Abstract
Proteins are translocated across or inserted into membranes by machines that are composed of soluble and membrane-anchored subunits. The molecular action of these machines and their evolutionary origin are at present the focus of intense research. For instance, our understanding of the mode of insertion of β-barrel membrane proteins into the outer membrane of endosymbiotically derived organelles has increased rapidly during the past few years. In particular, the identification of the Omp85/YaeT-involving pathways in Neisseria meningitidis, Escherichia coli and cyanobacteria, and homologues of Omp85/YaeT in chloroplasts and mitochondria, has provided new clues about the ancestral β-barrel protein insertion pathway. This review focuses on recent advances in the elucidation of the evolutionarily conserved concepts that underlie the translocation and insertion of β-barrel membrane proteins.
Keywords: β-barrel proteins, membrane insertion, Omp85, Sam50/Tob55, Toc75, transport evolution
Introduction
Protein translocation across and into membranes is a fundamental process: up to 50% of all proteins synthesized in the cytosol need to traverse at least one membrane to reach their place of function (Schatz & Dobberstein, 1996). The basic principles of protein translocation have been established, but the specific molecular mechanisms of each membrane system have been determined to varying degrees. However, some generalizations about the state of the substrate or the translocon can be made. Proteins translocated across the nuclear (Xu & Massague, 2004) and the peroxisomal membranes (Erdmann & Schliebs, 2005) and by the twin arginine translocation (TAT) pathway in bacteria and thylakoids (Robinson & Bolhuis, 2001) are folded. By contrast, proteins transported by the machines in the mitochondrial (Pfanner et al, 2004), chloroplast (Soll & Schleiff, 2004) and endoplasmatic reticulum membranes (Osborne et al, 2005) need to be unfolded. The systems also vary in the architecture of the translocon, as plastids, mitochondria, endoplasmic reticulum and nuclei contain pre-existing machines, whereas translocon formation in the peroxisomal or TAT pathway is substrate induced.
β-barrel membrane proteins are composed of antiparallel transmembrane β-strands connected by soluble loop regions (Schulz, 2000) and are inserted into the outer membrane of bacteria, mitochondria and chloroplasts by pre-existing translocation machineries. For the bacterial system, it was suggested that the β-barrel membrane proteins are at least partially folded before their insertion into the outer membrane as disulphide bond formation catalysed by periplasmic proteins precedes the insertion of some proteins (Eppens et al, 1997). In addition, the inactivation of the genes that encode the periplasmic chaperone Skp (7 kDa protein) and the periplasmic peptidyl-prolyl cis–trans isomerase SurA in Escherichia coli results in a synthetic lethal phenotype. This suggests that Skp and SurA have a redundant chaperone function that is essential for outer membrane protein (OMP) topology (Fig 1A; Rizzitello et al, 2001), but it remains open whether they keep proteins in an unfolded state or whether they are involved in folding preceding insertion. In this review, we highlight the similarities and differences between the insertion of β-barrel membrane proteins in bacteria and in eukaryotes.
Figure 1.
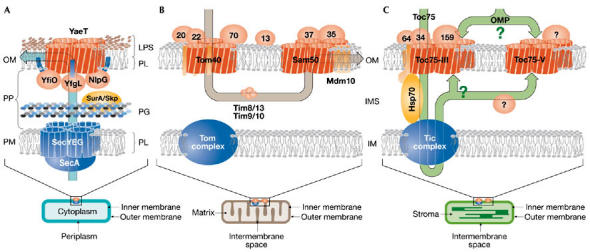
β-barrel outer membrane protein insertion. The translocation system for β-barrel outer membrane proteins (OMPs) in (A) bacteria, (B) mitochondria and (C) chloroplasts are shown. Arrows indicate the translocation pathway. Please note, the stoichiometry of the bacterial system is not experimentally supported. In the case of chloroplasts, the translocation path is dissected into that established for Toc75 and possible routes for other OMPs. The question marks indicate the current state of knowledge. IM, inner membrane; IMS, intermembrane space; LPS, lipopolysaccharide; OM, outer membrane; PG, peptidoglycan layer; PL, phospholipid; PM, plasma membrane; PP, periplasm.
Prokaryotic β-barrel-shaped transporters
The biogenesis of the outer membrane of Gram-negative bacteria remained elusive for a long time. In 1998, a homologous protein of the chloroplast translocation channel Toc75 (Soll & Schleiff, 2004) was identified in Synechocystis PCC6803 and was named slr1227 (Table 1; Bölter et al, 1998). This protein localizes to the outer membrane, is essential for viability of the bacteria (Reumann et al, 1999) and has similar electrophysiological properties to Toc75 from the pea (psToc75; Bölter et al, 1998). On the basis of this comparison, it was suggested that the protein had a function in the import or export of peptides. The authors also showed that the protein was related to Omp85 from Neisseria meningitidis, which was not functionally characterized at that time. Only later was it shown that Omp85 is an essential component for outer membrane biogenesis in N. meningitidis. Initially, two functions were suggested for Omp85: the export of lipids (Genevrois et al, 2003) and the assembly of OMPs (Voulhoux et al, 2003). The latter study showed that the depletion of Omp85 impaired the formation of complexes containing OMPs such as PorA, PorB, PilQ and FrpB. Moreover, the integration and subsequent folding of monomeric OMPs such as OmpLA or IgA1 was decreased. It was also shown that Omp85 interacts with unfolded OMPs such as PorA (Voulhoux et al, 2003). Therefore, the deleterious effect of Omp85 depletion on lipid export might be indirect because of the defective assembly of OMPs. This conclusion is supported by the identification of the OMP OstA/Imp (increased membrane permeability) in E. coli (Braun & Silhavy, 2002) and N. meningitidis (Bos et al, 2004), which is involved in the assembly of lipopolysaccharide in the outer membrane. However, the assembly state of Imp in the ΔOmp85 background is not yet known. In E. coli, the Omp85 homologue YaeT is also essential (Gerdes et al, 2003). Interestingly, the main protein of the inner membrane export system, SecA (Osborne et al, 2005), functions as a multicopy suppressor of the temperaturesensitive mutant of YaeT and partially restores OMP assembly (Doerrler & Raetz, 2005). This suppression may be caused by an increased secretion of the mutant YaeT or other OMPs across the inner membrane by the Sec complex. YaeT is part of a multi-component complex that also includes the outer membrane lipoproteins YfgL, YfiO and NlpB (Wu et al, 2005). In contrast to YfgL and YaeT, YfiO and NlpB are not essential but their depletion results in phenotypes that suggest they are important for maintaining the integrity of the cell envelope (Wu et al, 2005; Ruiz et al, 2005; Onofryk et al, 2005). NlpB and YfgL are genetically linked to SurA, which is a periplasmic chaperone specific for OMPs (Rouvière & Gross, 1996). It was suggested that these proteins function in a periplasmic folding pathway that parallels that of SurA (Onofryk et al, 2005). Mutations of YfgL and YfiO, but not of NlpB, reduce the amount of inserted OMPs such as LamB or OmpA (Wu et al, 2005; Ruiz et al, 2005). Combined with the results of structural predictions (Bölter et al, 1998; Voulhoux et al, 2003), it therefore seems that the prokaryotic machinery for OMP assembly is itself composed of a β-barrel OMP, which is associated with at least three outer membrane lipoproteins involved putatively in the perception and folding of the incoming OMPs. Although some evidence exists for pre-folding of the OMPs before membrane insertion, this issue requires further investigation.
Table 1.
Components involved in β-barrel protein insertion
| Prokaryotes | Eukaryotes | ||||||
|---|---|---|---|---|---|---|---|
| Machinery components | Mitochondria | Chloroplasts | |||||
| Primary translocon | Receptors | SecA | Tom20 | Toc34 | |||
| Tom22 | Toc64 | ||||||
| Tom70 | Toc159 | ||||||
| Tom5/6/7 | |||||||
| Pore | SecYEG | Tom40 | Toc75-III | ||||
| IMS machinery | Chaperone system | Skp | Tim8 & Tim13 | Toc12 & imsHsp70 | |||
| SurA | Tim9 & Tim10 | ||||||
| Secondary translocon | Species | Ec | Nm | S | N | ||
| Translocon | YaeT | Omp85 | slr1227 | alr2269 | Sam50/Tob55/mtOmp85 | Toc75-V/Oep80 | |
| Assisting proteins | YfiO | NI | NI | NI | Sam37/Mas37 | NI | |
| YfgL | Sam35/Tob38/Tom38 | ||||||
| NlpB | Mdm10 | ||||||
| Tom13/Mim1 | |||||||
Ec, Escherichia coli; IMS, intermembrane space; N, Nostoc PCC 7210; NI, not yet identified; Nm, Neisseria Meningitides; S, Synechocystis PCC6803; Sam, sorting and assembly of mitochondria outer membrane proteins; Tic, tranlocase on inner membrane of chloroplast; Tim, tranlocase on inner membrane of mitochondria; Tob, topogenesis of mitochondrial outer membrane β-barrel proteins; Toc, tranlocase on outer membrane of chloroplast; Tom, tranlocase on outer membrane of mitochondria. In the case of several names, the nomenclature used in the manuscript is the first term.
Besides the machinery for the assembly of OMPs, several proteins exist for transporting outer-membrane polypeptides, for example, FhaC in Bordetella pertussis (Guedin et al, 1998) and ShlB in Serratia marcescens (Poole et al, 1988). These systems are known as 'two-partner secretion pathways', as they are specific to their substrates, such as adhesins and hemolysins (Jacob-Dubuisson et al, 2001). The proteins seem to transport their substrate in its unfolded state, as has been shown for FhaC (Guedin et al, 1998). In addition, the investigation of ShlB suggests a dual function for this protein. The channel formed by ShlB facilitates the translocation of ShlA (Schiebel et al, 1989) and also changes its conformation—thereby inducing the transfer of phosphatidylethanolamine (Hertle et al, 1997; Walker et al, 2004), which is required for the activation of ShlA. Despite the topological and functional homology between proteins of this class, FhaC and ShlB are not interchangeable and therefore have molecular specificity (Jacob-Dubuisson et al, 1997). This class of transporters also has structural features similar to proteins of the Omp85 class (Surana et al, 2004).
Eukaryotic β-barrel-shaped transporters
Endosymbiotically derived organelles import most of their proteinaceous components from the cytosol. In mitochondria and plastids, two pore-forming proteins—Tom40 and Toc75, respectively—are involved in the translocation of proteins across the outer membrane. However, the evolutionary development of the two transport channels is distinct. The mitochondrial channel Tom40 seems to share its ancestral roots with porins (Gabriel et al, 2001), whereas Toc75 belongs to the Omp85 class (Moslavac et al, 2005). Interestingly, another translocation system specialized for the insertion of β-barrel OMPs (Fig 1B) was identified recently in the mitochondrial outer membrane (Wiedemann et al, 2003). The main component of this complex is Sam50 (Table 1; Paschen et al, 2003; Kozjak et al, 2003; Gentle et al, 2004; Humphries et al, 2005), which belongs to the Omp85 class and has the highest similarity with a subclass of Omp85 proteins previously annotated as Oma87 (Moslavac et al, 2005). The Sam50 protein is essential for the biogenesis of the mitochondrial outer membrane proteome. The evolutionary roots of this complex are also reflected in its mode of action: all β-barrel proteins first have to be translocated across the outer membrane, facilitated by the Tom40 machinery, followed by insertion into the outer membrane catalysed by the Tob/Sam complex (see, for example, Pfanner et al, 2004). In contrast to the bacterial system, no intermembrane space proteins that associate with Sam50 have been identified. However, Mdm10, an integral membrane protein previously identified as being involved in the maintenance of mitochondrial morphology (Sogo & Yaffe, 1994; Meisinger et al, 2004), and two proteins that associate with the cytosolic side of the complex, namely Sam37 (Gratzer et al, 1995; Kozjak et al, 2003; Waizenegger et al, 2004) and Sam35 (Waizenegger et al, 2004; Ishikawa et al, 2004; Milenkovic et al, 2004), have been found to be associated with Sam50. A third cytosolic-exposed component that influences β-barrel protein insertion, Tom13, seems to form part of a distinct, unidentified complex and is not a component of either Tom or Tob/Sam (Ishikawa et al, 2004; Waizenegger et al, 2005). The exact function of these extra components is yet to be established because the substrate approaches the translocon from the intermembrane space rather than the cytosol. The small mitochondrial Tim proteins, Tim8 and Tim13 (Hoppins & Nargang, 2004) or Tim9 and Tim10 (Wiedemann et al, 2004), might be the functional homologues of the factors acting in the periplasm of the bacterial system, as they are essential for the biogenesis of β-barrel OMPs.
The prokaryotic origin of Toc75, the central component of the translocation system in chloroplasts, was first suggested after the identification of a homologous protein in Synechocystis (Heins et al, 1998). Six homologues of Toc75 are encoded by the genome of Arabidopsis thaliana (Moslavac et al, 2005), which suggests functional differentiation, or substrate or tissue specificity of these proteins. Three isoforms are found in chloroplasts, namely Toc75-III, the main translocation pore of plastids (Soll & Schleiff, 2004), Toc75-IV (Baldwin et al, 2005) and Toc75-V (Eckert et al, 2002; Inoue & Potter, 2004). All proteins of the Toc75 family show a clear relation to proteins of the Omp85 class (Moslavac et al, 2005). So far, only the insertion of psToc75, which contains a bipartite targeting signal, has been investigated in detail. The bipartite signal directs the protein from the cytosol towards the stroma, where the first part of the signal is cleaved off by the stromal processing peptidase (Fig 1C; Tranel & Keegstra, 1996). The second portion of the signal functions as an intra-organellar targeting signal and is cleaved off in the intermembrane space. As all other β-barrel OMPs so far identified do not contain an amino-terminal signal, it is assumed that their mode of insertion differs from that of Toc75. Indeed, the insertion of outer envelope proteins Oep21 and Oep24 is independent of thermolysinsensitive factors and ATP (Schleiff & Klösgen, 2001), which excludes the use of the Toc-core translocon. However, it remains to be resolved whether Toc75-III itself or Toc75-V, which has the highest similarity to its bacterial ancestor (Moslavac et al, 2005), functions in the assembly of the OMPs. Interestingly, as in the bacterial system, an intermembrane space chaperone is involved in the translocation of chloroplast proteins (Becker et al, 2004). However, it remains to be established whether this system is specialized for β-barrel OMPs that are routed through the intermembrane space.
Translocation through β-barrel-shaped transporters
As summarized above, β-barrel-shaped transporters such as the bacterial Omp85 (Genevrois et al, 2003; Voulhoux et al, 2003), slr1227 (Bölter et al, 1998; Reumann et al, 1999), YaeT (Gerdes et al, 2003; Wu et al, 2005), alr2269 (Moslavac et al, 2005, Ertel et al, 2005), the mitochondrial Sam50 (Wiedemann et al, 2003; Paschen et al, 2003; Gentle et al, 2004; Humphries et al, 2005) and the plastidic Toc75 (Tranel et al, 1995; Eckert et al, 2002) are present in their respective outer membranes (Table 1) and facilitate the insertion of the proteins into the membrane. A recent comparison of prokaryotic and eukaryotic β-barrelshaped protein transporters revealed some explanations to support the evolutionary preference for this class of proteins as translocation channels (Ertel et al, 2005). It has been suggested that proteins of this family contain two distinct domains (Sanchez-Pulido et al, 2003): an N-terminal domain in which the βstrands are connected by long, soluble loops, and a pore-forming region at the carboxy-terminus (Schleiff et al, 2003; Voulhoux et al, 2003; Ertel et al, 2005). Indeed, the N-terminal region of psToc75 acts as a specific but low-affinity receptor for proteins that contain a targeting sequence (Ertel et al, 2005). Analysis of the Omp85 homologue from Nostoc PCC7120, alr2269, has revealed that this feature already exists in the cyanobacterial protein. This leads to the question whether the pore-forming proteins themselves formed the first binding site for proteins to be re-imported after the cyanobacterium was taken up by the host cell. In addition, the extreme N-terminal region of psToc75 and alr2269 is involved in the formation of the translocon (Ertel et al, 2005), although the partner and the mode of interaction differ between both proteins. A cytosolic loop of Toc75 is involved in hetero-oligomerization by recognizing the receptor Toc34, whereas the N-terminus of alr2269 facilitates homo-oligomerization (Ertel et al, 2005). The C-terminal regions of both proteins are also involved in homo-oligomerization (E.S., unpublished data). Interestingly, the C-terminal domains of psToc75 and alr2269 have similar pore characteristics to the full-length proteins, which is in agreement with a functional dissection of the molecules (Ertel et al, 2005). However, the gating of the channel is altered by the presence of the N-terminus and suggests a mechanistic relationship between receptor function and pore gating (Ertel et al, 2005).
Concluding remarks
The insertion and assembly pathway of β-barrel OMPs in prokaryotes and endosymbiotically derived organelles seems to be evolutionarily conserved. Hence, the mechanistic concepts are also expected to be similar. Indeed, in mitochondria and plastids, an intermediate has been identified in intermembrane space translocation. Therefore, OMPs are inserted in the outer membrane in the same direction as bacteria—that is from the 'periplasmic space', which supports the idea of mechanistic conservation. The identification of components with similar functions in all three systems (Fig 1; Table 1) further supports this concept.
In the future, it will be interesting to determine whether proteins of the Omp85 class have an extra lipid transport function and whether or not proteins are pre-folded before their insertion into these membranes. Furthermore, it is unlikely that the transfer of the OMPs out of the pore into the bilayer occurs by lateral diffusion because a lateral opening of the barrel would require the destruction of several hydrogen bonds and this would be energetically unfavourable. Alternatively, the β-barrel proteins of the Omp85 class that facilitate the insertion of the OMPs might serve as a seed or 'chaperone' for insertion, as a cooperative but specific insertion of β-barrel proteins has been suggested (Li & Colombini, 2002). In the latter case, the function of the reported channel activity remains elusive, as the electrophysiological properties of the eukaryotic proteins suggest a large pore diameter. To understand fully protein insertion into the outer membrane of endosymbiotically derived organelles, it will be important to discover which of the Omp85 homologues of the outer membrane of chloroplasts is the integrase for the OMPs. Nevertheless, the identification of bacterial homologues in cellular organelles has already answered a longstanding question in the endosymbiotic process—by providing the first experimental evidence that the origin of mitochondria and chloroplasts is indeed monophyletic.


Acknowledgments
This work was supported by grants from the Deutsche Forschungsgemeinschaft (SFB-TR01 and SFB 594), Fonds der Chemischen Industrie to E.S. and J.S. E.S. is also grateful to the Volkswagenstiftung for financial support.
References
- Baldwin A, Wardle A, Patel R, Dudley P, Park SK, Twell D, Inoue K, Jarvis P (2005) A molecular-genetic study of the Arabidopsis toc75 gene family. Plant Physiol 138: 715–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker T, Hritz J, Vogel M, Caliebe A, Bukau B, Soll J, Schleiff E (2004) Toc12, a novel subunit of the intermembrane space preprotein translocon of chloroplasts. Mol Biol Cell 15: 5130–5144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bölter B, Soll J, Schulz A, Hinnah S, Wagner R (1998) Origin of a chloroplast protein importer. Proc Natl Acad Sci USA 95: 15831–15836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos MP, Tefsen B, Geurtsen J, Tommassen J (2004) Identification of an outer membrane protein required for the transport of lipopolysaccharide to the bacterial cell surface. Proc Natl Acad Sci USA 101: 9417–9422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun M, Silhavy TJ (2002) Imp/OstA is required for cell envelope biogenesis in Escherichia coli. Mol Microbiol 45: 1289–1302 [DOI] [PubMed] [Google Scholar]
- Doerrler WT, Raetz CR (2005) Loss of outer membrane proteins without inhibition of lipid export in an Escherichia coli YaeT mutant. J Biol Chem 280: 27679–27687 [DOI] [PubMed] [Google Scholar]
- Eckart K, Eichacker L, Sohrt K, Schleiff E, Heins L, Soll J (2002) A Toc75-like protein import channel is abundant in chloroplasts. EMBO Rep 3: 557–562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eppens EF, Nouwen N, Tommassen J (1997) Folding of a bacterial outer membrane protein during passage through the periplasm. EMBO J 16: 4295–4301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdmann R, Schliebs W (2005) Peroxisomal matrix protein import: the transient pore model. Nat Rev Mol Cell Biol (in press) [DOI] [PubMed] [Google Scholar]
- Ertel F, Mirus O, Bredemeier R, Moslavac S, Becker T, Schleiff E (2005) The evolutionary related β-barrel polypeptide transporters from P. sativum and Nostoc PCC7120 contain two distinct functional domains. J Biol Chem 280: 28281–28289 [DOI] [PubMed] [Google Scholar]
- Gabriel K, Buchanan SK, Lithgow T (2001) The alpha and the beta: protein translocation across mitochondrial and plastid outer membranes. Trends Biochem Sci 26: 36–40 [DOI] [PubMed] [Google Scholar]
- Genevrois S, Steeghs L, Roholl P, Letesson JJ, van der Ley P (2003) The Omp85 protein of Neisseria meningitidis is required for lipid export to the outer membrane. EMBO J 22: 1780–1789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentle I, Gabriel K, Beech P, Waller R, Lithgow T (2004) The Omp85 family of proteins is essential for outer membrane biogenesis in mitochondria and bacteria. J Cell Biol 164: 19–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdes SY et al. (2003) Experimental determination and system level analysis of essential genes in Escherichia coli MG1655. J Bacteriol 185: 5673–5684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guedin S, Willery E, Locht C, Jacob-Dubuisson F (1998) Evidence that a globular conformation is not compatible with FhaC-mediated secretion of the Bordetella pertussis filamentous haemagglutinin. Mol Microbiol 29: 763–774 [DOI] [PubMed] [Google Scholar]
- Gratzer S, Lithgow T, Bauer RE, Lamping E, Paltauf F, Kohlwein SD, Haucke V, Junne T, Schatz G, Horst M (1995) Mas37p, a novel receptor subunit for protein import into mitochondria. J Cell Biol 129: 25–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heins L, Collinson I, Soll J (1998) The protein translocation apparatus of chloroplast envelopes. Trends Plant Sci 3: 56–61 [Google Scholar]
- Hertle R, Brutsche S, Groeger W, Hobbie S, Koch W, Konninger U, Braun V (1997) Specific phosphatidylethanolamine dependence of Serratia marcescens cytotoxin activity. Mol Microbiol 26: 853–865 [DOI] [PubMed] [Google Scholar]
- Hoppins SC, Nargang FE (2004) The Tim8–Tim13 complex of Neurospora crassa functions in the assembly of proteins into both mitochondrial membranes. J Biol Chem 279: 12396–12405 [DOI] [PubMed] [Google Scholar]
- Humphries AD, Streimann IC, Stojanovski D, Johnston AJ, Yano M, Hoogenraad NJ, Ryan MT (2005) Dissection of the mitochondrial import and assembly pathway for human Tom40. J Biol Chem 280: 11535–11543 [DOI] [PubMed] [Google Scholar]
- Inoue K, Potter D (2004) The chloroplastic protein translocation channel Toc75 and its paralog OEP80 represent two distinct protein families and are targeted to the chloroplastic outer envelope by different mechanisms. Plant J 39: 354–365 [DOI] [PubMed] [Google Scholar]
- Ishikawa D, Yamamoto H, Tamura Y, Moritoh K, Endo T (2004) Two novel proteins in the mitochondrial outer membrane mediate beta-barrel protein assembly. J Cell Biol 166: 621–627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob-Dubuisson F, Buisine C, Willery E, Renauld-Mongenie G, Locht C (1997) Lack of functional complementation between Bordetella pertussis filamentous hemagglutinin and Proteus mirabilis HpmA hemolysin secretion machineries. J Bacteriol 179: 775–783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob-Dubuisson F, Locht C, Antoine R (2001) Two-partner secretion in Gram-negative bacteria: a thrifty, specific pathway for large virulence proteins. Mol Microbiol 40: 306–313 [DOI] [PubMed] [Google Scholar]
- Kozjak V, Wiedemann N, Milenkovic D, Lohaus C, Meyer HE, Guiard B, Meisinger C, Pfanner N (2003) An essential role of Sam50 in the protein sorting and assembly machinery of the mitochondrial outer membrane. J Biol Chem 278: 48520–48523 [DOI] [PubMed] [Google Scholar]
- Li XX, Colombini M (2002) Catalyzed insertion of proteins into phospholipid membranes: specificity of the process. Biophys J 83: 2550–2559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin W, Rujan T, Richly E, Hansen A, Cornelsen S, Lins T, Leister D, Stoebe B, Hasegawa M, Penny D (2002) Evolutionary analysis of Arabidopsis, cyanobacterial, and chloroplast genomes reveals plastid phylogeny and thousands of cyanobacterial genes in the nucleus. Proc Natl Acad Sci USA 99: 12246–12251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meisinger C et al. (2004). The mitochondrial morphology protein Mdm10 functions in assembly of the preprotein translocase of the outer membrane. Dev Cell 7: 61–71 [DOI] [PubMed] [Google Scholar]
- Milenkovic D, Kozjak V, Wiedemann N, Lohaus C, Meyer HE, Guiard B, Pfanner N, Meisinger C (2004) Sam35 of the mitochondrial protein sorting and assembly machinery is a peripheral outer membrane protein essential for cell viability. J Biol Chem 279: 22781–22785 [DOI] [PubMed] [Google Scholar]
- Moslavac S, Mirus O, Bredemeier R, Soll J, von Haeseler A, Schleiff E (2005) Conserved pore-forming regions in polypeptide-transporting proteins. FEBS J 272: 1367–1378 [DOI] [PubMed] [Google Scholar]
- Onufryk C, Crouch ML, Fang FC, Gross CA (2005) Characterization of six lipoproteins in the sigmaE regulon. J Bacteriol 187: 4552–4561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne AR, Rapoport TA, van den Berg B (2005) Protein translocation by the Sec61/SecY channel. Annu Rev Cell Dev Biol 21: 529–550 [DOI] [PubMed] [Google Scholar]
- Paschen SA, Waizenegger T, Stan T, Preuss M, Cyrklaff M, Hell K, Rapaport D, Neupert W (2003) Evolutionary conservation of biogenesis of β-barrel membrane proteins. Nature 426: 862–866 [DOI] [PubMed] [Google Scholar]
- Pfanner N, Wiedemann N, Meisinger C, Lithgow T (2004) Assembling the mitochondrial outer membrane. Nat Struct Mol Biol 11: 1044–1048 [DOI] [PubMed] [Google Scholar]
- Poole K, Schiebel E, Braun V (1988) Molecular characterization of the hemolysin determinant of Serratia marcescens. J Bacteriol 170: 3177–3188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reumann S, Davila-Aponte J, Keegstra K (1999) The evolutionary origin of the protein-translocating channel of chloroplastic envelope membranes: identification of a cyanobacterial homolog. Proc Natl Acad Sci USA 96: 784–789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzitello AE, Harper JE, Silhavy TJ (2001) Genetic evidence for parallel pathways of chaperone activity in the periplasm of Escherichia coli. J Bacteriol 183: 6794–6800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson C, Bolhuis A (2001) Protein targeting by the twin-arginine translocation pathway. Nat Rev Mol Cell Biol 2: 350–356 [DOI] [PubMed] [Google Scholar]
- Rouvière PE, Gross CA (1996) SurA, a periplasmic protein with peptidyl-prolyl isomerase activity, participates in the assembly of outer membrane porins. Genes Dev 10: 3170–3187 [DOI] [PubMed] [Google Scholar]
- Ruiz N, Falcone B, Kahne D, Silhavy TJ (2005) Chemical conditionality: a genetic strategy to probe organelle assembly. Cell 121: 307–317 [DOI] [PubMed] [Google Scholar]
- Sanchez-Pulido L, Devos D, Genevrois S, Vicente M, Valencia A (2003) POTRA: a conserved domain in the FtsQ family and a class of beta-barrel outer membrane proteins. Trends Biochem Sci 28: 523–526 [DOI] [PubMed] [Google Scholar]
- Schatz G, Dobberstein B (1996) Common principles of protein translocation across membranes. Science 271: 1519–1526 [DOI] [PubMed] [Google Scholar]
- Schiebel E, Schwarz H, Braun V (1989) Subcellular location and unique secretion of the hemolysin of Serratia marcescens. J Biol Chem 264: 16311–16320 [PubMed] [Google Scholar]
- Schleiff E, Klösgen RB (2001) Without a little help from 'my' friends: direct insertion of proteins into chloroplast membranes? Biochim Biophys Acta 1541: 22–33 [DOI] [PubMed] [Google Scholar]
- Schleiff E, Eichacker LA, Eckart K, Becker T, Mirus O, Stahl T, Soll J (2003) Prediction of the plant β-barrel proteome: a case study of the chloroplast outer envelope. Protein Sci 12: 748–759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz G (2000) β-barrel membrane proteins. Curr Opin Struct Biol 10: 443–447 [DOI] [PubMed] [Google Scholar]
- Sogo LF, Yaffe MP (1994) Regulation of mitochondrial morphology and inheritance by Mdm10p, a protein of the mitochondrial outer membrane. J Cell Biol 126: 1361–1373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soll J, Schleiff E (2004) Protein import into chloroplasts. Nat Rev Mol Cell Biol 5: 198–208 [DOI] [PubMed] [Google Scholar]
- Surana NK, Grass S, Hardy GG, Li H, Thanassi DG, Geme JW 3rd (2004) Evidence for conservation of architecture and physical properties of Omp85-like proteins throughout evolution. Proc Natl Acad Sci USA 101: 14497–14502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tranel PJ, Keegstra K (1996) A novel, bipartite transit peptide targets OEP75 to the outer membrane of the chloroplastic envelope. Plant Cell 8: 2093–2104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tranel PJ, Froehlich J, Goyal A, Keegstra K (1995) A component of the chloroplastic protein import apparatus is targeted to the outer envelope membrane via a novel pathway. EMBO J 14: 2436–2446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voulhoux R, Bos MP, Geurtsen J, Mols M, Tommassen J (2003) Role of a highly conserved bacterial protein in outer membrane protein assembly. Science 299: 262–265 [DOI] [PubMed] [Google Scholar]
- Waizenegger T, Habib SJ, Lech M, Mokranjac D, Paschen SA, Hell K, Neupert W, Rapaport D (2004) Tob38, a novel essential component in the biogenesis of β-barrel proteins of mitochondria. EMBO Rep 5: 704–709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waizenegger T, Schmitt S, Zivkovic J, Neupert W, Rapaport D (2005) Mim1, a protein required for the assembly of the TOM complex of mitochondria. EMBO Rep 6: 57–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiedemann N, Kozjak V, Chacinska A, Schonfisch B, Rospert S, Ryan MT, Pfanner N, Meisinger C (2003) Machinery for protein sorting and assembly in the mitochondrial outer membrane. Nature 424: 565–571 [DOI] [PubMed] [Google Scholar]
- Wiedemann N, Truscott KN, Pfannschmidt S, Guiard B, Meisinger C, Pfanner N (2004) Biogenesis of the protein import channel Tom40 of the mitochondrial outer membrane: intermembrane space components are involved in an early stage of the assembly pathway. J Biol Chem 279: 18188–18194 [DOI] [PubMed] [Google Scholar]
- Walker G, Hertle R, Braun V (2004) Activation of Serratia marcescens hemolysin through a conformational change. Infect Immun 72: 611–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T, Malinverni J, Ruiz N, Kim S, Silhavy TJ, Kahne D (2005) Identification of a multicomponent complex required for outer membrane biogenesis in Escherichia coli. Cell 121: 235–245 [DOI] [PubMed] [Google Scholar]
- Xu L, Massague J (2004) Nucleocytoplasmic shuttling of signal transducers. Nat Rev Mol Cell Biol 5: 209–219 [DOI] [PubMed] [Google Scholar]


