecht S (2001) Intersubunit rotation and elastic power transmission in F0F1-ATPase. FEBS Lett 504: 152–160 [DOI] [PubMed] [Google Scholar]
- Kaim G, Dimroth P (1999) ATP synthesis by F-type ATP synthase is obligatorily dependent on the transmembrane voltage. EMBO J 18: 4118–4127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lolkema JS, Boekema EJ (2003) The A-type ATP synthase subunit K of Methanopyrus kandleri is deduced from its sequence to form a monomeric rotor comprising 13 hairpin domains. FEBS Lett 543: 47–50 [DOI] [PubMed] [Google Scholar]
- Matthey U, Kaim G, Braun D, Wüthrich K, Dimroth P (1999) NMR studies of subunit c of the ATP synthase from Propionigenium modestum in dodecylsulphate micelles. Eur J Biochem 261: 459–467 [DOI] [PubMed] [Google Scholar]
- Meier T, Matthey U, Henzen F, Dimroth P, Müller DJ (2001) The central plug in the reconstituted undecameric c cylinder of a bacterial ATP synthase consists of phospholipids. FEBS Lett 505: 353–356 [DOI] [PubMed] [Google Scholar]
- Meier T, Matthey U, von Ballmoos C, Vonck J, Krug von Nidda T, Kühlbrandt W, Dimroth P (2003) Evidence for structural integrity in the undecameric c-rings isolated from sodium ATP synthases. J Mol Biol 325: 389–397 [DOI] [PubMed] [Google Scholar]
- Meier T, Polzer P, Diederichs K, Welte W, Dimroth P (2005) Structure of the rotor ring of F-type Na+-ATPase from Ilyobacter tartaricus. Science 308: 659–662 [DOI] [PubMed] [Google Scholar]
- Mellwig C, Böttcher B (2003) A unique resting position of the ATPsynthase from chloroplasts. J Biol Chem 278: 18544–18549 [DOI] [PubMed] [Google Scholar]
- Mitome N, Suzuki T, Hayashi S, Yoshida M (2004) Thermophilic ATP synthase has a decamer c-ring: indication of noninteger 10:3 H+/ATP ratio and permissive elastic coupling. Proc Natl Acad Sci USA 101: 12159–12164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller DJ, Dencher NA, Meier T, Dimroth P, Suda K, Stahlberg H, Engel A, Seelert H, Matthey U (2001) ATP synthase: constrained stoichiometry of the transmembrane rotor. FEBS Lett 504: 219–222 [DOI] [PubMed] [Google Scholar]
- Murata N, Omata T (1988) Isolation of cyanobacterial plasma membranes. Methods Enzymol 167: 245–251 [Google Scholar]
- Murata T, Yamato I, Kakinuma Y, Leslie AG, Walker JE (2005) Structure of the rotor of the V-type Na+-ATPase from Enterococcus hirae. Science 308: 654–659 [DOI] [PubMed] [Google Scholar]
- Neisser A, Fromwald S, Schmatzberger A, Peschek GA (1994) Immunological and functional localization of both F-type and P-type ATPases in cyanobacterial plasma membranes. Biochem Biophys Res Commun 200: 884–892 [DOI] [PubMed] [Google Scholar]
- Nesterenko MV, Tilley M, Upton SJ (1994) A simple modification of Blum's silver stain method allows for 30 min detection of proteins in polyacrylamide gels. J Biochem Biophys Methods 28: 239–242 [DOI] [PubMed] [Google Scholar]
- Pogoryelov D, Sudhir PR, Kovács L, Gombos Z, Brown I, Garab G (2003) Sodium dependency of the photosynthetic electron transport in the alkaliphilic cyanobacterium Arthrospira platensis. J Bioenerg Biomembr 35: 427–437 [DOI] [PubMed] [Google Scholar]
- Seelert H, Poetsch A, Dencher NA, Engel A, Stahlberg H, Müller DJ (2000) Structural biology. Proton-powered turbine of a plant motor. Nature 405: 418–419 [DOI] [PubMed] [Google Scholar]
- Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC (1985) Measurement of protein using bicinchoninic acid. Anal Biochem 150: 76–85 [DOI] [PubMed] [Google Scholar]
- Stahlberg H, Müller DJ, Suda K, Fotiadis D, Engel A, Meier T, Matthey U, Dimroth P (2001) Bacterial Na+-ATP synthase has an undecameric rotor. EMBO Rep 2: 229–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stock D, Leslie AG, Walker JE (1999) Molecular architecture of the rotary motor in ATP synthase. Science 286: 1700–1705 [DOI] [PubMed] [Google Scholar]
- Vonck J, von Nidda TK, Meier T, Matthey U, Mills DJ, Kühlbrandt W, Dimroth P (2002) Molecular architecture of the undecameric rotor of a bacterial Na+-ATP synthase. J Mol Biol 321: 307–316 [DOI] [PubMed] [Google Scholar]


