Abstract
Metazoans limit origin firing to once per cell cycle by oscillations in cyclin-dependent kinases and the replication licensing inhibitor geminin. Geminin inhibits pre-replication complex assembly by preventing Cdt1 from recruiting the minichromosome maintenance proteins to chromatin. Geminin depletion results in genomic over-replication in Drosophila and human cell lines. Here, we show that loss of geminin affects other cell cycle-dependent events in addition to DNA replication. Geminin inactivation causes centrosome overduplication without passage through mitosis in human normal and cancer cells. Centrosomes are microtubule-organizing centres that are duplicated during S phase and have an important role in the fidelity of chromosome transmission by nucleating the mitotic spindle. Consistent with this, geminin-depleted cells show multiple mitotic defects, including multipolar spindles, when driven into mitosis by checkpoint abrogation. These results show that the consequences of geminin loss exceed its immediate role in DNA replication and extend to promoting chromosome mis-segregation in mitosis.
Keywords: centrosomes, DNA replication, geminin, mitotic spindle
Introduction
Centrosome duplication, similar to DNA replication, is a semiconservative process that occurs once and only once per cell cycle. The assembly of pre-replication complexes that are essential for DNA replication is limited to late mitosis and G1 phase, when the replication licensing inhibitor geminin is absent and cyclin-dependent kinase (CDK) activity is low (McGarry & Kirschner, 1998; Wohlschlegel et al, 2000; Tada et al, 2001; Diffley, 2004). Similarly, G1-phase cells contain one centrosome that is ‘licensed' for a single duplication event (Wong & Stearns, 2003). Centriole duplication and genomic replication initiate at the G1–S transition, and several studies indicate that cyclin A or E complexed to CDK2 couple these two processes to S-phase progression (Hinchcliffe et al, 1999; Matsumoto et al, 1999; Meraldi et al, 1999; Coverley et al, 2002; Sluder, 2004). The mechanisms that regulate centrosome duplication have been studied in part by the administration of DNA synthesis inhibitors such as aphidicolin and hydroxyurea, which lead to centrosome amplification in p53 mutant cell lines (Balczon et al, 1995). Likewise, subtoxic concentrations of anticancer drugs such as actinomycin D, cytosine arabinoside and 5-fluorouracil result in centrosome overduplication without DNA synthesis (Bennett et al, 2004). Centrosome amplification has also been observed in Rad51-deficient cells during a prolonged G2-phase arrest (Dodson et al, 2004). In most of these studies, DNA replication and centrosome duplication have been synthetically uncoupled, whereas they are normally linked to S-phase progression. Here, we show that the inhibition of geminin expression is sufficient to induce centrosome overduplication. This provides a novel approach to the study of centrosome duplication without a need for genotoxic chemicals, and indicates a new, more general role for geminin in coordinating the chromosome inheritance cycle in metazoans.
Results and Discussion
To investigate the function of geminin in cell-cycle progression, we examined the effects of inhibiting geminin expression using short interfering RNA (siRNA) in a variety of human cell lines. In U2OS osteosarcoma cells, geminin depletion resulted in a relative increase in cyclin A levels, as shown by western blotting of whole-cell lysates (Fig 1A). The same was observed in geminin-depleted HCT116 colorectal cells (Zhu et al, 2004), although the transfection efficiency was lower in HCT116 than in U2OS (Fig 1A). The increase in cyclin A levels could reflect the increased proportion of replicating cells that fail to exit S phase after geminin depletion (Fig 3A). However, indirect immunofluorescence of geminin-depleted cells shows that cyclin A protein levels are also upregulated on a per cell basis. This is particularly obvious in HCT116 cells because the lower transfection efficiency allows a direct comparison of geminin-depleted and untransfected cells in the same population (Fig 1B). Cyclin A levels are higher in the nuclei of some geminin-depleted cells, which can be clearly identified by their significant increase in size compared with control cells.
Figure 1.
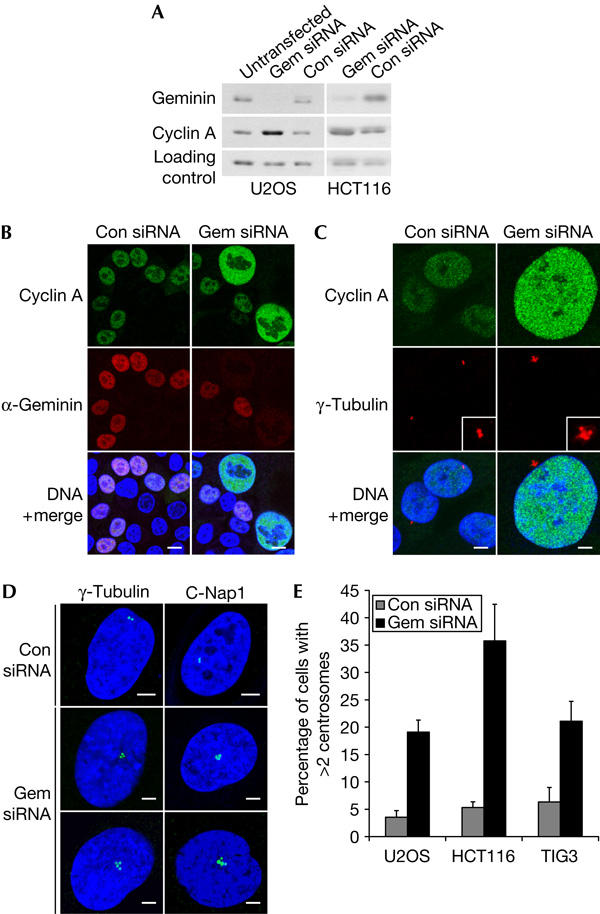
Depletion of geminin by short interfering RNA causes aberrations in centrosome number. (A) U2OS and HCT116 cells were treated with geminin (gem) or control (con) short interfering RNA (siRNA) and collected 48 h after transfection. Untransfected cells were included as a negative control. Western blots were probed with antibodies specific for geminin and cyclin A. The loading control is a crossreacting protein. (B) HCT116 cells were immunostained with cyclin A and geminin antibodies, and DNA was counterstained with Hoechst (blue). Scale bars, 10 μm. (C) HCT116 cells were immunostained with cyclin A and γ-tubulin antibodies. Scale bars, 5 μm. (D) U2OS cells were immunostained for centrosomes with γ-tubulin and C-Nap1 antibodies. Scale bars, 5 μm. (E) Quantification of supernumerary centrosomes in U2OS, HCT116 and TIG3 cells. The number of γ-tubulin spots was counted in at least 300 cells in three independent experiments. Error bars represent one standard deviation.
Figure 3.
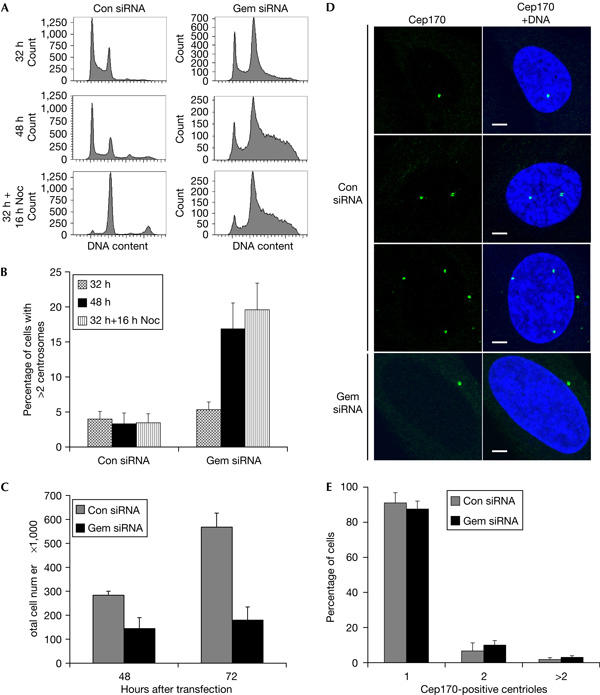
Loss of geminin induces centrosome overduplication and genomic over-replication without passage through mitosis. (A) Flow cytometric profiles of U2OS treated with geminin or control short interfering RNA (siRNA) for 32 or 48 h. Cells were either collected at 32 h after transfection or incubated for another 16 h with nocodazole (Noc). Cells were stained with propidium iodide for DNA content analysis. (B) Quantification of supernumerary centrosomes by γ-tubulin staining of cells treated with or without nocodazole. (C) Total cell number was counted at 48 and 72 h after transfection. (D) U2OS were treated with geminin or control siRNA for 48 h and immunostained with a Cep170 antibody to mark mature centrioles. Scale bars, 5 μm. (E) Comparison of the number of Cep170-positive centrioles in geminin and control siRNA-treated U2OS cells. Error bars represent one standard deviation.
As loss of geminin alters the relative levels of cyclins, we asked whether geminin depletion affects S-phase events other than DNA replication. Centriole duplication, similar to DNA replication, initiates at the G1–S transition and is tightly regulated by various kinases (Sluder, 2004). In particular, cyclin A/CDK2 has been implicated in centrosome duplication (Meraldi et al, 1999), and several studies have shown that its overexpression is linked to centrosome abnormalities in cell lines (Balczon et al, 2001; Faivre et al, 2002) and tissues (Hsu et al, 2004). Therefore, we analysed geminin siRNA-treated HCT116 cells for centrosome defects and found that cells with increased cyclin A levels also contain more than two centrosomes (Fig 1C). Cyclin A/CDK2 is a good candidate for mediating centrosome amplification in geminin-depleted cells, although cyclin E/CDK2 and other kinases might also be involved. Multiple centrosomes were also detected in geminin-depleted U2OS cells by two independent centrosome markers, γ-tubulin and centrosomal Nek2-associated protein 1 (C-Nap1; Fry et al, 1998; Fig 1D,E). A similar phenotype was also observed in TIG3 human diploid fibroblasts (Fig 1E), which indicates that loss of geminin affects centrosome duplication in both normal and cancer cells.
As a control for the specificity of the siRNA oligonucleotides, we used two different U2OS cell lines stably expressing haemagglutinin (HA)-tagged geminin in which two residues in the siRNA target sequence were changed in a way that altered the nucleotide sequence but not the amino acids (Melixetian et al, 2004). Treatment of these cell lines with geminin siRNA caused a decrease in endogenous geminin levels, but the HA-tagged geminin expression remained unaffected and the number of cells with more than two centrosomes remained at background levels (Fig 2A,B). This shows that multiple centrosomes are a consequence of geminin loss.
Figure 2.
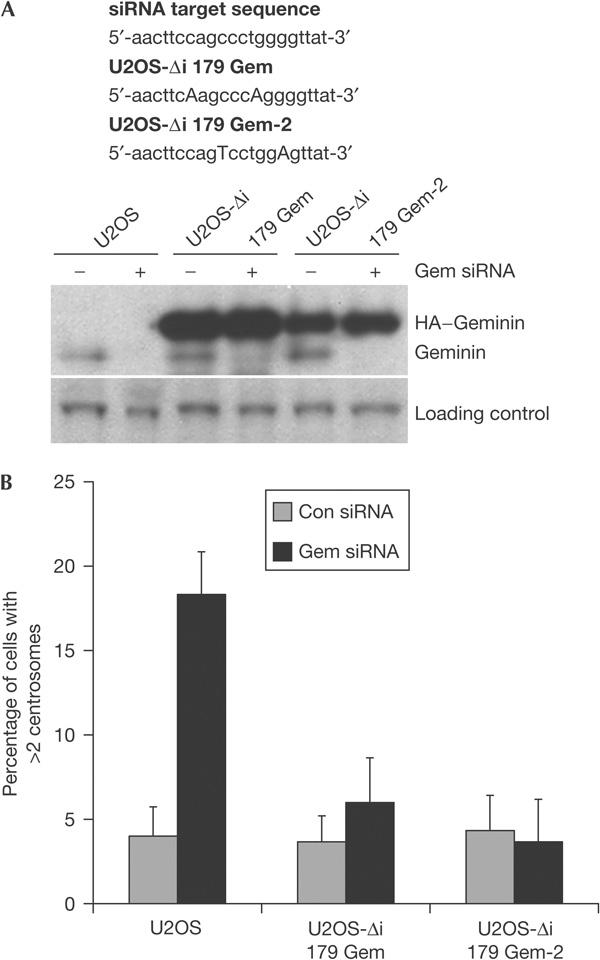
Rescue of geminin short interfering RNA phenotype in cells stably expressing haemagglutinin-tagged RNA interference mutants of geminin. (A) U2OS cell lines expressing two different mutant haemagglutinin (HA)-tagged RNA interference mutants of geminin in which two residues were changed in the short interfering RNA (siRNA) target sequence in a way that altered the nucleotide sequence but not the amino acids (Melixetian et al, 2004). The cell lines were treated with geminin siRNA for 48 h and analysed by western blot with a geminin antibody. (B) Quantification of supernumerary centrosomes by γ-tubulin staining in U2OS cell lines.
Cells can acquire multiple centrosomes by different mechanisms. We used several approaches to distinguish between centrosome overduplication and centrosome accumulation by cell division failure. Geminin depletion caused genomic over-replication in U2OS (Fig 3A) and HCT116, as has been reported recently (Melixetian et al, 2004; Zhu et al, 2004). We used nocodazole at a concentration that resulted in a G2–M arrest in control cells but did not result in significant microtubule depolymerization during interphase (Fig 3A; data not shown). Prolonged incubation with nocodazole did not affect over-replication (compare 48 h−nocodazole with 32 h+16 h nocodazole). Importantly, the addition of nocodazole did not prevent centrosome overduplication in geminin-depleted cells (Fig 3B). The insensitivity of geminin-depleted cells to nocodazole can be explained by loss of geminin preventing entry into mitosis (Melixetian et al, 2004) and, hence, cell division (Fig 3C).
The results indicate that centrosome overduplication occurs without passage through mitosis in geminin-depleted cells. We have investigated this further by examining the number of mature centrioles in control and geminin siRNA-treated cells. The centrosomal protein 170 (Cep170) is a marker of mature centrioles, and antibodies against this protein were recently used successfully to explore the mechanisms underlying centrosome amplification (Guarguaglini et al, 2005). Cep170 associates with a single mature centriole in G1-, S- and early-G2-phase cells, but with two mature centrioles in late G2 phase. A minority of control cells (<5%) contain more than two mature centrioles (Fig 3D,E), which have probably arisen owing to cell division failure. If multiple centrosomes detected by γ-tubulin staining in geminin-depleted cells (Fig 1D) arise from cell division failure, then these cells would also be expected to contain multiple Cep170-positive centrioles. However, most geminin-depleted cells contain only a single mature centriole, as shown by Cep170 staining (Fig 3D,E), which argues strongly in favour of bona fide centrosome overduplication.
Furthermore, we were interested to study the effects of overduplicated centrosomes on mitosis. Geminin depletion causes DNA damage and CHK1/2 activation, and metaphase spreads of geminin-depleted cells showed chromosome breaks (Melixetian et al, 2004; Zhu et al, 2004). On the basis of our data, we suggested that geminin-depleted cells would also have other mitotic defects that stem from overduplicated centrosomes. We used caffeine to override the G2–M DNA damage checkpoint and induced mitosis. Caffeine addition to geminin siRNA-treated cells caused a marked increase in the proportion of cells showing abnormal spindles (Fig 4A,B). Similar spindle defects were observed after 2 or 15 h incubation of geminin siRNA-treated cells with caffeine, although the number of mitotic cells was much higher after the longer treatment. Remarkably, some geminin-depleted cells were able to form nearly bipolar spindles despite the presence of multiple centrosomes, and unattached chromosomes could be detected frequently in these cells (Fig 4C). Other cells containing multiple centrosomes formed multipolar spindles, which may result in chromosome missegregation. To ensure that these phenotypes are specific to geminin loss rather than isolated aneuploid U2OS cells, checkpoint-abrogated cells were double labelled with geminin and α-tubulin to mark the mitotic spindle. In untreated and control cells, geminin was present in prometaphase and metaphase cells. Most of the prometaphase-like cells with abnormal spindles were negative for geminin, which confirmed that these mitotic defects are due to geminin depletion. Therefore, the loss of geminin may promote chromosome mis-segregation and aneuploidy, which are characteristics of human cancer (Nigg, 2002).
Figure 4.
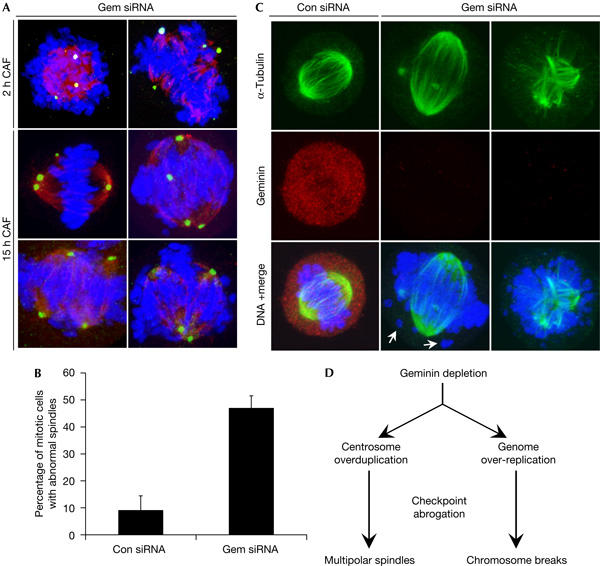
Checkpoint abrogation of geminin-depleted cells leads to multiple mitotic defects. (A) U2OS were incubated with geminin or control short interfering RNA (siRNA) for 48 h and subsequently treated with 5 mM caffeine (CAF) for 2 or 15 h. Cells were immunostained for γ-tubulin (green) and α-tubulin (red). (B) Quantification of mitotic cells with spindle abnormalities after 15 h caffeine treatment. Error bars represent one standard deviation. (C) U2OS were immunostained with geminin and α-tubulin antibodies. Arrows point to unattached chromosome fragments. (D) Summary of geminin depletion phenotypes in human cells.
In summary, our results show that loss of geminin has consequences beyond its immediate role in DNA replication. Geminin depletion causes continuous centrosome duplication without passage through mitosis (Fig 4D). It is possible that this is a downstream effect of genomic over-replication and activation of the G2–M DNA damage checkpoint pathway (Melixetian et al, 2004; Zhu et al, 2004), which is implicated in causing aberrations in centrosome number during a prolonged G2 arrest (Dodson et al, 2004). However, a direct role for geminin in regulating centrosome duplication cannot be excluded at present. Indeed, it is tempting to speculate that geminin functions as a licensing inhibitor of both DNA replication and centrosome duplication during S and G2 phases. Moreover, whereas chemicals that induce genotoxic stress cause centrosome overduplication in the absence of DNA synthesis (Balczon et al, 1995; Bennett et al, 2004), geminin depletion leaves the link between DNA replication and centrosome duplication intact. This offers the opportunity to explore further how these crucial cell cycle events are coupled.
Methods
Cell lines and drugs. Human U2OS osteosarcoma, HCT116 colorectal cancer cells and TIG3 human diploid fibroblasts were grown in 10% fetal calf serum in Dulbecco's modified Eagle's medium (Invitrogen, Paisley, UK). Cells were incubated with 5 mM caffeine (Sigma, Dorset, UK) or 500 ng/ml nocodazole (Sigma, UK).
RNA interference. siRNA oligonucleotides were made to the following target sequences: AACUUCCAGCCCUGGGGUUAU and AAUGCCAACUCUGGAAUCAAA for human geminin siRNA (MWG, Munich, Germany) and AATTCTCCGAACGTGTCACGT for control siRNA (Qiagen, West Sussex, UK). Transfections were carried out with 130 nM siRNA oligonucleotide duplexes with Oligofectamine (Invitrogen, UK), according to the manufacturer's instructions.
Antibodies, immunoblotting and immunofluorescence. Rabbit anti-geminin (Gonzalez et al, 2004), rabbit anti-C-Nap1 (Fry et al, 1998) and rabbit anti-Cep170 (Guarguaglini et al, 2005) antibodies were raised as described earlier. Mouse anti-α-tubulin (Sigma, UK), rabbit anti-γ-tubulin (Sigma, UK) and mouse anti-cyclin A antibodies (Novocastra, Newcastle, UK) were used for immunofluorescence and mouse anti-cyclin A antibody (Vector Labs, Peterborough, UK) was used for immunoblotting. For western blotting, whole-cell lysates were boiled in Laemmli buffer and proteins were resolved by SDS–polyacrylamide gel electrophoresis, transferred to nitrocellulose membranes and probed with appropriate antibodies. Immunofluorescence was carried out as follows. Cells were either grown on coverslips or spun through a sucrose cushion onto polylysine-coated coverslips and fixed with 4% paraformaldehyde. Cells were permeabilized with 0.1% Triton X-100/0.02% SDS in PBS for 10 min and blocked in 1% bovine serum albumin in PBS for 30 min. Coverslips were incubated with primary antibody for 1 h at 37°C, then washed and incubated with Alexa Fluor 546-conjugated goat anti-mouse, Alexa Fluor 546-conjugated goat anti-rabbit or Alexa Fluor 488-conjugated goat anti-rabbit secondary antibodies (Molecular Probes, Eugene, OR, USA) for 30 min at 37°C. DNA was counterstained with Hoechst (Sigma, UK). The cells were examined using a Zeiss 510 confocal microscope (Zeiss, Jena, Germany). For Z-projection analysis, sequential single-plane images were collected through cells at 0.2 μm intervals.
Fluorescence-activated cell sorting analysis. Cells were collected by trypsinization and fixed in 70% methanol overnight at −20°C. Cells were stained with 50 μg/ml propidium iodide (Sigma, UK) in 0.05% NP-40 (BDH, Poole, UK) and 100 μg/ml RNase A (Sigma, UK) in PBS for 1 h at 20°C. The cells were analysed on a Becton Dickinson flow cytometer using FACSDiva software (Becton Dickinson, Oxford, UK).
Acknowledgments
We thank M. Melixetian and K. Helin for generously providing us with their U2OS cell lines expressing HA–geminin. We also thank R. Habedanck, A. Mills and P. McMurtrie for helpful advice, G. Peters for the TIG3 cells and A. Venkitaraman for a critical review of the manuscript. We are grateful to the Medical Research Council (MRC) and Cancer Research UK for their support. K.K.T. is the recipient of an MRC Pre-Doctoral Fellowship, M.A.G. is the recipient of an MRC Clinical Research Training Fellowship and G.G. is the recipient of an FIRC (Italian Foundation for Cancer Research) Fellowship. The authors declare that they have no competing financial interests.
References
- Balczon R et al. (1995) Dissociation of centrosome replication events from cycles of DNA synthesis and mitotic division in hydroxyurea-arrested Chinese hamster ovary cells. J Cell Biol 130: 105–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balczon RC (2001) Overexpression of cyclin A in human HeLa cells induces detachment of kinetochores and spindle pole/centrosome overproduction. Chromosoma 110: 381–392 [DOI] [PubMed] [Google Scholar]
- Bennett RA, Izumi H, Fukasawa K (2004) Induction of centrosome amplification and chromosome instability in p53-null cells by transient exposure to subtoxic levels of S-phase-targeting anticancer drugs. Oncogene 23: 6823–6829 [DOI] [PubMed] [Google Scholar]
- Coverley D, Laman H, Laskey RA (2002) Distinct roles for cyclins E and A during DNA replication complex assembly and activation. Nat Cell Biol 4: 523–528 [DOI] [PubMed] [Google Scholar]
- Diffley JF (2004) Regulation of early events in chromosome replication. Curr Biol 14: R778–R886 [DOI] [PubMed] [Google Scholar]
- Dodson H, Bourke E, Jeffers LJ, Vagnarelli P, Sonoda E, Takeda S, Earnshaw WC, Merdes A, Morrison C (2004) Centrosome amplification induced by DNA damage occurs during a prolonged G2 phase and involves ATM. EMBO J 23: 3864–3873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faivre J et al. (2002) Centrosome overduplication, increased ploidy and transformation in cells expressing endoplasmic reticulum-associated cyclin A2. Oncogene 28: 1493–1500 [DOI] [PubMed] [Google Scholar]
- Fry AM, Meraldi P, Nigg EA (1998) C-Nap1, a novel centrosomal coiled-coil protein and candidate substrate of the cell cycle-regulated protein kinase Nek2. J Cell Biol 141: 1563–1574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez MA et al. (2004) Geminin predicts adverse clinical outcome in breast cancer by reflecting cell-cycle progression. J Pathol 204: 121–130 [DOI] [PubMed] [Google Scholar]
- Guarguaglini G, Duncan PI, Stierhof YD, Holmstrom T, Duensing S, Nigg EA (2005) The forkhead-associated domain protein Cep170 interacts with polo-like kinase 1 and serves as a marker for mature centrioles. Mol Biol Cell 16: 1095–1107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinchcliffe EH, Li C, Thompson EA, Maller JL, Sluder G (1999) of Cdk2–cyclin E activity for repeated centrosome reproduction in Xenopus egg extracts. Science 283: 851–854 [DOI] [PubMed] [Google Scholar]
- Hsu LC, Kapali M, Deloia JA, Gallion HH (2004) Centrosome abnormalities in ovarian cancer. Int J Cancer 113: 746–751 [DOI] [PubMed] [Google Scholar]
- Matsumoto Y, Hayashi K, Nishida E (1999) Cyclin-dependent kinase 2 (Cdk2) is required for centrosome duplication in mammalian cells. Curr Biol 9: 429–432 [DOI] [PubMed] [Google Scholar]
- McGarry TJ, Kirschner MW (1998) Geminin, an inhibitor of DNA replication, is degraded during mitosis. Cell 93: 1043–1053 [DOI] [PubMed] [Google Scholar]
- Melixetian M et al. (2004) Loss of Geminin induces rereplication in the presence of functional p53. J Cell Biol 24: 473–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meraldi P, Lukas J, Fry AM, Bartek J, Nigg EA (1999) Centrosome duplication in mammalian somatic cells requires E2F and Cdk2–cyclin A. Nat Cell Biol 1: 88–93 [DOI] [PubMed] [Google Scholar]
- Nigg EA (2002) Centrosome aberrations: cause or consequence of cancer progression? Nat Rev Cancer 2: 815–825 [DOI] [PubMed] [Google Scholar]
- Sluder G (2004) Centrosome duplication and its regulation in the higher animal cell. In Centrosomes in Development and Disease, Nigg EA (ed) pp 167–189. Weinheim, Germany: Wiley-VCH [Google Scholar]
- Tada S, Li A, Maiorano D, Mechali M, Blow JJ (2001) Repression of origin assembly in metaphase depends on inhibition of RLF-B/Cdt1 by geminin. Nat Cell Biol 3: 107–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohlschlegel JA, Dwyer BT, Dhar SK, Cvetic C, Walter JC, Dutta A (2000) Inhibition of eukaryotic DNA replication by geminin binding to Cdt1. Science 290: 2309–2312 [DOI] [PubMed] [Google Scholar]
- Wong C, Stearns A (2003) Centrosome number is controlled by a centrosome-intrinsic block to reduplication. Nat Cell Biol 5: 539–544 [DOI] [PubMed] [Google Scholar]
- Zhu W, Chen Y, Dutta A (2004) Rereplication by depletion of geminin is seen regardless of p53 status and activates a G2/M checkpoint. Mol Cell Biol 24: 7140–7150 [DOI] [PMC free article] [PubMed] [Google Scholar]


