Abstract
Hypoxia-Inducible Factor (HIF) prolyl hydroxylase domains (PHDs) have been proposed to act as sensors that have an important role in oxygen homeostasis. In the presence of oxygen, they hydroxylate two specific prolyl residues in HIF-α polypeptides, thereby promoting their proteasomal degradation. So far, however, the developmental consequences of the inactivation of PHDs in higher metazoans have not been reported. Here, we describe novel loss-of-function mutants of fatiga, the gene encoding the Drosophila PHD oxygen sensor, which manifest growth defects and lethality. We also report a null mutation in dHIF-α/sima, which is unable to adapt to hypoxia but is fully viable in normoxic conditions. Strikingly, loss-of-function mutations of sima rescued the developmental defects observed in fatiga mutants and enabled survival to adulthood. These results indicate that the main functions of Fatiga in development, including control of cell size, involve the regulation of dHIF/Sima.
Keywords: Drosophila, hypoxia-inducible factor, oxygen sensor, Sima
Introduction
Recent work has led to the definition of widely operative signalling systems that control the transcriptional response to hypoxia through hypoxia-inducible factor (HIF; Maxwell et al, 1993; Wang & Semenza, 1993). HIF proteins are a family of α/β-heterodimers in which the common βsubunit is constitutive and the α-subunits are oxygen-regulated by mechanisms that include transcriptional co-activator recruitment, subcellular localization and protein stabilization (Semenza, 2001; Bruick, 2003; Schofield & Ratcliffe, 2004). The regulation of proteasomal degradation of αsubunits has been well characterized in cell culture and in in vitro systems. In the presence of oxygen, a series of 2-oxoglutarate and iron-dependent dioxygenases termed PHDs (prolyl hydroxylase domains) hydroxylate specific prolyl residues in the HIF-α oxygen-dependent degradation domain (ODDD; Bruick & McKnight, 2001; Epstein et al, 2001), enabling its ubiquitination and proteasomal degradation (Maxwell et al, 1999; Ivan et al, 2001; Jaakkola et al, 2001). As molecular oxygen is absolutely required in the prolyl hydroxylation reaction and enzyme activity is sensitive to mild hypoxia, the PHDs have suitable characteristics that enable them to function as bona fide oxygen sensors that determine the half-life of HIF-α proteins (Semenza, 2001), thereby controlling hypoxia-dependent transcription. Analyses of ‘knockout' mouse strains have shown developmental roles of mammalian HIF proteins (Iyer et al, 1998; Adelman et al, 2000; Tomita et al, 2003; Covello & Simon, 2004; Pfander et al, 2004). They are required for the normal formation of the heart, brain, vasculature, cartilage and placenta, suggesting that fetal oxygen availability might have a role in these processes. However, this question remains open, and the developmental effects of genetic inactivation of the oxygensensitive PHD pathways have not yet been defined (Pugh & Ratcliffe, 2003).
We have previously reported that the Drosophila bHLH-PAS proteins Similar (Sima) and Tango (Tgo) are, respectively, the functional homologues of HIF-α and HIF-β in the fly (Lavista-Llanos et al, 2002; Gorr et al, 2004). We also described a lethal P-element insertional mutation (l(3)02255) in the Drosophila PHD gene (CG1114 in FlyBase) that fails to downregulate Sima protein in normoxia, thus driving constitutive activation of the transcriptional response to hypoxia. The aim of the present work was to investigate the developmental role of Drosophila PHD, which we have named fatiga (fga; Spanish for ‘fatigue') after its lack-of-oxygen phenotype.
Results and Discussion
As a first step in the study of the functions of fga in development, we generated new loss-of-function mutations by mobilizing the l(3)02255 P-element, which is located between the second and third exons of the fga gene. Precise excisions of the transposon led to a reconstitution of the wild-type hypoxic response, as shown by hypoxia-inducible expression of transcriptional reporters that are based on the murine LDH-A enhancer (Ldh-LacZ and Ldh-Gal4; Lavista-Llanos et al, 2002). Imprecise excisions of the P-element resulted in three novel fga alleles (fga1, fga9 and fga64; Fig 1A) that were characterized at the molecular level by Southern blot analysis and PCR experiments. fga9 conserved a 1.4 kb fragment of the original transposon; in fga64, a large genomic portion upstream of the insertion site was removed, and fga1 conserved a fragment of about 9 kb of the original P-element (Fig 1A). In normoxic wild-type embryos, Sima protein and induction of the Ldh-Gal4 reporter were not detected; upon exposure to hypoxia (5% O2), both Sima protein and reporter expression were observed, mainly in the tracheal system (Fig 1B; Lavista-Llanos et al, 2002). The molecular basis of this pattern of induction is now under investigation. Interestingly, fga loss-of-function alleles showed different levels of accumulation of Sima protein in normoxia, which was widely expressed in fga1 and fga64, and showed some prevalence in the tracheal system in fga9, correlating with the constitutive induction of the hypoxic reporter (Fig 1B). fga02255, fga1 and fga64 were lethal at the first larval instar and fga9 died in the pupal stage. As overexpression of Sima through a ubiquitous Gal4 driver provokes lethality in the larval stages, we reasoned that lethality in fga mutants could be due to overaccumulation of Sima protein in normoxia. To test this hypothesis and to determine if Fga is a dedicated regulator of Sima or whether, alternatively, it might modulate other molecular targets (Frei & Edgar, 2004; Frei et al, 2005), we sought to analyse fga phenotypes in a sima-free genetic background. Loss-of-function mutations of sima have not been reported so far, but two different P-element insertions mapping within the sima locus were available from the Public Stock Centers. One of these insertion lines was able to respond to hypoxia and, thus, was indistinguishable from the wild type (data not shown). In contrast, embryos homozygous for the other insertion, sima07607 (Fig 2A), did not express sima mRNA (Fig 2B) and failed to induce the Ldh-LacZ reporter in hypoxia (Fig 2C). Introduction of a UASsima transgenic element under the control of an hs-Gal4 driver was able to rescue induction of reporter expression, which was expressed in a wild-type pattern (Fig 2C), indicating that the absence of Sima was indeed responsible for the lack of hypoxic response. Altogether, these results indicate that sima07607 is a sima loss-of-function allele.
Figure 1.
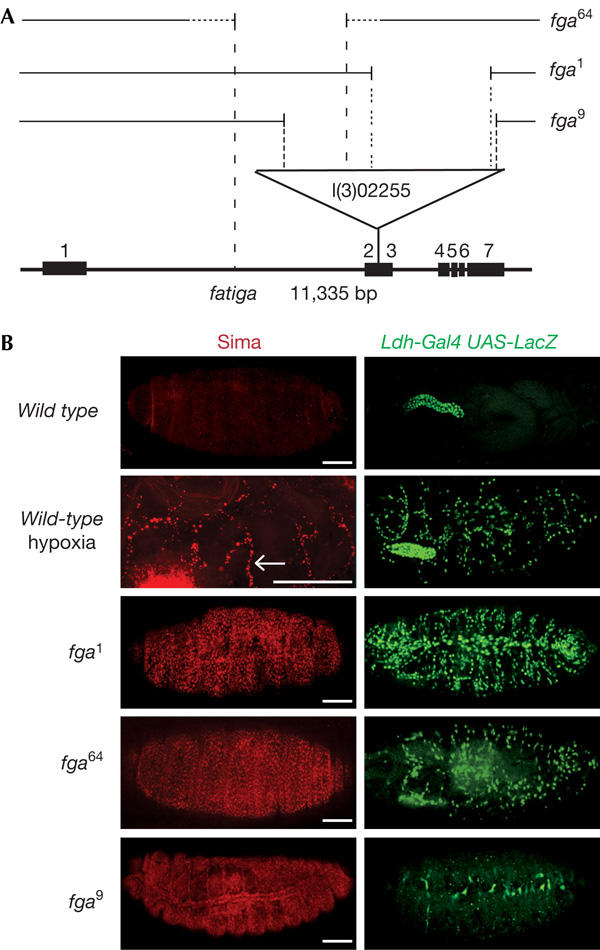
Phenotypes of fatiga mutations. (A) Schematic representation of the fga locus; the P-element insertional allele fga02255 and the three novel alleles fga1, fga9 and fga64 generated by imprecise excisions are indicated. (B) Sima protein (red) and Ldh-Gal4 UAS-LacZ reporter expression (green) were not observed in wild-type embryos in normoxia, but were turned on in hypoxia (5% O2) in a pattern that followed tracheal branches (arrow). In fga mutants, Sima protein accumulation and induction of the reporter occurred in normoxia. On the basis of the expression of the reporter, the relative strength of the alleles was fga1>fga64>fga9, although Sima levels looked similar in fga1 and fga64 embryos. Scale bars, 50 μm.
Figure 2.
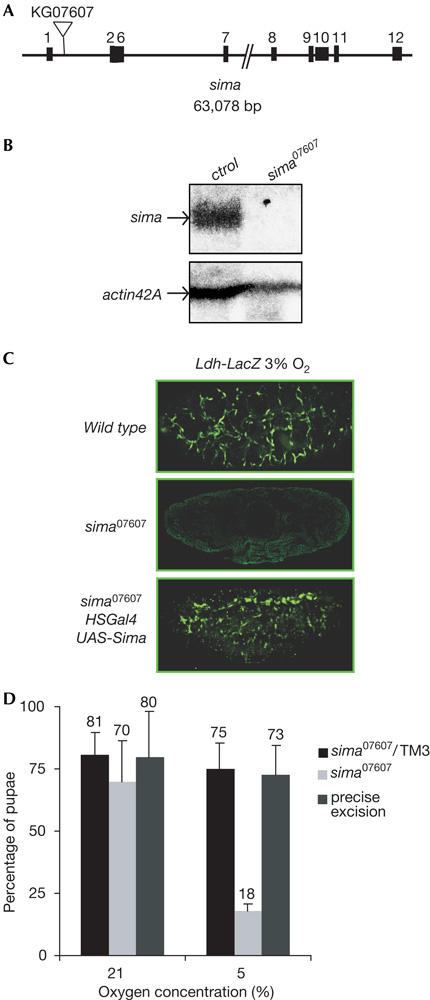
Characterization and phenotypes of sima mutants. (A) Schematic representation of the sima locus showing sima07607 P-element insertion. (B) Northern blot analysis showing that sima mRNA is expressed in wild-type but not in sima07607 homozygous flies in normoxia. (C) The Ldh-LacZ reporter was induced following 5 h exposure to 3% O2 in control embryos but not in sima07607 homozygotes; a UASsima element rescued reporter induction in sima07607 mutants. (D) Only a small proportion of sima07607 homozygotes attained the pupal stage at 5% O2 as compared with the control larvae. Precise excision of the P-element led to complete rescue of survival in hypoxia (5% O2).
To explore whether the absence of Sima protein, and thus the inability to respond to hypoxia, affects developmental progression, we analysed phenotypes in sima07607 mutants. Homozygous mutant embryos developed without any obvious difference from the controls, and the first-instar larvae looked healthy and motile. Next, we placed homozygous mutant or control larvae in vials containing fresh food, which were then exposed to 21% or 5% O2 until individuals attained the pupal stage. We observed that sima07607 mutants were viable and fertile in normoxia, but virtually unable to develop in hypoxia (Fig 2D). Precise excision of the P-element totally reverted hypoxia-dependent lethality, which indicated that the insertion was indeed responsible for this phenotype (Fig 2D). Thus, we conclude that, unlike Tango that participates as a common bHLH-PAS partner in several developmental processes in normoxia (Sonnenfeld et al, 1997), Sima is necessary for developmental progression in hypoxia but not in normoxia.
Recently, Frei & Edgar (2004) reported that fga mutations caused a reduction in cell size, but it is unclear whether this effect depends on overaccumulation of Sima. The availability of sima07607 as a sima loss-of-function allele enabled us to answer this particular question and, more generally, to address the extent to which the developmental defects of fga loss-of-function mutations are due to the de-regulated accumulation of Sima protein. As expected, in fga1sima07607 double homozygous mutants, Sima protein was undetectable and embryos did not show any expression of hypoxia-inducible reporters in normoxia (Fig 3A). Consistent with previously described growth defects of fga mutants (Frei & Edgar, 2004), fga9 pupae were smaller than their heterozygous siblings (Fig 3B,C) and, interestingly, they exhibited a delay in larval development, taking 2 additional days to reach the pupariation stage (Fig 3D). Strikingly, fga1sima07607 double homozygous mutants were indistinguishable from the controls, both in their pupal weight and in the duration of larval development (Fig 3B–D). Thus, the loss of Sima provoked the complete reversion of growth defects occurring in fga mutants. To answer whether overaccumulation of Sima is sufficient to account for the autonomous reduction in cell size that was reported for fga mutant cells (Frei & Edgar, 2004), we overexpressed Sima protein in random clones using the flipase-induced recombination (FLP-OUT) technique, and the effect on cell size was analysed. As shown in Fig 3E (see also supplementary Fig S1 online), overexpression of Sima in isolated cells caused a marked autonomous reduction of cell size, which correlated with smaller nuclei, as shown by 4,6-diamidino-2-phenylindole (DAPI) staining. Taken together, these results indicate that Sima is a downstream effector of Fga as a regulator of cell growth. Further analyses were carried out on the tracheal system; once again defects (particularly, air-filling impairment) that were observed in fga mutants, were corrected in fga sima double mutants (see the supplementary information online and supplementary Fig S2 online).
Figure 3.
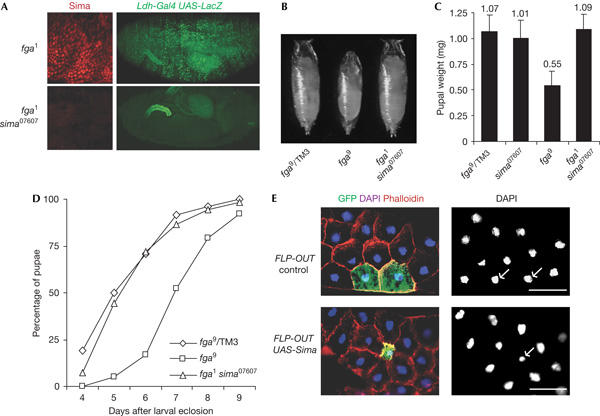
sima loss-of-function reverts phenotypic defects of fatiga mutants. (A) Normoxic accumulation of Sima and induction of the Ldh-Gal4 hypoxia-inducible reporter occurred in fga1 homozygous embryos but not in fga1 sima07607 double mutants. (B,C) fga9 homozygous pupae were smaller than their heterozygous siblings and (D) larval development until pupariation lasted 2 days more than that in the controls (100% refers to the individuals from each genotype that attained the pupal stage). Both pupal weight (N>13) and duration of larval stages of fga1sima07607 (N>120) were indistinguishable from the those of the controls (B–D). (E) Sima was randomly overexpressed in isolated cells using the ‘flip-out' method. Sima-expressing cells in the fat body (marked with green fluorescent protein (GFP)) were smaller than neighbouring cells that did not express Sima (cells unmarked with GFP). Similar flip-out control experiments in which GFP alone was expressed in random cells did not cause any growth alteration. Note that nuclei from cells overexpressing Sima were smaller than those from neighbouring cells. DAPI, 4,6-diamidino-2-phenylindole. Scale bars, 50 μm.
Given the reversion of the analysed fga phenotypes in fga sima double mutants, we wanted to test whether lethality that occurred following fga loss-of-function is also due to overaccumulation of Sima. Data in Fig 4 show that this is indeed the case, as, in normoxia, fga1 sima07607 double homozygous mutants were viable to adulthood, even when many of these adults failed to complete emergence from the pupal case, and those that emerged (24%; N>160) looked weak and frequently died shortly afterwards. As expected, in hypoxia, this reversion of lethality did not occur, and fga or sima single mutants, as well as fga sima double mutant flies, died in the first larval stage. Overall, these results show that Drosophila development can proceed in the absence of PHD oxygen sensors, provided that the HIF-α subunit is absent and oxygen availability is not compromised. Thus, we conclude that the most fundamental functions of Fatiga/PHD in development probably involve the downregulation of Sima protein levels. However, fga sima exarate adults show defects in wing and ovary development (supplementary Fig S3 online), which may imply that Fga is involved in patterning these organs in a Sima-independent manner. Detailed genetic and molecular analysis of fga sima double mutants should help to define Sima-independent developmental functions of the oxygen sensor in Drosophila.
Figure 4.
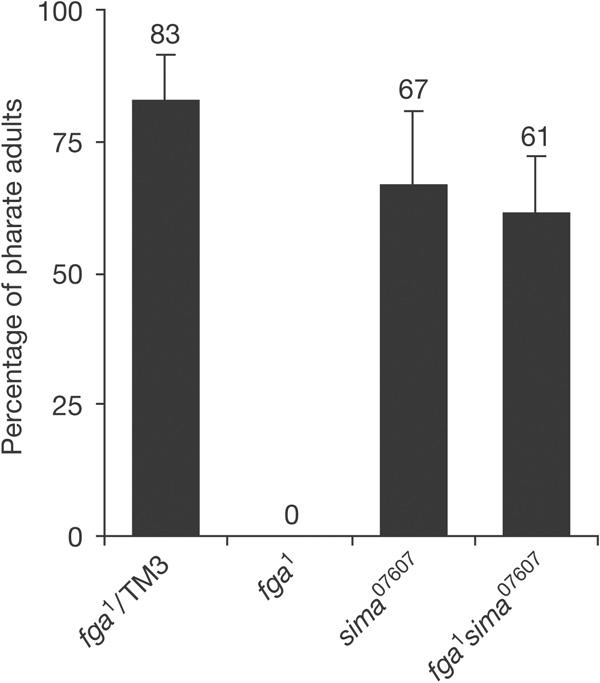
sima loss of function reverts lethality of fatiga mutants in normoxia. A large proportion of control or sima07607 homozygous first-instar larvae, but none of the fga1 homozygotes, reached the adult stage in normoxia. Viability to adulthood was restored in fga1 sima07607 double homozygous mutants (N>160).
The relationship between developmental processes and inducible systems dedicated to maintaining an organism's homeostasis following environmental challenges is only beginning to be explored (Britton et al, 2002). The current work on oxygen signalling shows that although aberrant activation of the hypoxia-responsive machinery has the potential to disturb development and cause lethality, total inactivation of the system is compatible with developmental progression that is sufficient to maintain life—at least in the basal state. The work suggests the operation of a hierarchical organization, in which inducible oxygen signalling is superimposed on ‘hard-wired' developmental programmes that already make a significant contribution to the physiological challenge of oxygen homeostasis (Jarecki et al, 1999; Frazier et al, 2001). We speculate that a similar hierarchy and common functionality between ontogenic processes and inducible-response pathways may apply to other systems and may be an important principle facilitating adaptation. Interestingly, several other examples have been reported in which removal of one regulatory component of a pathway that is crucial for maintenance of homeostasis leads to the constitutive activation of a downstream effector, causing lethality, whereas the simultaneous ablation of the effector together with the regulator restores the ability of the organism to develop: (i) Mdm2 knockout mice fail to develop beyond embryonic day 6.5, but concomitant genetic ablation of its dedicated target molecule, p53, allows the development of normal and fertile adult mice (Jones et al, 1995; Montes de Oca Luna et al, 1995). (ii) Drosophila PTEN mutations are lethal but if such mutations are combined with mutations in the protein kinase B PH domain, developmental progression is largely restored (Stocker et al, 2002). Thus, the de-regulation of pathways that maintain cell homeostasis is apparently more detrimental than inactivating the whole pathway, suggesting that despite the ability of these pathways to perturb development, their physiological operation is essentially superimposed on a relatively sufficient ‘hard-wired' programme.
Methods
Fly strains and genetics. Fly stocks used in this study were Ldh-Gal4 UAS-GFPLacZ and Ldh-LacZ (Lavista-Llanos et al, 2002), Hs-Gal4, btl-Gal4 (Shiga et al, 1996), Term-Gal4 (Jarecki et al, 1999), escargot-Gal4 (Steneberg et al, 1998), UASsima (Bacon et al, 1998), sima07607 (kindly provided by Hugo Bellen), fga02255 (Spradling et al, 1999), Hs-FLP122, act>CD2>GAL4 and the TM3 act-GFP balancer chromosome (Bloomington Stock Center, Bloomington, IN, USA).
Generation of fatiga alleles: fga02255 P [lacZ, ry+] element was mobilized through crosses with a Δ2-3 line, overexpressing transposase. rosy− males from the offspring were individually crossed with females carrying a TM3ry balancer to establish balanced stocks, and were tested for lethality against the chromosomal deficiency Df(3)R3-4.
Precise excision of P{SUP or P}sima07607: the sima07607 homozygous viable P[y+w+] element was mobilized as above and the resulting y− w− males were used to establish independent lines. Homozygous flies from these stocks were tested for survival at 5% O2.
Generation of fga1sima07607double mutants: heterozygous y w; fga1/TM3Sb flies were crossed with y w; sima07607 individuals and non-Sb females from the offspring were crossed with y w; Xa/TM3 males. Males carrying the sima07607 mutation, identified by dark body colour and red eyes, were tested for lethality by crossing them individually with Df(3)R3-4/TM3Sb females. The presence of the fga1 mutation in candidate double mutant strains was confirmed by Southern blot.
Molecular analysis of fatiga mutations. For Southern blots, genomic DNA extracted from fga1, fga9, fga64 and fga02255 heterozygous larvae or from wild-type controls was digested with different restriction enzymes, run on agarose gels, transferred to a Zeta-Probe GT Genomic membrane (Bio-Rad Laboratories, Hercules, CA, USA) and hybridized with 32P-labelled genomic fragments flanking or including the P-element insertion site.
Immunostaining and bright-field microscopy. Rabbit anti-β-gal antibody (Cappel MP Biomedicals, Irvine, CA, USA) was used at a dilution of 1:1200 and rat anti-Sima antibody (Lavista-Llanos et al, 2002) was used at a 1:150 dilution. Cy™2-conjugated anti-rabbit and Cy™3-conjugated anti-rat secondary antibodies were used at a 1:150 dilution (Jackson Laboratories, West Grove, PA, USA). Observations were made by fluorescence microscopy in a BX-60 Olympus microscope or by confocal microscopy in a Zeiss LSM5 Pascal microscope.
Northern blot. Total RNA was isolated from sima07607 or y w adult flies using the Trizol reagent (Life Technologies, Carlsbad, CA, USA); 25 μg of total RNA was run on a formaldehyde gel, transferred to Zeta-Probe GT Genomic membrane (Bio-Rad) and hybridized with 32P-CTP-labelled sima or actin42A probes. A storm 840 phosphoimager (Molecular Dynamics, Sunnyvale, CA, USA) was used to detect 32P emission.
Hypoxia treatment. Hypoxia was applied in a Forma Scientific 3131 incubator, by regulating the proportions of oxygen and nitrogen at 25°C. For studies of survival, adults carrying a mutation balanced with TM3 act-GFP (green fluorescent protein) were placed in egg-laying bottles and the first-instar larvae from the offspring were sorted under a fluorescence microscope. The same number of homozygous mutants or GFP-expressing heterozygous larvae were placed in vials containing fresh food, which were then exposed to 21% or 5% O2 until individuals attained pupal or adult stages.
Analysis of growth defects. The first-instar larvae of different genotypes were sorted and placed in fresh vials, as described above. To measure the duration of larval development, pupae were counted daily until day 10 after the first-instar larval sorting. For the experiments described in Fig 3C, pupae were weighed 24 h after pupariation using a Mettler AE 240 electronic balance. For ‘flip-out' experiments, hsFLP; act>CD2>Gal4/UASGFP or hsFLP; act>CD2>Gal4/UAS-GFP UASsima flies were heat shocked during embryogenesis for 15 min at 35°C and then allowed to develop at 25°C; third-instar larvae were dissected, fixed in 4% paraformaldehyde, stained with 4,6-diamidino-2-phenylindole and/or phalloidin and mounted in 40% glycerol.
Supplementary information is available at EMBO reports online (http://www.emboreports.org).
Supplementary Material
Supplementary Information
Acknowledgments
We are grateful to H. Bellén, M. Krasnow, M. Llimargas, B.Z. Shilo and Bloomington Stock Center for fly stocks and to J. Riesgo-Escovar, G. Boccaccio, M. Muzzopappa and members of Wappner's and Ceriani's labs for helpful discussions. This work was supported by the Wellcome Trust grant 070161/Z/03/Z to P.W. and P.J.R. and Universidad de Buenos Aires X411 and ANPCyT 01-10839 to P.W. L.C. is a fellow of the Fundación Antorchas and P.W. is a career investigator of CONICET.
References
- Adelman DM, Gertsenstein M, Nagy A, Simon MC, Maltepe E (2000) Placental cell fates are regulated in vivo by HIF-mediated hypoxia responses. Genes Dev 14: 3191–3203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacon NC, Wappner P, O'Rourke JF, Bartlett SM, Shilo B, Pugh CW, Ratcliffe PJ (1998) Regulation of the Drosophila bHLH-PAS protein Sima by hypoxia: functional evidence for homology with mammalian HIF-1α. Biochem Biophys Res Commun 249: 811–816 [DOI] [PubMed] [Google Scholar]
- Britton JS, Lockwood WK, Li L, Cohen SM, Edgar BA (2002) Drosophila's insulin/PI3-kinase pathway coordinates cellular metabolism with nutritional conditions. Dev Cell 2: 239–249 [DOI] [PubMed] [Google Scholar]
- Bruick RK (2003) Oxygen sensing in the hypoxic response pathway: regulation of the hypoxia-inducible transcription factor. Genes Dev 17: 2614–2623 [DOI] [PubMed] [Google Scholar]
- Bruick RK, McKnight SL (2001) A conserved family of prolyl-4-hydroxylases that modify HIF. Science 294: 1337–1340 [DOI] [PubMed] [Google Scholar]
- Covello KL, Simon MC (2004) HIFs, HYpoxia, and vascular development. Curr Top Dev Biol 62: 37–54 [DOI] [PubMed] [Google Scholar]
- Epstein AC et al. (2001) C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell 107: 43–54 [DOI] [PubMed] [Google Scholar]
- Frazier MR, Woods HA, Harrison JF (2001) Interactive effects of rearing temperature and oxygen on the development of Drosophila melanogaster. Physiol Biochem Zool 74: 641–650 [DOI] [PubMed] [Google Scholar]
- Frei C, Edgar BA (2004) Drosophila cyclin D/Cdk4 requires Hif-1 prolyl hydroxylase to drive cell growth. Dev Cell 6: 241–251 [DOI] [PubMed] [Google Scholar]
- Frei C, Galloni M, Hafen E, Edgar BA (2005) The Drosophila mitochondrial ribosomal protein mRpL12 is required for Cyclin D/Cdk4-driven growth. EMBO J 24: 623–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorr TA, Tomita T, Wappner P, Bunn HF (2004) Regulation of Drosophila hypoxia-inducible factor (HIF) activity in SL2 cells: identification of a hypoxia-induced variant isoform of the HIFα homolog gene similar. J Biol Chem 279: 36048–36058 [DOI] [PubMed] [Google Scholar]
- Ivan M, Kondo K, Yang H, Kim W, Valiando J, Ohh M, Salic A, Asara JM, Lane WS, Kaelin WG Jr (2001) HIFα targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science 292: 464–468 [DOI] [PubMed] [Google Scholar]
- Iyer NV et al. (1998) Cellular and developmental control of O2 homeostasis by hypoxia-inducible factor 1α. Genes Dev 12: 149–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaakkola P et al. (2001) Targeting of HIF-α to the von Hippel–Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science 292: 468–472 [DOI] [PubMed] [Google Scholar]
- Jarecki J, Johnson E, Krasnow MA (1999) Oxygen regulation of airway branching in Drosophila is mediated by branchless FGF. Cell 99: 211–220 [DOI] [PubMed] [Google Scholar]
- Jones SN, Roe AE, Donehower LA, Bradley A (1995) Rescue of embryonic lethality in Mdm2-deficient mice by absence of p53. Nature 378: 206–208 [DOI] [PubMed] [Google Scholar]
- Lavista-Llanos S, Centanin L, Irisarri M, Russo DM, Gleadle JM, Bocca SN, Muzzopappa M, Ratcliffe PJ, Wappner P (2002) Control of the hypoxic response in Drosophila melanogaster by the basic helix–loop–helix PAS protein similar. Mol Cell Biol 22: 6842–6853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell PH, Pugh CW, Ratcliffe PJ (1993) Inducible operation of the erythropoietin 3′ enhancer in multiple cell lines: evidence for a widespread oxygensensing mechanism. Proc Natl Acad Sci USA 90: 2423–2427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell PH, Wiesener MS, Chang GW, Clifford SC, Vaux EC, Cockman ME, Wykoff CC, Pugh CW, Maher ER, Ratcliffe PJ (1999) The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature 399: 271–275 [DOI] [PubMed] [Google Scholar]
- Montes de Oca Luna R, Wagner DS, Lozano G (1995) Rescue of early embryonic lethality in mdm2-deficient mice by deletion of p53. Nature 378: 203–206 [DOI] [PubMed] [Google Scholar]
- Pfander D, Kobayashi T, Knight MC, Zelzer E, Chan DA, Olsen BR, Giaccia AJ, Johnson RS, Haase VH, Schipani E (2004) Deletion of Vhlh in chondrocytes reduces cell proliferation and increases matrix deposition during growth plate development. Development 131: 2497–2508 [DOI] [PubMed] [Google Scholar]
- Pugh CW, Ratcliffe PJ (2003) Regulation of angiogenesis by hypoxia: role of the HIF system. Nat Med 9: 677–684 [DOI] [PubMed] [Google Scholar]
- Schofield CJ, Ratcliffe PJ (2004) Oxygen sensing by HIF hydroxylases. Nat Rev Mol Cell Biol 5: 343–354 [DOI] [PubMed] [Google Scholar]
- Semenza GL (2001) HIF-1, O2, and the 3 PHDs: how animal cells signal hypoxia to the nucleus. Cell 107: 1–3 [DOI] [PubMed] [Google Scholar]
- Shiga Y, Tanaka-Matakatu M, Hayashi S (1996) A nuclear GFP/β-galactosidase fusion protein as a marker for morphogenesis in living Drosophila. Dev Growth Differ 38: 99–106 [Google Scholar]
- Sonnenfeld M, Ward M, Nystrom G, Mosher J, Stahl S, Crews S (1997) The Drosophila tango gene encodes a bHLH-PAS protein that is orthologous to mammalian Arnt and controls CNS midline and tracheal development. Development 124: 4571–4582 [DOI] [PubMed] [Google Scholar]
- Spradling AC, Stern D, Beaton A, Rhem EJ, Laverty T, Mozden N, Misra S, Rubin GM (1999) The Berkeley Drosophila Genome Project gene disruption project: single P-element insertions mutating 25% of vital Drosophila genes. Genetics 153: 135–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steneberg P, Englund C, Kronhamn J, Weaver TA, Samakovlis C (1998) Translational readthrough in the hdc mRNA generates a novel branching inhibitor in the Drosophila trachea. Genes Dev 12: 956–967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker H, Andjelkovic M, Oldham S, Laffargue M, Wymann MP, Hemmings BA, Hafen E (2002) Living with lethal PIP3 levels: viability of flies lacking PTEN restored by a PH domain mutation in Akt/PKB. Science 295: 2088–2091 [DOI] [PubMed] [Google Scholar]
- Tomita S et al. (2003) Defective brain development in mice lacking the Hif-1α gene in neural cells. Mol Cell Biol 23: 6739–6749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GL, Semenza GL (1993) General involvement of hypoxia-inducible factor 1 in transcriptional response to hypoxia. Proc Natl Acad Sci USA 90: 4304–4308 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information


