Abstract
Conventional centrosomes are absent from a female meiotic spindle in many animals. Instead, chromosomes drive spindle assembly, but the molecular mechanism of this acentrosomal spindle formation is not well understood. We have screened female sterile mutations for defects in acentrosomal spindle formation in Drosophila female meiosis. One of them, remnants (rem), disrupted bipolar spindle morphology and chromosome alignment in non-activated oocytes. We found that rem encodes a conserved subunit of Cdc2 (Cks30A). As Drosophila oocytes arrest in metaphase I, the defect represents a new Cks function before metaphase–anaphase transition. In addition, we found that the essential pole components, Msps and D-TACC, were often mislocalized to the equator, which may explain part of the spindle defect. We showed that the second cks gene cks85A, in contrast, has an important role in mitosis. In conclusion, this study describes a new pre-anaphase role for a Cks in acentrosomal meiotic spindle formation.
Keywords: Cdk, chromosome, meiosis, microtubule, Msps
Introduction
Spindle formation in female meiosis is unique in terms of the absence of conventional centrosomes (McKim & Hawley, 1995). Instead, chromosomes have a central role in the assembly of spindle microtubules. This acentrosomal (also called acentriolar or anastral) spindle formation is common in female meiosis for many animals including mammals, insects and worms. Despite potential medical implications, this spindle formation is much less studied than centrosome-mediated spindle formation in mitosis.
Drosophila provides a valuable tool to study the acentrosomal spindle formation in vivo. Unlike many other species, mature non-activated Drosophila oocytes arrest in metaphase of meiosis I until ovulation, which coincides with fertilization (Mahowald & Kambysellis, 1980). This provides a unique opportunity to study spindle formation, without interference from chromosome segregation or meiotic exit.
We have previously identified two components of acentrosomal spindle poles, Msps and D-TACC, which physically interact and are crucial for spindle bipolarity (Cullen & Ohkura, 2001). Other studies have identified essential components for spindle formation, such as kinesin-like proteins (Ncd and Sub; Endow et al, 1990; McDonald et al, 1990; Giunta et al, 2002), γ-tubulin (Tavosanis et al, 1997) and a membrane protein surrounding the spindle (Axs; Kramer & Hawley, 2003). Some of these spindle components are probably modulated by cell-cycle regulators, but our knowledge of the regulation is limited. To identify essential components and regulators, we carried out a cytological screen for mutants defective in acentrosomal spindle formation of non-activated oocytes.
Through the screen, we identified remnants and found that it is a mutant of a Drosophila Cks/Suc1 homologue, Cks30A. Cks is the third subunit of the Cdc2 (Cdk1)–cyclin B complex, but the role of Cks is less clearcut than that of other subunits of the complex. It is implicated in entry into mitosis/meiosis, metaphase–anaphase transition, exit from mitosis/meiosis and inactivation of Cdk inhibitors (Patra & Dunphy, 1996; Polinko & Strome, 2000; Ganoth et al, 2001; Spruck et al, 2001, 2003). Here, we show that Cks30A is required for spindle morphogenesis and chromosome alignment in the metaphase I spindle in arrested mature oocytes. This requirement of a Cks before metaphase–anaphase transition represents a new function that has not previously been identified. Furthermore, we found that essential spindle pole components Msps and D-TACC mislocalize in the mutant, which may be partly responsible for the spindle defects.
Results
Screening for spindle mutants in female meiosis
For molecular analysis of the acentrosomal spindle in Drosophila female meiosis, we screened female sterile mutants for spindle defects in non-activated oocytes. Female sterile mutants on the second chromosome have previously been isolated (Schupbach & Wieschaus, 1989). We focused on classes of mutants that lay eggs that do not develop beyond the blastoderm stage (class 1 and 2 in Schupbach & Wieschaus, 1989). This category of mutants includes known meiotic mutants affecting spindle formation, such as fs(2)TW1 (γ-tubulin 37C; Tavosanis et al, 1997) and subito (a kinesin-like protein; Giunta et al, 2002).
As many of the stocks gained background lethal mutations, we recombined them out or placed them over appropriate deficiencies (large chromosomal deletions) before examination. Non-activated oocytes from each mutant were dissected from mature adult females, and spindle and chromosomes were stained using α-tubulin antibody and DAPI. This study focused on one of the mutants, remnants1 (rem1 or remRA74), which showed an abnormal spindle and chromosome misalignment (see below).
Molecular identification of the remnants gene
For understanding the molecular role, we determined the identity of the remnants (rem) gene. The rem gene was previously mapped to 30A-C using a deficiency (Df(2L)30AC; Schupbach & Wieschaus, 1989). We confirmed spindle defects in rem over the deficiency. Further deficiency mapping located the rem1 mutation between the left breakpoints of two deficiencies, Df(2L)N22-3 and Df(2L)gamma7 (Fig 1A). As these breakpoints had been mapped only cytologically, we physically mapped them more precisely (data not shown). To further narrow the region, we used synthetic deficiencies that have defined breakpoints (Golic & Golic, 1996). They were created either by us—using stocks obtained from the Drosdel project—or by Exelixis. Complementation tests using these deficiencies limited the location of the rem gene to within a 175 kb region (Fig 1A).
Figure 1.
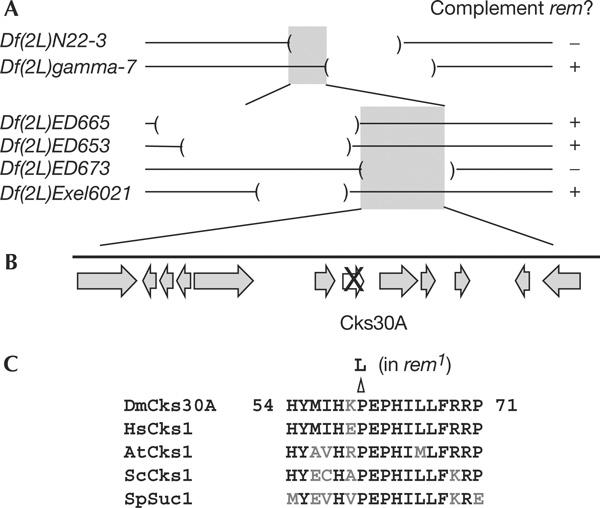
Molecular identification of the remnants gene. (A) Deficiency mapping of the rem gene. The rem gene is located within the 175 kb region (grey boxes). (B) Physical map around the rem gene. The arrows represent the predicted genes in the region. One gene (cks30A) contains a missense mutation. (C) Sequence comparison among Cks homologues. At, Arabidopsis thaliana; Dm, Drosophila melanogaster; Hs, Homo sapiens; Sc, Saccharomyces cerevisiae; Sp, Schizosaccharomyces pombe.
We amplified and sequenced all predicted coding regions and splicing junctions within the region from the rem1 mutant. We found one missense mutation in the gene CG3738 (cks, hereafter called cks30A; Finley & Brent, 1994). There were no other mutations within coding sequences and splicing junctions in the region. In addition, we tested the amount and size of the transcripts that are known to be expressed in adult females, and found no differences between rem and wild type (data not shown).
Cks30A is one of two Drosophila homologues of Saccharomyces cerevisiae Cks1/Schizosaccharomyces pombe Suc1, a conserved subunit of the Cdc2 (Cdk1)/cyclin B complex, and has been shown to interact with Cdc2 (Finley & Brent, 1994). The mutation in rem1 results in a conversion of the 61st amino acid from proline to leucine. This proline is completely conserved among all Cks homologues (Fig 1C), further confirming that the mutation is not a polymorphism. Crystal structure analysis has indicated that this residue forms part of the interaction surface with Cdc2 (Bourne et al, 1996). Immunoblots using an anti-human Cks1 antibody indicated that this mutation disrupts the stability of the Cks30A protein (see below).
Cks30A is essential for metaphase I in female meiosis
To explain the role of Cks30A, we focused our analysis of the rem1 mutant on non-activated oocytes, which arrest in metaphase I. Non-activated oocytes were dissected from wild type and the rem1 mutant, and chromosomes and spindles were visualized by immunostaining.
In wild type, non-activated mature oocytes contain a single bipolar spindle around chromosomes. Bivalent chromosomes align symmetrically with chiasmatic chromosomes at the equator and achiasmatic chromosomes that are located nearer the poles (Fig 2A). The rem1 mutant was able to enter meiosis, condense chromosomes and assemble microtubules around chromosomes. However, only a minority of spindles showed normal spindle morphology and chromosome alignment.
Figure 2.
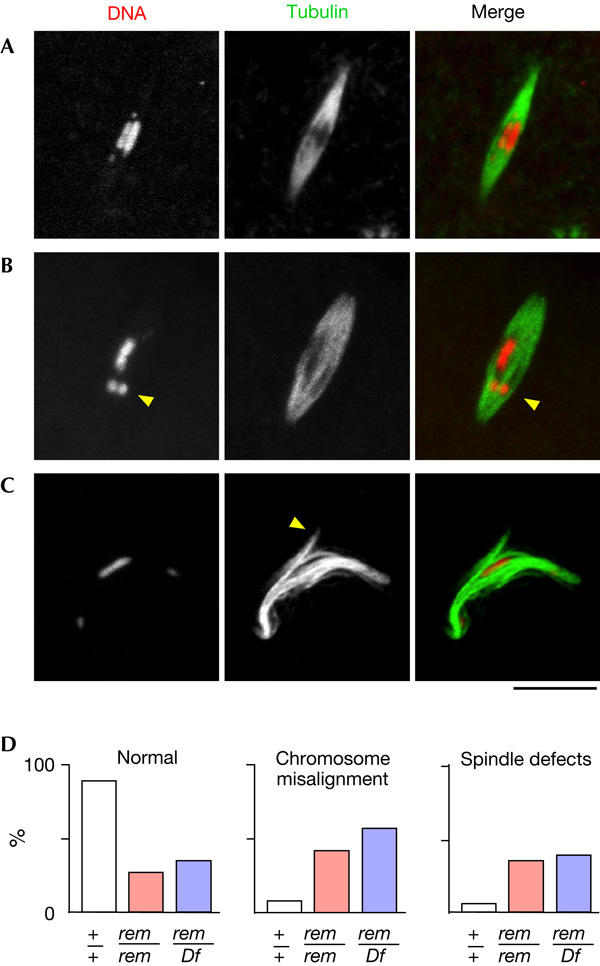
Cks30A is essential for spindle morphogenesis and chromosome alignment. The spindle and chromosomes in non-activated oocytes were visualized by immunostaining in wild type (A) and in the rem1 mutant (B,C). Spindle abnormality and chromosome misalignment in the rem1 mutant. The arrowheads in (B,C) indicate a mispositioned/misorientated chromosome and an ectopic spindle pole formed near the equator, respectively. Spindles are longer in the rem+ mutant (21.1±5.7 μm) than in wild type (14.7±5.8 μm). (D) Quantification of the spindle and chromosome defects in wild type (+/+), rem1 homozygotes (rem/rem) and hemizygotes (rem/Df). Between 29 and 135 spindles were counted for each genotype. The differences between wild type and rem1 homozygotes (or hemizygotes) are significant (P<0.001), whereas the differences between rem homozygotes and hemizygotes are not (P=0.84). Scale bar, 10 μm.
The most prominent defect in the rem1 mutant was chromosome misalignment. This defect was observed in about a half of the spindles (Fig 2D). Even in the cases in which the spindle remained well organized, chiasmatic chromosomes often moved away from the equator and lost overall symmetrical distribution (Fig 2B). The second class of defect in the rem1 mutant was abnormal spindle morphology. Although the abnormality varied from spindle to spindle in the rem1 mutant, the most typical defect was the formation of ectopic poles near the spindle equator (Fig 2C). The focusing of spindle poles seemed to be unaffected.
Further quantitative analysis showed no significant difference between the phenotypes of rem1 homozygotes (rem1/rem1) and hemizygotes (rem1/Df). This indicates that the rem1 mutation is genetically amorphic. A recent independent study has indicated that another weaker allele remHG24 (Schupbach & Wieschaus, 1989) shows similar abnormalities at a lower frequency (Swan et al, 2005).
These results indicate that Cks30A is required before the metaphase–anaphase transition for spindle morphology and chromosome alignment.
Cks30A is essential for proper localization of Msps
To gain an insight into the spindle defects in female meiosis, we examined the localization of Msps. Msps protein belongs to a conserved family of microtubule regulators, including XMAP215, and is the first protein identified at the acentrosomal poles in Drosophila (Cullen & Ohkura, 2001; Ohkura et al, 2001). An msps mutation often leads to the formation of a tripolar spindle in female meiosis I.
In wild type, Msps protein is accumulated at the acentrosomal poles of the metaphase I spindle in female meiosis (Fig 3A), although the localization sometimes spreads to the spindle microtubules. In the rem1 mutant, although the Msps protein is still concentrated at the poles, it is often accumulated around the equator of the spindle (Fig 3B). Mislocalization of this important pole protein to the equator in the rem1 mutant may sometimes lead to the formation of ectopic spindle poles near the equator.
Figure 3.
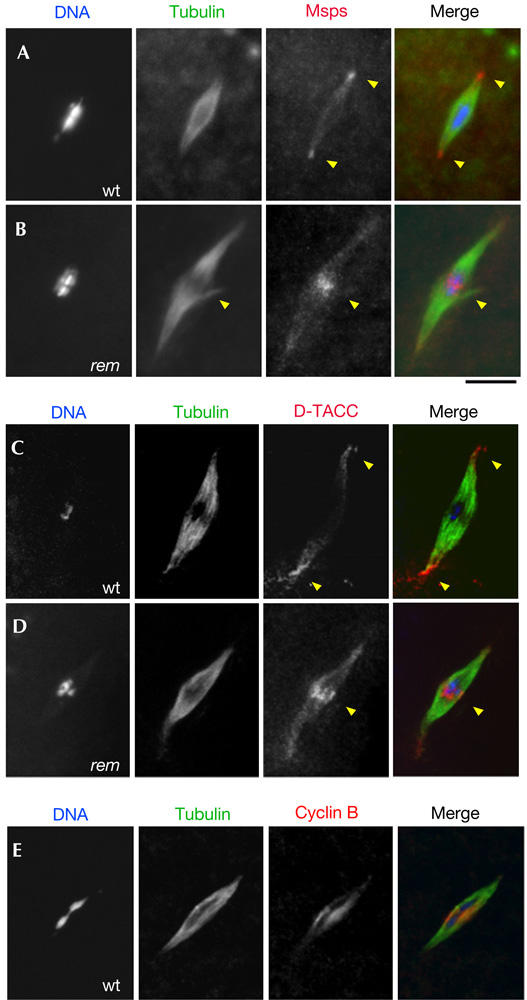
Mislocalization of Msps and D-TACC in the cks30A mutant. (A) Localization of Msps protein to the spindle poles (the arrowheads) in wild type (wt). (B) Mislocalization of Msps protein to the spindle equator (the arrowhead) in the rem1 mutant. (C) Localization of D-TACC to the spindle poles (the arrowheads) in wild type. (D) Mislocalization of D-TACC to the spindle equator (the arrowhead) in the rem1 mutant. (E) Concentration of cyclin B at the spindle equator in wild type. Scale bar, 10 μm.
We have previously shown that Msps localization is dependent on another pole protein D-TACC, which binds to Msps (Cullen & Ohkura, 2001). To test whether D-TACC also mislocalizes, we examined the localization of D-TACC in the rem1 mutant. In wild type, D-TACC is highly concentrated at the acentrosomal poles (Fig 3C). In the rem1 mutant, D-TACC often accumulated at the spindle equator (Fig 3D), although it is still concentrated around the poles to some degree.
In summary, Cks30A is required for correct localization of the essential pole proteins, Msps and D-TACC.
Cyclin B is concentrated at the spindle equator
To gain an insight into how the defect in the Cdc2 complex leads to Msps or D-TACC mislocalization to the spindle equator, we studied the localization of cyclin B. Cyclin B is considered to be the main determinant of the activity and cellular localization of the Cdc2 complex. Immunostaining in non-activated oocytes showed that cyclin B is localized to the metaphase I spindle, with a concentration around the spindle equator (Fig 3E). This cyclin B localization could suggest a possible regulatory role of the Cdc2 complex in the transport of Msps and D-TACC from the spindle equator to the poles. The cyclin B localization is not affected in the rem mutant, suggesting that Cks30A mainly affects the substrate specificity of the Cdc2 complex, as shown in other systems (Patra & Dunphy, 1998).
Two Drosophila Cks homologues
The Drosophila genome contains one more predicted cks homologue (CG9790), which we called cks85A. Although mammalian genomes also have two Cks genes, they are more similar in sequence to each other than to either of the two cks genes in Drosophila (Fig 4A).
Figure 4.
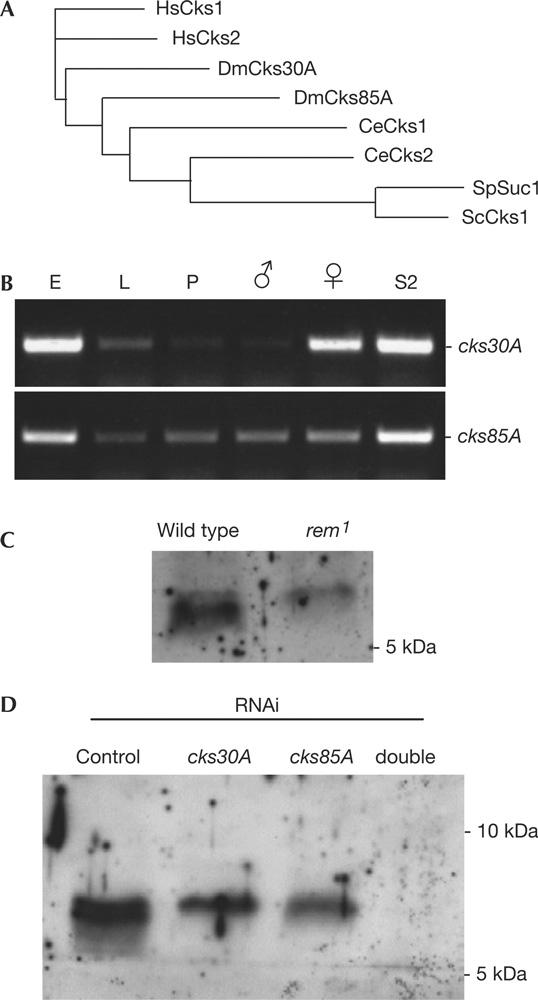
Two Cks homologues in Drosophila. (A) Phylogram of the Cks homologues in various organisms. The cores of 66 amino acids were compared by ClustalW at EBI. At, Arabidopsis thaliana; Ce, Caenorhabditis elegans, Dm, Drosophila melanogaster; Hs, Homo sapiens; Sc, Saccharomyces cerevisiae; Sp, Schizosaccharomyces pombe. (B) Expression of the two cks genes during Drosophila development. RNA was isolated from embryos (E), third-instar larvae (L), early pupae (P), adult males, adult females and S2 cells (from left to right). Transcripts of cks30A and cks85A were detected from 0.2 μg of RNA by reverse transcription–PCR with 30 cycles. (C) Cks proteins in embryos laid by wild type and rem1. Embryos (0–12 h) were used for immunoblots that were probed with an antibody against human Cks1. The lower band is greatly reduced in the rem1 mutant. (D) Identification of Cks proteins in S2 cells. S2 cells were treated with doublestranded RNA corresponding to cks30A and cks85A for 7 days before analysis by immunoblots using an antibody against human Cks1. From the left: control RNA interference (RNAi; bacterial β-lactamase), cks30A single RNAi, cks85A single RNAi and cks30A cks85A double RNAi.
We next examined the gene expression pattern of the two cks genes during Drosophila development. RNAs were isolated from various stages of development and analysed by reverse transcription–PCR (RT–PCR) using primers that correspond to each of the cks genes. cks30A gave strong signals in adult females and embryos, whereas it gave only weak signals in adult males, larvae and pupae (Fig 4B). This maternal expression pattern is consistent with the observed female sterile phenotype of the cks30A (rem1) mutant. In contrast, cks85A signals were obtained more uniformly throughout the development without sex specificity in adults (Fig 4B). In S2 cultured cells, which originated from embryos, both genes were well expressed.
To identify the Cks proteins, we used an anti-human Cks1 antibody for immunoblots of protein extracts from embryos and S2 cells. Although the antibody recognized many proteins, two bands were detected within a range of molecular weights consistent with the Cks proteins (Fig 4C,D). In embryos laid by the rem1 mutant, the amount of the smaller band was greatly reduced. To further confirm their identity, S2 cells were subjected to RNA interference (RNAi) using doublestranded RNAs (dsRNAs) corresponding to the cks genes. We found that both of the bands disappeared when both genes were simultaneously knocked down by RNAi (Fig 4D). It indicated that, consistent with our RT–PCR results, S2 cells produced both the Cks proteins and that RNAi effectively depleted them.
Cytological analysis showed that cks85A RNAi resulted in a significant increase in chromosome misalignment/missegregation and spindle abnormality in mitosis after an extended time, whereas cks30A RNAi had a lesser impact on mitotic progression (Fig 5D). About a half of anaphase or telophase cells had lagging chromosomes or chromosome bridges after cks85A RNAi (Fig 5A). In some cases, spindles contained scattered chromosomes the sister chromatids of which were either attached or detached (Fig 5B). The frequency of multipolar spindles was also increased (Fig 5C).
Figure 5.
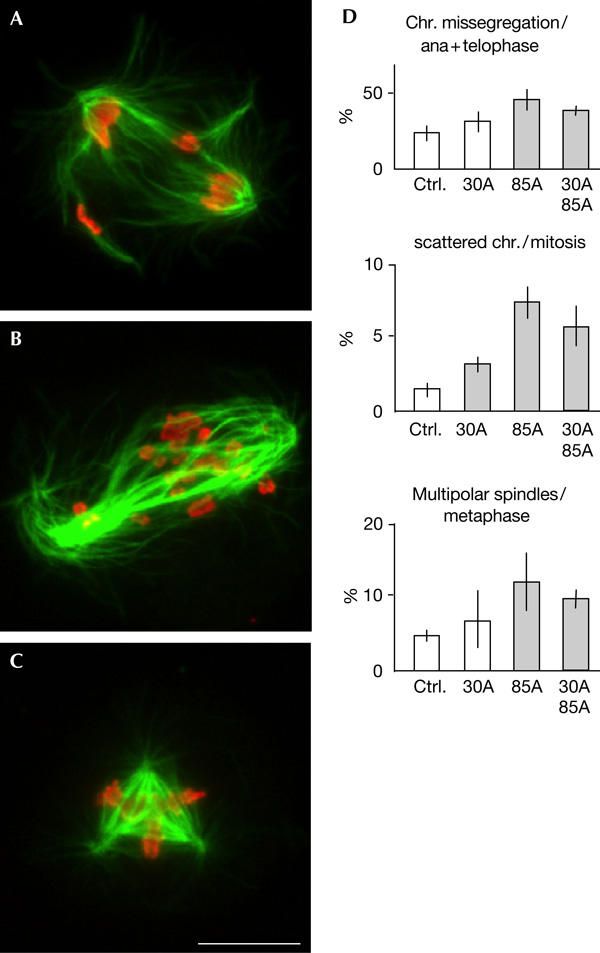
Mitotic abnormalities after cks85A RNA interference. (A) Anaphases with lagging chromosomes after cks85A RNA interference (RNAi) in S2 cells. (B) Chromosomes are scattered within a bipolar spindle after cks85A RNAi. In this case, sister chromatids have not been separated. (C) A tripolar spindle after cks85A RNAi. Scale bars, 10 μm. (D) Statistics of mitotic abnormalities after RNAi of the cks genes in S2 cells. From the left in each graph: control RNAi (bacterial β-lactamase), cks30A single RNAi, cks85A single RNAi and double RNAi of cks30A and cks85A. The mean values and standard deviations from four experiments are shown as bars and lines. Shaded bars represent significant differences (P<0.02) from control RNAi.
The genetic and RNAi results indicated that cks85A has an important function in mitotic progression, whereas cks30A mainly functions in female meiosis.
Discussion
In this study, we showed a new pre-anaphase function of a Cks protein in acentrosomal spindle formation during Drosophila female meiosis. Through a cytological screen, we found spindle defects in remnants among female sterile mutants and discovered that remnants encodes one of two Cks proteins (Cks30A) in Drosophila. Cytological analysis showed that Cks30A is required for correct formation of the acentrosomal spindle and chromosome alignment in female meiosis I. Our observation on mislocalization of the essential pole components, Msps and D-TACC, in the mutant provides a molecular insight into a role of Cks30A in spindle morphogenesis.
Cks/Suc1 protein is the third subunit of the Cdc2–cyclin B complex, which is conserved across eukaryotes (Brizuela et al, 1987; Draetta & Beach, 1988). Although it has been known to be essential for the cell cycle, the function seems to be less straightforward than that of the other subunits of the Cdc2 complex. One reason is that Cks also interacts with other Cdks and has Cdk-independent functions (Ganoth et al, 2001; Spruck et al, 2001). Even if Cks is limited to roles in mitosis/meiosis, Cks proteins are implicated in entry into mitosis/meiosis, metaphase–anaphase transition and also exit from mitosis/meiosis (Patra & Dunphy, 1996; Polinko & Strome, 2000; Spruck et al, 2003). Furthermore, the roles of Cks were further complicated by the fact that animal genomes encode two Cks homologues.
Studies in Caenorhabditis elegans and mice showed that one of two cks genes is required for female fertility (Polinko & Strome, 2000; Spruck et al, 2003). Similarly, our results indicated that one of two Drosophila cks homologues, cks30A, is expressed maternally and is required for female meiosis. Further analysis indicated that Cks30A is required for proper bipolar spindle formation and chromosome alignment in mature oocytes arrested in metaphase I. In C. elegans, depletion of one of the Cks proteins by RNAi results in a failure to complete meiosis I (Polinko & Strome, 2000). Similarly, in mice, oocytes from a Cks2 knockout cannot progress past metaphase I and a small percentage of oocytes show chromosome congression failure (Spruck et al, 2003). In both cases, the defects were interpreted mainly as post-metaphase defects. As Drosophila non-activated oocytes are arrested in metaphase I until ovulation, we can distinguish pre-anaphase function of Cks30A from possible post-metaphase function. Our study clearly showed that Drosophila Cks30A has a function in establishing metaphase I, in addition to later functions that were reported recently (Swan et al, 2005).
At the moment, we do not know how the cks30A mutation disrupts spindle formation and chromosome alignment in female meiosis. It has been thought that a loss of Cks function affects the Cdc2 activity towards certain substrates. We found that the essential pole components, Msps and D-TACC, mislocalize to the spindle equator in the mutant. Previously, we hypothesized that Msps is transported by the Ncd motor and anchored to the poles by D-TACC (Cullen & Ohkura, 2001). D-TACC localizes to the poles independently from Ncd, but may also be transported from the spindle equator along microtubules by other motors. Cks30A-dependent Cdc2 activity may be required for activating the transport system at the onset of spindle formation in female meiosis. Consistently, we found that cyclin B is concentrated around the equator of the metaphase I spindle. Msps is the XMAP215 homologue and belongs to a family of conserved microtubule-associated proteins (Cullen et al, 1999; Ohkura et al, 2001). It is a major microtubule regulator, both in mitosis/meiosis and interphase (Gard & Kirschner, 1987; Ohkura et al, 2001; Brittle & Ohkura, 2005). The mislocalization of this microtubule-regulating activity could lead to the disruption of spindle organization in the mutant.
Methods
Drosophila genetics. Standard techniques for fly manipulation were followed (Ashburner, 1989). All stocks were grown at 25°C in standard cornmeal media. w1118 flies were used as wild type. Details of mutations have been described previously (Lindsley & Zimm, 1992). Female sterile chromosomes were recombined with rucuca chromosome to remove background lethal mutations. rem1 mutation was kept over CyO.
Molecular and immunological techniques. Standard molecular techniques were followed throughout (Harlow & Lane, 1988; Sambrook et al, 1989). Onestep RT–PCR was carried out using Taq polymerase and a reverse transcriptase (Invitrogen, Paisley, UK). Non-activated oocytes were prepared for immunostaining, as described previously (Cullen & Ohkura, 2001). RNAi was carried out as described previously (Brittle & Ohkura, 2005; Dzhindzhev et al, 2005). Mitotic abnormalities were counted after incubating with dsRNA for 11–18 days. A human Cks1 antibody was purchased from Santa Cruz Biotechnology (CA, USA). A Drosophila cyclin B antibody (Whitfield et al, 1990) was obtained from Dr W. Whitfield (Dundee, UK). Immunofluorescence microscopy was carried out, as described (Brittle & Ohkura, 2005).
Acknowledgments
We thank Bloomington Stock Centre, Drosdel project and Exelixis for fly stocks, W. Whitfield for an antibody and T. Schupbach for sharing unpublished data. This work is supported by The Wellcome Trust. N.J.P., N.S.D. and H.O. receive a Wellcome Trust studentship, a Darwin Trust studentship and a Wellcome Trust senior research fellowship, respectively.
References
- Ashburner M (1989) Drosophila. Cold Spring Harbor, NY, USA: Cold Spring Harbor Laboratory Press [Google Scholar]
- Bourne Y, Watson MH, Hickey MJ, Holmes W, Rocque W, Reed SI, Tainer JA (1996) Crystal structure and mutational analysis of the human CDK2 kinase complex with cell cycle-regulatory protein CksHs1. Cell 84: 863–874 [DOI] [PubMed] [Google Scholar]
- Brittle AL, Ohkura H (2005) Mini spindles, the XMAP215 homologue, suppresses pausing of interphase microtubules in Drosophila. EMBO J 24: 1387–1396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brizuela L, Draetta G, Beach D (1987) p13suc1 acts in the fission yeast cell division cycle as a component of the p34cdc2 protein kinase. EMBO J 6: 3507–3514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen CF, Ohkura H (2001) Msps protein is localized to acentrosomal poles to ensure bipolarity of Drosophila meiotic spindles. Nat Cell Biol 3: 637–642 [DOI] [PubMed] [Google Scholar]
- Cullen CF, Deak P, Glover DM, Ohkura H (1999) Mini spindles: a gene encoding a conserved microtubule-associated protein required for the integrity of the mitotic spindle in Drosophila. J Cell Biol 146: 1005–1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draetta G, Beach D (1988) Activation of cdc2 protein kinase during mitosis in human cells: cell cycle-dependent phosphorylation and subunit rearrangement. Cell 54: 17–26 [DOI] [PubMed] [Google Scholar]
- Dzhindzhev NS, Rogers SL, Vale RD, Ohkura H (2005) Distinct mechanisms govern the localisation of Drosophila CLIP-190 to unattached kinetochores and microtubule plus ends. J Cell Sci 118: 3781–3790 [DOI] [PubMed] [Google Scholar]
- Endow SA, Henikoff S, Soler-Niedziela L (1990) Mediation of meiotic and early mitotic chromosome segregation in Drosophila by a protein related to kinesin. Nature 345: 81–83 [DOI] [PubMed] [Google Scholar]
- Finley RL Jr, Brent R (1994) Interaction mating reveals binary and ternary connections between Drosophila cell cycle regulators. Proc Natl Acad Sci USA 91: 12980–12984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganoth D, Bornstein G, Ko TK, Larsen B, Tyers M, Pagano M, Hershko A (2001) The cell-cycle regulatory protein Cks1 is required for SCF(Skp2)-mediated ubiquitinylation of p27. Nat Cell Biol 3: 321–324 [DOI] [PubMed] [Google Scholar]
- Gard DL, Kirschner MW (1987) A microtubule-associated protein from Xenopus eggs that specifically promotes assembly at the plus-end. J Cell Biol 105: 2203–2215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giunta KL, Jang JK, Manheim EA, Subramanian G, McKim KS (2002) subito encodes a kinesin-like protein required for meiotic spindle pole formation in Drosophila melanogaster. Genetics 160: 1489–1501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golic KG, Golic MM (1996) Engineering the Drosophila genome: chromosome rearrangements by design. Genetics 144: 1693–1711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow E, Lane D (1988) Antibodies: A Laboratory Manual. Cold Spring Harbor, NY, USA: Cold Spring Harbor Laboratory Press [Google Scholar]
- Kramer J, Hawley RS (2003) The spindle-associated transmembrane protein Axs identifies a membranous structure ensheathing the meiotic spindle. Nat Cell Biol 5: 261–263 [DOI] [PubMed] [Google Scholar]
- Lindsley DL, Zimm GG (1992) The Genome of Drosophila malanogaster. New York, NY, USA: Academic Press [Google Scholar]
- Mahowald AP, Kambysellis MP (1980) Oogenesis In The Genetics and Biology of Drosophila, Ashburner M, Wright TRF (eds) pp 141–224. New York, NY, USA: Academic Press [Google Scholar]
- McDonald HB, Stewart RJ, Goldstein LS (1990) The kinesin-like ncd protein of Drosophila is a minus end-directed microtubule motor. Cell 63: 1159–1165 [DOI] [PubMed] [Google Scholar]
- McKim KS, Hawley RS (1995) Chromosomal control of meiotic cell division. Science 270: 1595–1601 [DOI] [PubMed] [Google Scholar]
- Ohkura H, Garcia MA, Toda T (2001) Dis1/TOG universal microtubule adaptors—one MAP for all? J Cell Sci 114: 3805–3812 [DOI] [PubMed] [Google Scholar]
- Patra D, Dunphy WG (1996) Xe-p9, a Xenopus Suc1/Cks homolog, has multiple essential roles in cell cycle control. Genes Dev 10: 1503–1515 [DOI] [PubMed] [Google Scholar]
- Patra D, Dunphy WG (1998) Xe-p9, a Xenopus Suc1/Cks protein, is essential for the Cdc2-dependent phosphorylation of the anaphase-promoting complex at mitosis. Genes Dev 12: 2549–2559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polinko ES, Strome S (2000) Depletion of a Cks homolog in C. elegans embryos uncovers a post-metaphase role in both meiosis and mitosis. Curr Biol 10: 1471–1474 [DOI] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T (1989) Molecular Cloning: A Laboratory Manual. Cold Spring Harbor, NY, USA: Cold Spring Harbor Laboratory Press [Google Scholar]
- Schupbach T, Wieschaus E (1989) Female sterile mutations on the second chromosome of Drosophila melanogaster. I. Maternal effect mutations. Genetics 121: 101–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spruck C, Strohmaier H, Watson M, Smith AP, Ryan A, Krek TW, Reed SI (2001) A CDK-independent function of mammalian Cks1: targeting of SCF(Skp2) to the CDK inhibitor p27Kip1. Mol Cell 7: 639–650 [DOI] [PubMed] [Google Scholar]
- Spruck CH, de Miguel MP, Smith AP, Ryan A, Stein P, Schultz RM, Lincoln AJ, Donovan PJ, Reed SI (2003) Requirement of Cks2 for the first metaphase/anaphase transition of mammalian meiosis. Science 300: 647–650 [DOI] [PubMed] [Google Scholar]
- Swan A, Barcelo G, Schupbach T (2005) Drosophila Cks30A interacts with Cdk1 to target Cyclin A for destruction in the female germline. Development 132: 3669–3678 [DOI] [PubMed] [Google Scholar]
- Tavosanis G, Llamazares S, Goulielmos G, Gonzalez C (1997) Essential role for γ-tubulin in the acentriolar female meiotic spindle of Drosophila. EMBO J 16: 1809–1819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield WG, Gonzalez C, Maldonado-Codina G, Glover DM (1990) The A- and B-type cyclins of Drosophila are accumulated and destroyed in temporally distinct events that define separable phases of the G2–M transition. EMBO J 9: 2563–2572 [DOI] [PMC free article] [PubMed] [Google Scholar]


