Abstract
Immunostaining with endothelial and pericyte markers was used to evaluate the cellular composition of angiogenic sprouts in several types of tumors and in the developing retina. Confocal microscopy revealed that, in addition to conventional endothelial tubes heavily invested by pericytes, all tissues contained small populations of endothelium-free pericyte tubes in which nerve/glial antigen 2 (NG2) positive, platelet-derived growth factor beta (PDGF β) receptor-positive perivascular cells formed the lumen of the microvessel. Perfusion of tumor-bearing mice with FITC-dextran, followed by immunohistochemical staining of tumor vasculature, demonstrated direct apposition of pericytes to FITC-dextran in the lumen, confirming functional connection of the pericyte tube to the circulation. Transplantation of prostate and mammary tumor fragments into NG2-null mice led to the formation of tumor microvasculature that was invariably NG2-negative, demonstrating that pericytes associated with tumor microvessels are derived from the host rather than from the conversion of tumor cells to a pericyte phenotype. The existence of pericyte tubes reflects the early participation of pericytes in the process of angiogenic sprouting. The ability to study these precocious contributions of pericytes to neovascularization depends heavily on the use of NG2 and PDGF β-receptor as reliable early markers for activated pericytes.
Keywords: angiogenesis, endothelium, neovascularization, NG2, PDGF β-receptor, pericyte, sprout, tube, tumor, transplantation
Introduction
Angiogenesis is essential for almost all aspects of normal development, as well as for many pathological processes, including tumor growth and metastasis [1, 2]. The walls of typical angiogenic microvessels are composed of two principal cell types: endothelial cells, which form the inner lining of the vascular tube, and pericytes (mural cells) which form an outer sheath around the endothelium [2-4]. While endothelial cells have been studied extensively, much less is known about the pericyte, a name (‘peri’ around and ‘cyto’ cell) that denotes the cell’s periendothelial location at the abluminal aspect of microvessels [5]. A recent search of the PUBMED database at http://www.ncbi.nlm.nih.gov reveals a 118-fold difference between the number of papers published on these two vascular cell types. Since the cellular processes underlying neovascular sprout formation remain incompletely understood [6, 7], increased attention to pericytes and their interaction with endothelial cells will be required not only to attain a better understanding of vascular biology, but also to realize the full potential of anti-angiogenic therapy in oncology.
The relationship between endothelial cells and pericytes varies from tissue to tissue. As one example, the density of pericyte investment of the microvascular endothelium is quite high in the developing central nervous system, but very low in skeletal muscle [8, 9]. In tumor neovasculature this relationship may be even more variable. Underscoring the heterogeneity of tumor vasculature, as many as nine distinct angiogenic vessel classes have been described in neoplasms, based on both morphology and the cellular composition of the vessel wall (Table 1) [10]. One of these vessel classes (class 5) describes vascular walls in which the endothelium is discontinuous. Examples of class-5 vessels have recently become more numerous with the description of mosaic vessels [11, 12] and the phenomenon of vasculogenic mimicry [13-15].
Table 1.
The varieties and classification of blood vessels in the substance of neoplasm. (From Warren BA [10]).
| Class 1. Arteries and arterioles |
| Class 2. Capillaries |
| Class 3. Capillary sprouts |
| Class 4. Sinusoidal vessels |
| Class 5. Blood channels without endothelial lining |
| Class 6. Giant capillaries |
| Class 7. Capillaries with fenestrated endothelium |
| Class 8. Venules and veins |
| Class 9. Arterio-venous anastomoses |
These vessel types call to mind a recent suggestion from our studies that vascular pericytes can contribute to the heterogeneous nature of microvessels, even in non-tumor situations. In a model of corneal angiogenesis, we found endothelial cell-free segments of microvessels in which pericytes appeared to comprise the lumen of the microvascular tube [16]. In the current study we further analyze the ontogeny, morphology, and functional properties of pericyte tubes in normal retinal vasculature and in several types of tumors (melanoma, mammary, prostate, lung, glioma). This study expands our understanding of the contribution of pericytes to the process of angiogenic sprouting, demonstrating the early participation of these perivascular cells in microvascular tube formation.
Materials and methods
Tumors
Lewis lung carcinoma [17] and B16F1 melanoma [18] tumors resulted from subcutaneous injection of 5 × 106 cells into C57Bl/6 mice. Human prostatic carcinoma cell line derived from a bone metastasis of a patient (PC-3) prostate [19], protatic carcinoma cell line derived from a supraclavicular lymph node (LNCaP) prostate [20] and U87MG glioma tumors [21] were grown subcutaneously in athymic (Crl nu:nu) mice. Mammary tumors were obtained from female MMTV/PyMT mice, which spontaneously develop neoplasms due to activation of the polyoma middle T oncogene (MT) under control of the mouse mammary tumor virus (MMTV) promoter [22]. Prostate tumors were obtained from transgenic adenoma of prostate (TRAMP) mice [23].
Retinal vascular development
Vascular development in the retina progresses from the optic nerve head, extending peripherally along the inner retinal surface and finally invading the outer retinal layers. The laminar anatomy of the retina and the fact that vascularization largely occurs postnatally make this tissue a very useful model for the study of neovascular sprouting [24, 25]. Eyes were taken from postnatal days 2 and 7 (P2 and P7) C57Bl/6 mice following daily intraperitoneal bromodeoxyuridine (BrdU) (Sigma, St. Louis, Missouri) injections (80 μg/g body weight) to allow subsequent identification of proliferating vascular cells. Eyes were sectioned in a plane oriented sagitally to the optic nerve, so that each section represented a slice of the entire eyeball and retina. Immunostaining was then used to identify the tips of the most peripheral blood vessels in the primary vascular plexus, marking the transition between the vascular and avascular retinal tissue.
Transplantation of transgenic prostate and breast tumors
Breast and prostate tumors, respectively, were dissected from sacrificed MMTV/PyMT and TRAMP mice, both of which have an nerve/glial antigen 2 (NG2)+/+, C57Bl/6 genetic background. As described below, tumor fragments were transplanted into C57Bl/6, NG2-/-(NG2 knockout) mice [26] to determine whether the pericytes in tumor neovasculature are host or tumor-derived.
For mammary tumor transplantation, female NG2 knockout mice were anesthetized with Avertin, and an inverted Y-shaped incision was made through the abdominal skin [27]. The number-four mammary fat pads were exposed by a blunt dissection, and a small incision (1 mm) was made in each fat pad to accommodate 1 mm3 PyMT tumor fragments. The incision was closed using metal clips, and the mice were followed for 3 months to allow tumors to grow to a size of 1 cm3.
The cornea is a transparent, avascular tissue which enables real-time identification of new pathologic vessels that form as a result of implantation of tumor fragments [28]. NG2 knockout mice were anesthetized with Avertin, and tumor fragments (0.027 mm3) from 8-month-old TRAMP mice were implanted into corneal stromal micropockets created using a modified von Graefe knife [29]. The mice were monitored by biomicroscopic examination for two weeks to follow the progress of tumor vascularization within the corneal stroma.
Immunohistochemistry and vascular imaging
Following fixation of all tissues in 4% paraformaldehyde, cryopreservation in 20% sucrose in phosphate-buffered saline (PBS) at 4° C, embedding in O.C.T. compound (Sakura Inc., Torrance, California), and snap-freezing, frozen histologic sections (40-80 μm) were cut using a Reichert cryostat (Reichert Inc., Buffalo, New York).
Endothelial cells have been shown to express different cell surface markers as a function of developmental age [30]. We therefore identified endothelial cells using a cocktail of antibodies against endoglin (CD105), PECAM-1 (CD31), and VEGF receptor-2 (flk-1) (Pharmingen, San Diego, California), a strategy that has been previously utilized to maximize labeling of all vascular endothelial cells, both immature and mature [11]. Pericytes were identified by labeling with affinity-purified rabbit polyclonal antibodies prepared in our laboratory against the NG2 proteoglycan or the platelet-derived growth factor beta receptor (PDGF β-receptor) [9, 16]. Both NG2 and PDGF β-receptor are regarded as specific markers for pericytes [12, 31]. Proliferative pericyte and endothelial cell populations were identified immunohistochemically using anti-BrdU antibody (Fitzgerald Industries, Concord, Massachusetts) [32-34]. Briefly, frozen sections were digested with 0.005% pepsin (Sigma, St. Louis, Missouri) in 0.01 HCl for 30 min at 37° C followed by treatment with 4N HCl for 30 min at room temperature. Sections were then blocked by incubation in 5% goat serum in PBS for 30 min [35] prior to incubation with antibody.
Confocal microscopic imaging of combined endothelial (CD31 + CD105 +VEGF receptor-2, flk1) [11, 30] and pericyte markers (NG2 or PDGF β-receptor) was performed as described previously [9, 16, 36]. Briefly, optical sections were obtained from the specimens using the Bio-Rad MRC-1024MP confocal microscope. Serial optical sections (1 μm each) across the entire thickness (40-80 μm) of the histological specimens were overlaid (Z-stack) to provide reconstructions of entire vessels. This allowed unambiguous identification of the spatial relationship between pericytes and endothelial cells in the vessel wall. In order to estimate the frequency of vessels in any given tissue that contained endothelium-free segments (pericyte tubes), we performed systematic random sampling [37] to obtain 10 histologic sections for each tissue type to represent the corresponding frozen tissue block. Following immunohistochemistry, we counted the number of such endothelium-free segments (pericyte tubes) by microscopy, and divided the number of pericyte tubes by the total number of vessels in immunostained sections.
Fluorescein microangiography integrated with immunohistochemistry
To determine the functionality of pericyte tubes in a subcutaneous B16F1 melanoma, we performed fluorescein angiography using high molecular weight (two million daltons) dextran conjugated with fluorescein isothiocyanate (Sigma, St. Louis, Missouri). Since FITC-dextran remains completely within the vascular lumen without diffusion or decay [38], this procedure identifies only those vessels that are actively perfused by the circulation. FITC-dextran-perfused tissues were sectioned and immunostained for PDGF β-receptor or NG2 (red fluorochrome) to identify vessel walls in which pericytes interface directly with the intraluminal FITC-dextran.
Results
Pericyte tubes in early angiogenic sprouts
Examination of sections taken from spontaneous mammary tumors in female MMTV-PyMT mice reveals that a subpopulation of angiogenic tumor microvessels (1%) contain endothelium-free segments in which the vascular lumen is formed by a pericyte tube (Figures 1a-c). This phenomenon is remarkably similar to that observed in our corneal angiogenesis studies [16]. The Z-stack overlay demonstrates the absence of endothelial markers associated with the pericyte tube across the entire extent of the vessel in question (arrows and Δ in panel c). In the more typical vessels containing endothelial elements, we consistently noted extensive pericyte investment of the endothelium (panel c). Since it has been proposed that activated pericytes may be involved in creating/marking pathways for invading endothelial cells [4, 39], we wondered if pericyte tubes might represent an early developmental stage of microvessel development at which formation of the vascular endothelium is not complete. To test this possibility, we examined much younger (12 days old) tumors produced by subcutaneous grafts of mouse Lewis lung carcinoma cells in C57Bl/6 mice. Sections of these tumors contain even more striking examples in which entire vessels appear to be composed of pericyte tubes (Figures 1d-f). As before, the Z-stack demonstrates that these tubes are devoid of endothelial markers across their entire width. Even younger tumors (7 days) produced by subcutaneous xenografts of human PC-3 prostate tumor cells into athymic male mice (Figures 1g-i) contain examples not only of endothelium-free pericyte tubes (arrows and Δ in panel i), but also large numbers of individual pericytes invading the tumor in the absence of endothelial cells. TRAMP, LNCaP, and U87MG glioma tumors were also examined and found to contain populations of pericyte tubes (data not shown).
Figure 1.

Pericyte tubes are a component of tumor microvasculature. Frozen sections were taken from spontaneous MMTV-PyMT breast carcinoma (a-c), subcutaneous Lewis lung carcinoma (d-f), and subcutaneous PC-3 prostate tumor (g-i). Vascular endothelial elements (red) are identified by combined immunohistochemical staining for CD31, CD105, and flk-1. Pericytes (green) are identified by staining for NG2 proteoglycan. All panels are Z-stack confocal images. All three tumor types contain endothelium-free pericyte tubes, labeled by Δ in the merged images of panels c, f and i. The margins of complete pericyte tubes are indicated with arrows in c and i. In f the entire segment is a pure pericyte tube. Note the absence of endothelium (a, d, g and c, f, i) in regions that contain pericyte tubes (b, e, h). Scale bars indicate 10 μm.
To investigate the occurrence of pericyte tubes in normally developing microvasculature, we examined the mouse retina, in which a primary vascular plexus develops postnatally at the interface between the retina and vitreous. In addition to comparing endothelial and pericyte-specific markers, we also evaluated these samples for cell proliferation by immunohistochemical staining for BrdU incorporation. The sagital orientation of the sections made it possible to identify the tips of angiogenic sprouts growing toward more peripheral regions of the retina (arrow in Figure 2a). We used Nomarski interference microscopy (as in Figure 2c) to help choose sections that appeared to contain intact sprout tips. Between postnatal days 2 and 7 it is clear that pericytes and endothelial cells are both present at the growing tip of the vascular sprout. Using confocal microscopy, we found examples of endothelium-free segments of growing sprouts that appeared to be formed by pericytes (Δ in panel b), demonstrating the occurrence of pericyte tubes in normal as well as pathological microvasculature. The frequency of pericyte tubes in retinal microvasculature was similar to that seen in the various tumor models (1%). Strikingly, both endothelial cells (arrows) and pericytes (arrowheads) in growing retinal vessel tips have nuclei that are BrdU-positive. The pericyte nucleus characteristically fills almost the entire soma of the cell and bulges away from the lumen of the microvessel [8]. These factors explain why some pericyte nuclei in Figure 2b occupy an extreme peripheral position relative to the vessel. It is clear from the accompanying Nomarski image (Figure 2c) however, that these nuclei belong to pericytes that are closely associated with the microvessel. The presence of these BrdU-labeled nuclei is consistent with the nascent character of the vessel and shows that both cell populations are mitotically active at this stage. In microvessels such as these that contain both pericytes and endothelial cells, we always noted extensive investment of the endothelium by PDGF β-receptor-positive, NG2-positive pericytes. This is a general characteristic of microvessels in the central nervous system [3, 8, 31].
Figure 2.
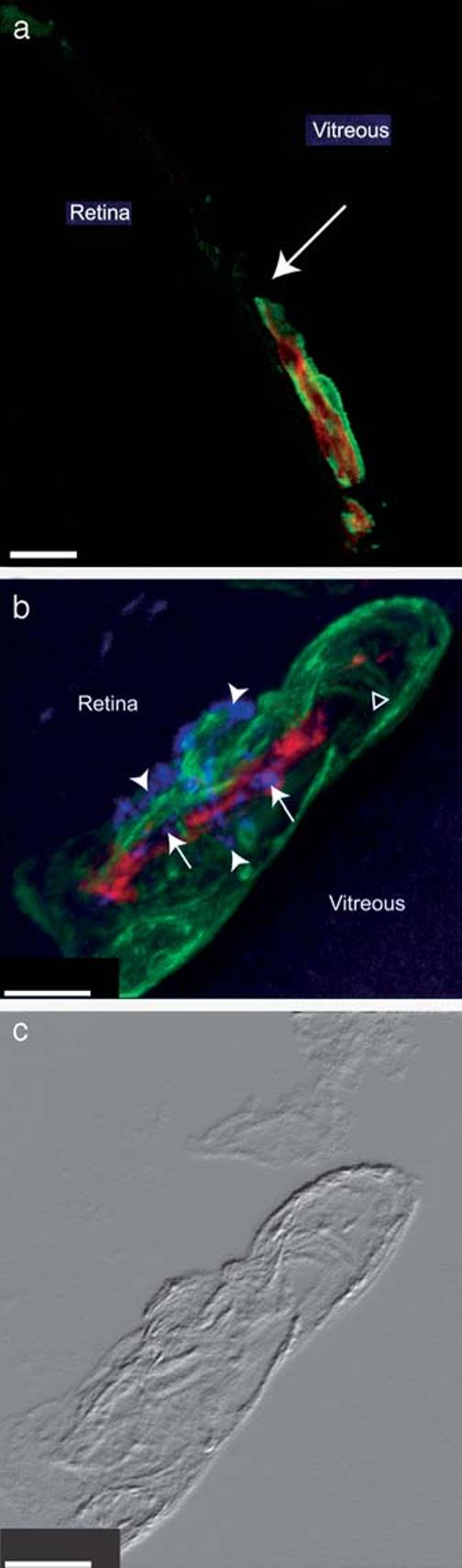
Early pericyte association with angiogenic microvessels in newborn mouse retina. Frozen sections of P2 (a) and P7 (b and c) mouse retina were processed by immunohistochemistry to identify the vascular endothelium (combined CD31 + CD105 + flk-1, red), pericytes (NG2, green), and nuclei of mitotic cells (BrdU, purple, panel b). Z-stack confocal images show that vascular pericytes extensively invest the angiogenic sprouts at both ages. Arrow in panel a indicates the tip of the growing angiogenic sprout at the vascular/avascular junction in the P2 peripheral retina. At P7 (panels b and c) both pericytes (arrowheads) and endothelial cells (arrows) contain BrdU-positive nuclei. Δ marks an endothelium free, pericyte tube-containing region of the angiogenic sprout. Panel c is the Nomarski image of a single focal plane from the specimen shown in panel b. Scale bars indicate 20 μm (a) and 5 μm (b, c).
Pericyte tubes are perfused by the tumor circulation
Sections of a subcutaneous B16F1 melanoma tumor perfused with FITC-dextran were examined to evaluate whether pericyte tubes are a functional component of the circulation. In 1% of the angiogenic vessels in these specimens, we observed a direct interface (Figure 3c) between pericytes (Figure 3a) and luminal FITC-dextran (Figure 3b), indicative of patency of the pericyte tube. This phenomenon was seen using either PDGF β-receptor immunostaining (Figure 3) or NG2 immunostaining (not shown) to identify pericytes. Since FITC-dextran does not leak and diffuse from blood vessels, the only way for a pericyte tube to be filled with the dye is to be connected to the active circulation.
Figure 3.

Functional (perfused) pericyte tube. In sections obtained from a subcutaneous B16F1 melanoma perfused with FITC-dextran, a pericyte tube immunostained (red) for PDGF β-receptor (a), is in direct contact with the vascular lumen filled with FITC-dextran (green) (b). In the merged image (c), arrowheads identify pericytes forming the vessel wall. Arrows indicate FITC-dextran in the lumen of the pericyte tube. The direct interface between these two labels precludes the presence of an intervening endothelial cell layer. Scale bar indicates 10 μm.
Pericytes in transplanted tumor microvessels are of host origin
Just as it has been suggested that tumor cells can display endothelial markers and contribute to forming the lumen of tumor vessels, it is formally possible that our observations could be explained by the acquisition of pericyte markers (such as NG2 and PDGF β-receptor) by tumor cells at the vascular lumen. Arguing against this possibility, B16F1, PC3, Lewis lung carcinoma, LNCaP, and U87MG cells were all found to be negative for CD105, CD31, flk-1, NG2 and PDGF β-receptor expression both in vivo and in vitro. TRAMP and PyMT tumor cells were also negative for NG2 expression (Figures 4b, d and f). Still, this does not preclude the possibility that rare, specialized tumor cells at the vascular lumen might be induced to express NG2 by virtue of the novel environment. Tumor transplantation experiments were performed to address this possibility.
Figure 4.
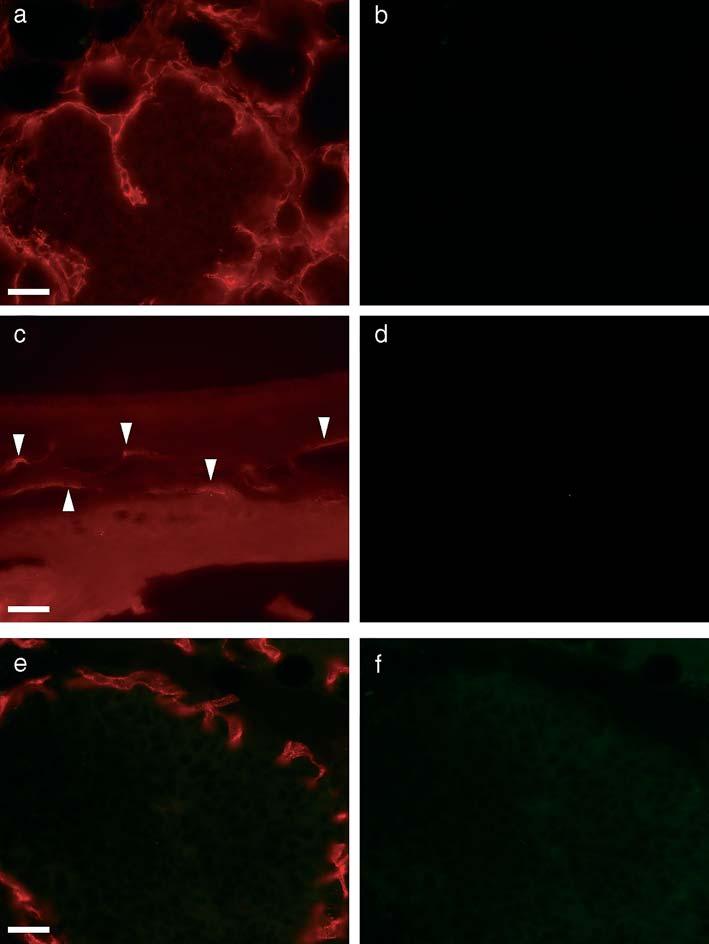
Host origin of pericytes in tumors. (a, b) Section from a PyMT breast tumor fragment transplanted to the fat pad of an NG2 knockout mouse is double-stained for PDGF β-receptor (a) and NG2 (b). Vascular pericytes invading the tumor are immunostained for PDGF β-receptor (a) but not for NG2 (b). (c, d) Section of a TRAMP prostate tumor fragment transplanted to the corneal stroma of an NG2 knockout mouse is double-stained for PDGF β-receptor (c) and NG2 (d). Neovascular pericytes are immunostained for PDGF β-receptor (c) but not for NG2 (d). Arrowheads indicate feeder vessels invading the cornea. (e, f) Section of PyMT breast tumor fragment transplanted to the fat pad of NG2 knockout mouse is double-stained for endothelial markers (CD105 + CD31 + flk1) (e) and NG2 (f). Angiogenic vessels exhibit immunostaining for endothelial markers (e) but not for NG2 (f). Tumor cells are not immunostained for either endothelial markers (e) or NG2 (f). Scale bars indicate 20 μm.
Analysis of breast tumor fragments transplanted from an NG2-positive (MMTV/PyMT) donor into the fat pads of an NG2 knockout host revealed PDGF β-receptor-positive pericytes associated with angiogenic sprouts invading the tumor from the periphery (Figure 4a). These pericytes were invariably NG2-negative (Figure 4b). Likewise, examination of prostate tumor fragments transplanted from an NG2-positive donor (TRAMP) into the cornea of an NG2 knockout host revealed PDGF β-receptor-positive pericytes (Figure 4c) associated with the corneal neovasculature. Once again, these pericytes were always negative for NG2 expression (Figure 4d). If the lumenal NG2-positive cells we have observed in our experiments with NG2-wildtype mice were of tumor origin, we would also expect to find such cells in the tumor vasculature of NG2-null mice. The fact that PDGF β-receptor-positive lumenal cells express NG2 in tumors grown in wildtype mice but not in tumors grown in NG2-null mice argues strongly that lumenal pericytes of the tumor microvasculature are derived from the host rather than from any component of the donor tumor.
Discussion
Two of the observations presented in our study seem especially noteworthy. First, mitotically active pericytes are associated with angiogenic sprouts during the early phases of neovascularization in both pathologic (tumors) and normal (retinal) tissues. Second, pericytes alone can invade tissues in the absence of endothelial cells and can form functional, endothelium-free tubes that may be sub-classified as ‘pericyte tubes’ under the category of class-5 vessels.
These observations support the idea that activated, mitotic pericytes play an early role in the development of angiogenic sprouts and vessels. Coupled with our previous finding of pericytes associated with angiogenic sprouts in the embryonic central nervous system and in postnatal neovasculature formed in response to ischemia or growth factors [9, 16], these results emphasize the early participation of pericytes in both physiological and pathological angiogenesis. Activated pericytes are present as early as mitotic endothelial cells, and in some instances appear to take the lead in establishing not only pathways of invasion, but also the formation of actual tubes. These findings are not entirely consistent with data suggesting that pericytes have only a late role in angiogenesis [40-42], but instead provide support for reports concerning an early role for pericytes in the formation of angiogenic sprouts [4, 12, 31, 39, 43-47]. Our identification of perfused, endothelium-free pericyte tubes comprising up to 1% of the total number of microvessels in our various preparations may be a reflection of the kinetics of angiogenic development. While pericytes may have a leading role in establishing pathways of invasion and formation of angiogenic tubes, endothelial cell participation cannot lag far behind, and may in fact occur almost simultaneously. Thus in most cases, especially in normal tissues, we observe pericytes and endothelial cells working in concert to form angiogenic microvessels composed of endothelial tubes extensively invested by pericytes. In this sense, pericyte tubes and pericyte-invested endothelial tubes do not represent distinct functional entities, but instead different developmental stages of the same process. In only a small number of instances do we catch a glimpse of pericyte tubes that are not yet fully lined by endothelial cells. It seems possible that sufficient disregulation could occur during tumor angiogenesis to render the early contributions of pericytes more easily detectable.
An alternative mechanism for the formation of pericyte tubes could be the loss of endothelial cells as part of the process of vascular regression/pruning and remodeling. We cannot definitively rule out this possibility. However, it seems the less likely of the two alternatives, based on the following observations. In the developing retina, neovascular remodeling is primarily seen in the central retina, rather than in peripheral areas of the retina that contain the newest angiogenic sprouts [48]. The examples shown in Figure 2 are taken from the peripheral retina at the outermost boundary between the vascular and avascular retina. The structures shown are therefore likely to be nascent sprouts rather than vessels undergoing remodeling. The presence of BrdU-positive endothelial cell nuclei further supports the idea that these are new sprouts rather than regressing vessels. In the tumor studies, it is more difficult to evaluate the ontogeny of vessels in the absence of the stereotypical tissue architecture found in the retina. On the basis of tumor age and the possible existence of hypoxic areas within the tumor, it seems possible that regressing vessels might contribute to the labeling pattern seen in the mature mammary tumors (Figures 1a-c). It seems much less likely that endothelial cell regression can explain the patterns seen in the small, nascent tumor xenografts examined only 12 (d-f) and 7 (g-i) days after tumor cell injection. Endothelial cells from regressing vascular segments usually do not disappear (for example via apoptosis), but instead are re-utilized for the formation of new vessels [49]. Thus, the observation of pericytes invading the seven-day-old PC-3 tumor and forming tubes in the absence of endothelial cells is more suggestive of the formation of new microvessels than the regression of old ones.
Although pericytes are widely regarded to be the microvascular equivalent of smooth muscle cells, the origin, development, and function of these cells seem to be variable and complex [8, 50, 51]. Reliable identification of pericytes is important for understanding the role of these cells in angiogenic sprout formation and vascular heterogeneity. Our ability to detect the precocious contribution of pericytes to microvascular development depends heavily on the use of PDGF β-receptor and NG2 proteoglycan as markers for these activated mural cells at an early stage of their development [12, 31]. One of the traditional markers for pericyte identification has been the expression of alpha-smooth muscle actin (α-SMA). However, a growing body of evidence suggests that α-SMA is a late marker for differentiated pericytes in rodents and therefore may be poorly expressed in developing angiogenic microvasculature [12, 31]. Since only a fraction of developing pericytes can be identified on the basis of α-SMA expression [4, 12, 52-55], the absence of immunoreactivity for α-SMA does not necessarily indicate the absence of pericytes. By comparison, NG2 is more widely expressed by pericytes, especially during early stages of development. Pericytes expressing NG2 also express other pericyte markers such as PDGF β-receptor [9, 16, 36, 56-58] and aminopeptidase A [45]. Therefore, NG2 is an established, specific marker for identification of pericytes. It is expressed by pericytes in angiogenic microvasculature during pre- and postnatal development [9, 59], during tumor angiogenesis [43, 44, 60], in granulation tissue [43, 44, 58, 61], and in corneal and retinal angiogenesis models [16].
Our results more firmly establish endothelium-free pericyte tubes as a valid species of angiogenic vessel. Our tumor fragment transplantation experiments support previous results demonstrating a host origin for pericytes in tumor neovasculature [62]. Direct visualization of the invasion of host-derived, GFP-negative cells into GFP-positive tumors has provided additional evidence for the host origin of tumor vasculature [63]. Our combined fluorescein angiography-immunohistochemistry analysis of tumor vasculature is also of interest in this regard, since it provides an additional means of visualizing functional, newly formed pericyte tubes that have recently invaded the tumor.
In summary, our study provides evidence that pericytes contribute to the early phases of angiogenic sprout formation during both neoplastic and non-neoplastic neovascularization. The early participation of pericytes in microvascular development has important implications for therapeutic intervention in the many pathologies in which angiogenesis is a factor. As early players in angiogenesis, pericytes represent an additional target for treatments designed either to up-regulate (for example in ischemic disorders) or down-regulate (for example in cancer) vascularization. Recent studies suggest that dual targeting of pericytes and endothelial cells improves the efficacy of treatments aimed at the destruction of tumor masses [64, 65]. Clearly, the design of improved dual targeting strategies aimed at both pericytes and endothelial cells will depend on further understanding of the role of pericytes in neovascularization.
Acknowledgements
This work was supported by postdoctoral fellowship 0120036Y from the American Heart Association Western States Affliate, New Investigator Grant PC020822 from the US Department of Defense Prostate Cancer Research Program DAMD, and NIH grant RO3 HD044783-01 to Dr Ozerdem, and by NIH grant RO1 CA95287 to Dr Stallcup.
Footnotes
Presented in part at the vascular biology symposium of Federation of American Societies for Experimental Biology (FASEB), 2003, Annual Meeting in San Diego, California, USA.
- BrdU
- bromodeoxyuridine
- CD31
- PECAM-1
- CD105
- endoglin
- flk 1
- VEGF receptor-2
- LNCaP
- prostatic carcinoma cell line derived from a supraclavicular lymph node metastasis
- NG2
- nerve/glial antigen 2
- PBS
- phosphate-buffered saline
- PC-3
- prostatic carcinoma cell line derived from a bone metastasis of a patient
- PDGF β-receptor
- platelet-derived growth factor beta receptor
- α-SMA
- alpha isoform of smooth muscle actin
- TRAMP
- transgenic adenocarcinoma of mouse prostate
References
- Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995;1:27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- Risau W. Mechanisms of angiogenesis. Nature. 1997;386:671–4. doi: 10.1038/386671a0. [DOI] [PubMed] [Google Scholar]
- Sims DE. Recent advances in pericyte biology - implications for health and disease. Can J Cardiol. 1991;7:431–43. [PubMed] [Google Scholar]
- Nehls V, Denzer K, Drenckhahn D. Pericyte involvement in capillary sprouting during angiogenesis in situ. Cell Tissue Res. 1992;270:469–74. doi: 10.1007/BF00645048. [DOI] [PubMed] [Google Scholar]
- Rhodin JA. Ultrastructure of mammalian venous capillaries, venules, and small collecting veins. J Ultrastruct Res. 1968;25:452–500. doi: 10.1016/s0022-5320(68)80098-x. [DOI] [PubMed] [Google Scholar]
- Bergers G, Benjamin LE. Tumorigenesis and the angiogenic switch. Nat Rev Cancer. 2003;3:401–10. doi: 10.1038/nrc1093. [DOI] [PubMed] [Google Scholar]
- Darland DC, D’Amore PA. Blood vessel maturation: Vascular development comes of age. J Clin Invest. 1999;103:157–8. doi: 10.1172/JCI6127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims DE. The pericyte - a review. Tissue Cell. 1986;18:153–74. doi: 10.1016/0040-8166(86)90026-1. [DOI] [PubMed] [Google Scholar]
- Ozerdem U, Grako KA, Dahlin-Huppe K, et al. NG2 proteoglycan is expressed exclusively by mural cells during vascular morphogenesis. Dev Dyn. 2001;222:218–27. doi: 10.1002/dvdy.1200. [DOI] [PubMed] [Google Scholar]
- Warren BA. The vascular morphology of tumors. In: Peterson H-I, editor. Tumor Blood Circulation: Angiogenesis, Vascular Morphology and Blood Flow of Experimental and Human Tumors. CRC Press; Boca Raton, Florida: 1979. pp. 31–9. [Google Scholar]
- Chang YS, di Tomaso E, McDonald DM, et al. Mosaic blood vessels in tumors: Frequency of cancer cells in contact with flowing blood. Proc Natl Acad Sci USA. 2000;97:14608–13. doi: 10.1073/pnas.97.26.14608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald DM, Choyke PL. Imaging of angiogenesis: From microscope to clinic. Nat Med. 2003;9:713–25. doi: 10.1038/nm0603-713. [DOI] [PubMed] [Google Scholar]
- Folberg R, Hendrix MJ, Maniotis AJ. Vasculogenic mimicry and tumor angiogenesis. Am J Pathol. 2000;156:361–81. doi: 10.1016/S0002-9440(10)64739-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrix MJ, Seftor EA, Hess AR, Seftor RE. Angiogenesis: Vasculogenic mimicry and tumour-cell plasticity: Lessons from melanoma. Nat Rev Cancer. 2003;3:411–21. doi: 10.1038/nrc1092. [DOI] [PubMed] [Google Scholar]
- Sood AK, Seftor EA, Fletcher MS, et al. Molecular determinants of ovarian cancer plasticity. Am J Pathol. 2001;158:1279–88. doi: 10.1016/S0002-9440(10)64079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozerdem U, Monosov E, Stallcup WB. NG2 proteoglycan expression by pericytes in pathological microvasculature. Microvasc Res. 2002;63:129–34. doi: 10.1006/mvre.2001.2376. [DOI] [PubMed] [Google Scholar]
- O’Reilly MS, Holmgren L, Shing Y, et al. Angiostatin: A novel angiogenesis inhibitor that mediates the suppression of metastases by a Lewis lung carcinoma. Cell. 1994;79:315–28. doi: 10.1016/0092-8674(94)90200-3. [DOI] [PubMed] [Google Scholar]
- Fidler IJ. Biological behavior of malignant melanoma cells correlated to their survival in vivo. Cancer Res. 1975;35:218–24. [PubMed] [Google Scholar]
- Kaighn ME, Narayan KS, Ohnuki Y, et al. Establishment and characterization of a human prostatic carcinoma cell line (PC-3) Invest Urol. 1979;17:16–23. [PubMed] [Google Scholar]
- Horoszewicz JS, Leong SS, Chu TM, et al. The LNCaP cell line - a new model for studies on human prostatic carcinoma. Prog Clin Biol Res. 1980;37:115–32. [PubMed] [Google Scholar]
- Ponten J, Macintyre EH. Long term culture of normal and neoplastic human glia. Acta Pathol Microbiol Scand. 1968;74:465–86. doi: 10.1111/j.1699-0463.1968.tb03502.x. [DOI] [PubMed] [Google Scholar]
- Guy CT, Cardiff RD, Muller WJ. Induction of mammary tumors by expression of polyomavirus middle T oncogene: A transgenic mouse model for metastatic disease. Mol Cell Biol. 1992;12:954–61. doi: 10.1128/mcb.12.3.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg NM, DeMayo F, Finegold MJ, et al. Prostate cancer in a transgenic mouse. Proc Natl Acad Sci USA. 1995;92:3439–43. doi: 10.1073/pnas.92.8.3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaki H, Seo MS, Ozaki K, et al. Blockade of vascular endothelial cell growth factor receptor signaling is sufficient to completely prevent retinal neovascularization. Am J Pathol. 2000;156:697–707. doi: 10.1016/S0002-9440(10)64773-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campochiaro PA. Retinal and choroidal neovascularization. J Cell Physiol. 2000;184:301–10. doi: 10.1002/1097-4652(200009)184:3<301::AID-JCP3>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Grako KA, Ochiya T, Barritt D, et al. PDGF (alpha)-receptor is unresponsive to PDGF-AA in aortic smooth muscle cells from the NG2 knockout mouse. J Cell Sci. 1999;112:905–15. doi: 10.1242/jcs.112.6.905. [DOI] [PubMed] [Google Scholar]
- Daniel CW, De Ome KB, Young JT, et al. The in vivo life span of normal and preneoplastic mouse mammary glands: A serial transplantation study. Proc Natl Acad Sci USA. 1968;61:53–60. doi: 10.1073/pnas.61.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimbrone MA, Jr, Cotran RS, Leapman SB, Folkman J. Tumor growth and neovascularization: An experimental model using the rabbit cornea. J Natl Cancer Inst. 1974;52:413–27. doi: 10.1093/jnci/52.2.413. [DOI] [PubMed] [Google Scholar]
- Kenyon BM, Voest EE, Chen CC, et al. A model of angiogenesis in the mouse cornea. Invest Ophthalmol Vis Sci. 1996;37:1625–32. [PubMed] [Google Scholar]
- Drake CJ, Fleming PA. Vasculogenesis in the day 6.5 to 9.5 mouse embryo. Blood. 2000;95:1671–9. [PubMed] [Google Scholar]
- Gerhardt H, Betsholtz C. Endothelial-pericyte interactions in angiogenesis. Cell Tissue Res. 2003;314:15–23. doi: 10.1007/s00441-003-0745-x. [DOI] [PubMed] [Google Scholar]
- Dolbeare F, Gratzner H, Pallavicini MG, Gray JW. Flow cytometric measurement of total DNA content and incorporated bromodeoxyuridine. Proc Natl Acad Sci USA. 1983;80:5573–7. doi: 10.1073/pnas.80.18.5573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean PN, Dolbeare F, Gratzner H, et al. Cell-cycle analysis using a monoclonal antibody to BrdUrd. Cell Tissue Kinet. 1984;17:427–36. doi: 10.1111/j.1365-2184.1984.tb00601.x. [DOI] [PubMed] [Google Scholar]
- Nowakowski RS, Lewin SB, Miller MW. Bromodeoxyuridine immunohistochemical determination of the lengths of the cell cycle and the DNA-synthetic phase for an anatomically defined population. J Neurocytol. 1989;18:311–8. doi: 10.1007/BF01190834. [DOI] [PubMed] [Google Scholar]
- Ezaki T, Baluk P, Thurston G, et al. Time course of endothelial cell proliferation and microvascular remodeling in chronic inflammation. Am J Pathol. 2001;158:2043–55. doi: 10.1016/S0002-9440(10)64676-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl P, Johansson BR, Leveen P, Betsholtz C. Pericyte loss and microaneurysm formation in PDGF-B-deficient mice. Science. 1997;277:242–5. doi: 10.1126/science.277.5323.242. [DOI] [PubMed] [Google Scholar]
- Dawson B, Trapp RG. Basic and Clinical Biostatistics. 3rd ed McGraw-Hill; New York: 2001. [Google Scholar]
- D’Amato R, Wesolowski E, Smith LE. Microscopic visualization of the retina by angiography with high-molecular-weight fluorescein-labeled dextrans in the mouse. Microvasc Res. 1993;46:135–42. doi: 10.1006/mvre.1993.1042. [DOI] [PubMed] [Google Scholar]
- Rhodin JA, Fujita H. Capillary growth in the mesentery of normal young rats. Intravital video and electron microscope analyses. J Submicrosc Cytol Pathol. 1989;21:1–34. [PubMed] [Google Scholar]
- Sato Y, Rifkin DB. Inhibition of endothelial cell movement by pericytes and smooth muscle cells: Activation of a latent transforming growth factor-beta 1-like molecule by plasmin during co-culture. J Cell Biol. 1989;109:309–15. doi: 10.1083/jcb.109.1.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck L, Jr, D’Amore PA. Vascular development: Cellular and molecular regulation. FASEB J. 1997;11:365–73. [PubMed] [Google Scholar]
- Hirschi KK, Rohovsky SA, Beck LH, et al. Endothelial cells modulate the proliferation of mural cell precursors via platelet-derived growth factor-BB and heterotypic cell contact. Circ Res. 1999;84:298–305. doi: 10.1161/01.res.84.3.298. [DOI] [PubMed] [Google Scholar]
- Schlingemann RO, Rietveld FJ, de Waal RM, et al. Expression of the high molecular weight melanoma-associated antigen by pericytes during angiogenesis in tumors and in healing wounds. Am J Pathol. 1990;136:1393–405. [PMC free article] [PubMed] [Google Scholar]
- Schlingemann RO, Rietveld FJ, Kwaspen F, et al. Differential expression of markers for endothelial cells, pericytes, and basal lamina in the microvasculature of tumors and granulation tissue. Am J Pathol. 1991;138:1335–47. [PMC free article] [PubMed] [Google Scholar]
- Schlingemann RO, Oosterwijk E, Wesseling P, et al. Aminopeptidase a is a constituent of activated pericytes in angiogenesis. J Pathol. 1996;179:436–42. doi: 10.1002/(SICI)1096-9896(199608)179:4<436::AID-PATH611>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Wesseling P, Schlingemann RO, Rietveld FJ, et al. Early and extensive contribution of pericytes/vascular smooth muscle cells to microvascular proliferation in glioblastoma multiforme: An immuno-light and immuno-electron microscopic study. J Neuropathol Exp Neurol. 1995;54:304–10. doi: 10.1097/00005072-199505000-00003. [DOI] [PubMed] [Google Scholar]
- Morikawa S, Baluk P, Kaidoh T, et al. Abnormalities in pericytes on blood vessels and endothelial sprouts in tumors. Am J Pathol. 2002;160:985–1000. doi: 10.1016/S0002-9440(10)64920-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhardt H, Golding M, Fruttiger M, et al. VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J Cell Biol. 2003;161:1163–77. doi: 10.1083/jcb.200302047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes S, Chang-Ling T. Roles of endothelial cell migration and apoptosis in vascular remodeling during development of the central nervous system. Microcirculation. 2000;7:317–33. [PubMed] [Google Scholar]
- Le Lievre CS, Le Douarin NM. Mesenchymal derivatives of the neural crest: Analysis of chimaeric quail and chick embryos. J Embryol Exp Morphol. 1975;34:125–54. [PubMed] [Google Scholar]
- Allt G, Lawrenson JG. Pericytes: Cell biology and pathology. Cells Tissues Organs. 2001;169:1–11. doi: 10.1159/000047855. [DOI] [PubMed] [Google Scholar]
- Nehls V, Drenckhahn D. Heterogeneity of microvascular pericytes for smooth muscle type alpha-actin. J Cell Biol. 1991;113:147–54. doi: 10.1083/jcb.113.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balabanov R, Dore-Duffy P. Role of the CNS microvascular pericyte in the blood-brain barrier. J Neurosci Res. 1998;53:637–44. doi: 10.1002/(SICI)1097-4547(19980915)53:6<637::AID-JNR1>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Boado RJ, Pardridge WM. Differential expression of alpha-actin mRNA and immunoreactive protein in brain microvascular pericytes and smooth muscle cells. J Neurosci Res. 1994;39:430–5. doi: 10.1002/jnr.490390410. [DOI] [PubMed] [Google Scholar]
- Alliot F, Rutin J, Leenen PJ, Pessac B. Pericytes and periendothelial cells of brain parenchyma vessels co-express aminopeptidase N, aminopeptidase A, and nestin. J Neurosci Res. 1999;58:367–78. [PubMed] [Google Scholar]
- Reuterdahl C, Sundberg C, Rubin K, et al. Tissue localization of beta receptors for platelet-derived growth factor and platelet-derived growth factor B chain during wound repair in humans. J Clin Invest. 1993;91:2065–75. doi: 10.1172/JCI116429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundberg C, Ljungstrom M, Lindmark G, et al. Microvascular pericytes express platelet-derived growth factor-beta receptors in human healing wounds and colorectal adenocarcinoma. Am J Pathol. 1993;143:1377–88. [PMC free article] [PubMed] [Google Scholar]
- Rajkumar VS, Sundberg C, Abraham DJ, et al. Activation of microvascular pericytes in autoimmune Raynaud’s phenomenon and systemic sclerosis. Arthritis Rheum. 1999;42:930–41. doi: 10.1002/1529-0131(199905)42:5<930::AID-ANR11>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Grako KA, Stallcup WB. Participation of the NG2 proteoglycan in rat aortic smooth muscle cell responses to platelet-derived growth factor. Exp Cell Res. 1995;221:231–40. doi: 10.1006/excr.1995.1371. [DOI] [PubMed] [Google Scholar]
- Chekenya M, Enger PO, Thorsen F, et al. The glial precursor proteoglycan. NG2, is expressed on tumour neovasculature by vascular pericytes in human malignant brain tumours. Neuropathol Appl Neurobiol. 2002;28:367–80. doi: 10.1046/j.1365-2990.2002.00412.x. [DOI] [PubMed] [Google Scholar]
- Sims DE. Diversity within pericytes. Clin Exp Pharmacol Physiol. 2000;27:842–6. doi: 10.1046/j.1440-1681.2000.03343.x. [DOI] [PubMed] [Google Scholar]
- Abramsson A, Berlin O, Papayan H, et al. Analysis of mural cell recruitment to tumor vessels. Circulation. 2002;105:112–7. doi: 10.1161/hc0102.101437. [DOI] [PubMed] [Google Scholar]
- Yang M, Baranov E, Wang JW, et al. Direct external imaging of nascent cancer, tumor progression, angiogenesis, and metastasis on internal organs in the fluorescent orthotopic model. Proc Natl Acad Sci USA. 2002;99:3824–9. doi: 10.1073/pnas.052029099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergers G, Song S, Meyer-Morse N, et al. Benefits of targeting both pericytes and endothelial cells in the tumor vasculature with kinase inhibitors. J Clin Invest. 2003;111:1287–95. doi: 10.1172/JCI17929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saharinen P, Alitalo K. Double target for tumor mass destruction. J Clin Invest. 2003;111:1277–80. doi: 10.1172/JCI18539. [DOI] [PMC free article] [PubMed] [Google Scholar]


