Abstract
In this study, we report the interaction of the Drosophila transcription factors Trithorax-like (GAGA) and tramtrack (TTK). This interaction is documented both in vitro, through GST pull-down assays, as well as in vivo, in yeast and Schneider S2 cells. GAGA and TTK share in common the presence of an N-terminal POZ/BTB domain that was found to be necessary and sufficient for GAGA–TTK interaction. Structural models that could account for this interaction are discussed. GAGA is known to activate the expression of many genes in Drosophila. On the other hand, TTK was proposed to act as a maternally provided repressor of several pair-rule genes, such as even-skipped (eve). As with many Drosophila genes, eve contains at its promoter region binding sites for GAGA and TTK. Here, in transient expression experiments, we showed that GAGA activates transcription from the eve stripe 2 promoter element and that TTK inhibits this GAGA-dependent activation. Repression by TTK of the eve promoter requires its activation by GAGA and depends on the presence of the POZ/BTB domains of TTK and GAGA. These results indicate that GAGA–TTK interaction contributes to the regulation of gene expression in Drosophila.
INTRODUCTION
Many genes in Drosophila contain, at their regulatory regions, binding sites for the transcription factors Trithorax-like (GAGA) and tramtrack (TTK). GAGA and TTK share in common the presence of an N-terminal POZ/BTB domain (1,2). POZ/BTB is a highly conserved structural motif that has been found in proteins participating in a variety of biological processes from the formation of ion-selective channels to the organisation of the cytoskeleton and the nuclear matrix, or the regulation of transcription (reviewed in 3). Typical of a structurally independent domain that plays specific functions, the POZ/BTB domain is most frequently found in combination with other domains such as kelch repeats, ankryn repeats, transmembrane domains or zinc-finger domains (3). The POZ/BTB domain mediates protein–protein interactions. In several proteins, the POZ/BTB domain has been found to participate in the formation of homo-oligomers (4–7) and, based on in vitro co-expression experiments, it was proposed that POZ/BTB domains can also form hetero-oligomers (4). In addition, in several transcription factors, the POZ/BTB domain was also shown to be involved in interactions with various co-repressor proteins (8–14). The POZ/BTB domain of the human promyelocytic leukaemia zinc finger (PLZF) protein was crystallised and its structure was solved (15,16). The crystal structure shows a tightly interwound dimer and, therefore, provides a general structural basis for the participation of POZ/BTB domains in homomeric interactions. Some features of the PLZF dimer are strongly reminiscent of an obligate homo-dimer. Dimerisation occurs through an extensive hydrophobic interface that involves approximately one quarter of the monomer accessible surface (15,16) and unfolding of the POZ/BTB dimer of PLZF occurs as a single coupled two-state transition that is irreversible (7). These considerations raise the question as to what extent POZ/BTB domains can actually support the formation of hetero-oligomers in vivo. On the other hand, heterophilic interactions with co-repressors were proposed to occur through a cluster of conserved residues that lie at a surface-exposed groove formed at the dimer interface (16).
In this study, we report that GAGA519 and TTK69 isoforms interact in vitro as well as in vivo, both in yeast and Schneider S2 cells, and that their POZ/BTB domains are necessary and sufficient for this interaction to occur. Structural models that could account for this interaction are discussed. The various GAGA and TTK isoforms all share the corresponding POZ/BTB domain (17,18) and, therefore, are likely to support similar GAGA–TTK interactions. The potential contribution of this interaction to the regulation of transcription is also discussed. As with many Drosophila promoters, the even-skipped (eve) stripe 2 regulatory region contains several binding sites for GAGA and TTK which are in relatively close proximity (2,19,20), suggesting that the GAGA–TTK interaction described here might participate in its functional regulation. GAGA and TTK appear to have opposite effects on transcription. The expression of many genes in Drosophila is positively regulated by GAGA (21) and it has been shown that GAGA acts as a transcription activator in vitro, or upon transient transfection in cultured S2 cells (2,18,22). On the other hand, during early embryo development, TTK was shown to function as a transcription repressor of several pair-rule genes, including eve (23–25). Here, we show that GAGA activates transcription from the eve stripe 2 promoter and that TTK inhibits this GAGA-dependent activation. Repression by TTK of the eve promoter depends on the presence of GAGA at the promoter and requires the contribution of the POZ/BTB domain.
MATERIALS AND METHODS
Proteins
The proteins used in these experiments correspond to GAGA519 (2,18) and TTK69 forms (17,26). Several truncated peptides derived from them were constructed: ΔPOZGAGA and ΔPOZTTK, which are missing the first 122 and 115 residues of GAGA519 and TTK69, respectively, and POZGAGA and POZTTK which carry the first 122 and 114 residues of GAGA519 and TTK69, respectively. Proteins were expressed in BL21LysS or BL21 Escherichia coli cells as 6×His- tagged proteins, using pET14 expression vectors (Novagen), and/or as GST-fusions proteins, using pGEX-KG vectors (Amersham).
Rabbit polyclonal antibodies raised against bacterially expressed GAGA519 and TTK69 proteins were obtained and purified by conventional methods.
Immunoprecipitation experiments
For co-immunoprecipitation experiments, Schneider S2 cells were co-transfected by the calcium phosphate method. For each transfection, 7–8 × 106 cells were plated onto 100 mm diameter tissue culture dishes 1 day before transfection. Cells were co-transfected with 15 µg of pAct5CPPA expression vectors carrying the full-length cDNAs encoding GAGA519 and TTK69. The total amount of transfected DNA was adjusted to 30 µg by the addition of pUC19. Cell extracts were prepared 48 h after transfection. Cells were washed with 1 ml of ice-cold PBS and lysed with 0.5 ml of IPH buffer [50 mM Tris–HCl (pH 8.0), 150 mM NaCl, 5 mM EDTA, 0.5% NP-40, 0.1 mM PMSF, 0.1 mM aprotinin and 0.1 mM leupeptin] for 30 min on ice. Extracts were cleared by centrifugation. Immunoprecipitation was carried out at 300 mM NaCl by the addition of affinity-purified αGAGA or αTTK rabbit polyclonal antibodies. When the preimmune serum was used, the IgG fraction was prepared. Incubation was performed at 4°C for 2 h. Twenty microlitres of Protein A–Sepharose beads (Amersham) were then added and incubated at 4°C for 2 h. Complexes were pelleted by centrifugation, washed four times with IPH buffer and analysed by western blotting with affinity-purified αGAGA or αTTK antibodies and detected by ECL (Amersham).
Yeast two-hybrid experiments
For the yeast two-hybrid assays, the various constructs used were cloned into plasmids pGBT9 and pGAD424 (Clontech) to express fusion proteins containing either the GAL4 DNA-binding domain or the GAL4 activation domain, respectively. Appropriate plasmids were then transformed into yeast HF7c strain (MATa, ura3-52, his3-200, ade2-101, lys2-801, trp1-901, leu2-3,112, gal4-542, gal80-538, LYS2::GAL1UAS-GAL1TATA-HIS3, URA3::GAL417mers(3x)-CyC1TATA-HIS3) and the transformants were tested for their ability to grow on selective medium lacking histidine. For quantitative analysis, transformants were tested for activation of the GAL4-dependent LacZ reporter gene.
GST pull-down assays
GST-fusion proteins expressed in E.coli were bound to glutathione–Sepharose-4B beads. Protein concentrations, determined by gel electrophoresis, were adjusted by dilution with unbound beads. Agarose-bound GST fusion proteins were equilibrated in 25 mM HEPES (pH 7.5), 150 mM KCl, 12.5 mM MgCl2, 20% glycerol, 0.1% NP-40 and incubated for 1 h at room temperature with 35S-labelled full-length TTK or ΔPOZTTK proteins that were produced in vitro, using the TNT-coupled reticulocyte lysate system (Promega). Beads were washed four times with 1 ml of 20 mM Tris–HCl (pH 8.0), 100 mM NaCl, 1 mM EDTA, 0.5% NP-40. Bound proteins were analysed on SDS–PAGE gels by autoradiography.
Transcription assays in S2 cells
The effects of GAGA and TTK on the transcription activity of the eve stripe 2 promoter were analysed by transient expression experiments in cultured S2 cells using a pGL3 reporter plasmid (Promega) that carries a luciferase reporter gene fused to a 1.8 kb fragment of the eve promoter (from position +102 to position –1759), which was found to drive transcription within the limits of stripe 2 (19). The structure of the reporter plasmid carrying the 5×GAL4-hsp70 promoter was described earlier (22). Seven micrograms of each reporter plasmid was co-transfected to S2 cells together with increasing amounts of pAct5CPPA vectors expressing GAGA519, TTK69, ΔPOZTTK, ΔPOZGAGA, GAL4Q or POZGAGAGAL4Q as indicated in each case. GAL4Q was described earlier (22). POZGAGAGAL4Q was constructed by fusing the POZ/BTB domain of GAGA (122 amino acids) to the N-terminus of GAL4Q using a 6×His-tag as linker. Three micrograms of pCMVβGal plasmid (Stratagene) was added to the transfection mixture to allow correction for variations in transfection efficiencies. The total amount of plasmid added was adjusted to 20 µg by the addition of pAct5CPPA. Transfection was carried out by the calcium phosphate method. Cells were recovered 48 h after transfection and lysed according to the β-galactosidase gene reporter kit (Roche Molecular Biochemicals). Luciferase activity was determined according to the luciferase activity assay kit (Promega) and normalised with respect to β-galactosidase activity.
Model building
The models of the POZ/BTB domain complexes analysed in this study were built using the program MODELLER v.4, kindly provided by Dr Sali, which models protein structures by satisfying spatial restraints, starting from the extended polypeptide chain conformation (27). For comparative modelling of the POZ/BTB domains, these restraints were derived from the aligned sequences of the modelled domains with that of PLZF, whose crystal structure (15,16) was taken as a template (PDB no. 1buo).
Four kinds of models were built. For the POZ/BTB domains of GAGA and TTK, homo-dimers were built using the whole crystallographic PLZF homo-dimer as template. No symmetry restraints were applied, so that each monomer was solely constrained to keep in the dimer, but not to have exactly the same conformation as its partner. A model of a putative GAGA–TTK hetero-dimer was obtained following the same principles as for the homo-dimers. The models of GAGA and TTK homo-tetramers were built on the basis of the crystallographic PLZF tetramer, as observed in the same PDB entry indicated above. Again, no symmetry restraints were imposed to the monomers. Finally, a GAGA–TTK hetero-tetramer was constructed by the same procedure, but using POZGAGA and POZTTK homo-dimers. The flexible loops that for GAGA and TTK lie in the exposed groove at the dimer interface, were generated by using internal co-ordinates from the CHARMM topology library included in MODELLER. All the models were refined by the program using thorough simulated annealing with molecular dynamics. During the modelling process the target structures were internally checked for their consistency with the restraints used to calculate them. Five models were generated in each round, and that with the best self-consistency score was selected.
Global analysis of the models was performed with the programs ProsaIIv.3 (28), to assess the compatibility of residues with their environment, and WHATCHECK (29), to elucidate its stereochemical correctness. Only the WHATCHECK packing quality scores were found to be significantly worse in the models, which was also reflected in slightly higher ProsaII z-scores. These values were, however, in the expected range for theoretical models. The sc program (30) from the CCP4 suite was used to calculate the ‘surface complementarity’ statistics, as well as to incorporate their values to the solvent accessible surface generated by GRASP (31).
RESULTS
GAGA and TTK interact in vivo
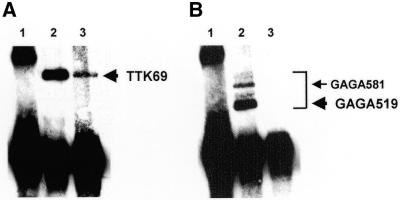
The question of whether GAGA and TTK interact in vivo was addressed by co-immunoprecipitation experiments performed with total extracts prepared from Schneider S2 cells that were co-transfected with plasmids expressing GAGA519 and TTK69 forms (Fig. 1). Immunoprecipitation with αGAGA antibodies brings down a significant amount of TTK (Fig. 1A, lane 3). Under these conditions, GAGA complexes are efficiently immunoprecipitated (Fig. 1B, lane 2). In S2 cells, the majority of GAGA appears as two doublets of roughly the same intensity (13,18). The doublet of slower electrophoretic migration corresponds to the endogenous GAGA581 form, while the species of fast mobility correspond to the GAGA519 form that was selectively overexpressed in these experiments. This co-immunoprecipitation is specific since no TTK was observed when immunoprecipitation was performed with an excess of preimmune serum (Fig. 1A, lane 1). On the other hand, no significant amount of GAGA is detected when immunoprecipitation is carried out with αTTK antibodies (Fig. 1B, lane 3) though, under these conditions, TTK is efficiently immunoprecipitated (Fig. 1A, lane 2). The lack of co-immunoprecipitation observed with αTTK antibodies might arise from their inability to recognise GAGA–TTK complexes or the distortion of such complexes in their presence. As described below, the interaction of GAGA and TTK was corroborated by yeast two-hybrid assays (Fig. 2) and, in vitro, through GST pull-down assays (Fig. 3).
Figure 1.
Co-immunoprecipitation of GAGA and TTK. Extracts from S2 cells overexpressing GAGA519 and TTK69 forms were immunoprecipitated with affinity-purified αTTK (A, lane 2 and B, lane 3) and αGAGA (A, lane 3 and B, lane 2) antibodies, or with the IgG fraction of a preimmune serum (lanes 1). Immunoprecipitates were analysed by western blotting with αTTK antibodies (A) or αGAGA antibodies (B). The positions corresponding to TTK69, GAGA519 and GAGA581 forms are indicated.
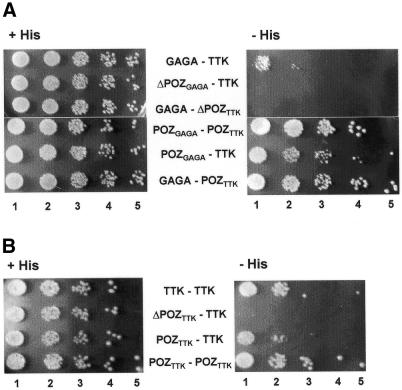
Figure 2.
Yeast two-hybrid assay of GAGA–TTK (A) and TTK–TTK (B) interaction. Twenty microlitres of cultures containing the constructs indicated in each case were plated on His+ (left) and His– (right) plates at a cell density of 2 × 106 cells/ml (lanes 1) and serial 10-fold dilutions (lanes 2–5).
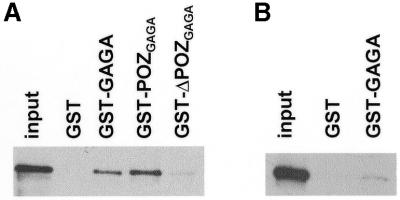
Figure 3.
GST pull-down assays of the interaction of recombinant TTK (A) and ΔPOZTTK (B) with the GST-fusion proteins indicated in each case. Lanes labelled input show 10% of the input protein.
The POZ/BTB domains of GAGA and TTK are necessary and sufficient for GAGA–TTK interaction
The POZ/BTB domain defines a conserved structural motif of approximately 110–120 residues that, in GAGA and TTK, is located at the N-terminus. The region of homology between the POZ/BTB domains of GAGA and TTK extends up to residue 118 of the GAGA sequence (115 of the TTK sequence), showing an overall identity of 40% (16). The contribution of the POZ/BTB domain to GAGA–TTK interaction was analysed with truncated proteins missing such a domain. The various constructs used in these experiments were designed according to the sequence comparison described above. ΔPOZGAGA and ΔPOZTTK are missing the first 122 and 115 residues of GAGA and TTK, respectively. Similarly, POZGAGA and POZTTK correspond to polypeptides containing the first 122 and 114 residues of GAGA and TTK.
The interaction between GAGA and TTK was analysed by yeast two-hybrid assays (Fig. 2). Significant growth on selective medium lacking histidine is observed when GAGA– BDGAL4 and TTK–ADGAL4 fusions are used as baits (Fig. 2A, row GAGA–TTK), confirming the interaction between GAGA and TTK also in yeast cells. This interaction is strictly dependent on the presence of the POZ/BTB domains of GAGA and TTK. Deletion of the POZ/BTB domain of GAGA fully abolishes its interaction with TTK (Fig. 2A, row ΔPOZGAGA–TTK) as, vice versa, deletion of the POZ/BTB domain of TTK does (Fig. 2A, row GAGA–ΔPOZTTK). On the other hand, the POZ/BTB domains of GAGA and TTK are, by themselves, capable of interacting with TTK and GAGA, respectively (Fig. 2A, rows POZGAGA–TTK and GAGA– POZTTK). Moreover, POZGAGA and POZTTK interact strongly (Fig. 2A, row POZGAGA–POZTTK). These results were cor roborated when the interactions were analysed quantitatively through β-galactosidase assay (data not shown).
Similar results were obtained in vitro through GST pull-down assays (Fig. 3). Recombinant TTK was specifically bound by GST–GAGA or GST–POZGAGA but not by GST–ΔPOZGAGA (Fig. 3A) fusion proteins. On the other hand, GST–GAGA does not bind ΔPOZTTK efficiently (Fig. 3B).
It was shown earlier that the POZ/BTB domain also mediates the formation of GAGA–GAGA homo-oligomers (6,32). As judged by yeast two-hybrid assays, TTK also forms homo-oligomers (Fig. 2B, row TTK–TTK). Homomeric TTK–TTK interaction is also dependent on the POZ/BTB domain, which is necessary (Fig. 2B, row ΔPOZTTK–TTK) and sufficient (Fig. 2B, rows POZTTK–TTK and POZTTK– POZTTK) for the interaction to occur. Altogether, these results indicate that the POZ/BTB domain, that mediates the formation of GAGA–GAGA and TTK–TTK homo-oligomers, is also responsible for GAGA–TTK interaction, both in vitro and in vivo.
TTK inhibits GAGA-dependent activation of the eve stripe 2 promoter element
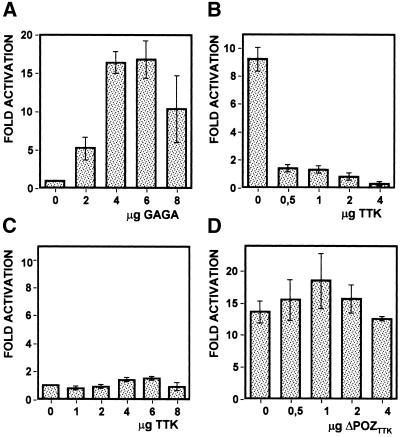
The functional consequences of GAGA–TTK interaction were evaluated in the context of the eve promoter. In the cellular blastoderm, the pair-rule eve gene is expressed in seven defined stripes, each spanning five to six cells. It was shown that a truncated eve promoter spanning 1.7 kb of the region immediately upstream from the transcription start site carries the basal promoter elements, being sufficient to drive expression within the limits of stripe 2 (19). This cis-regulatory element was used to analyse the potential contribution of GAGA–TTK interaction to the regulation of transcription since it contains several binding sites for both GAGA and TTK (20). For this purpose, a plasmid carrying the eve stripe 2 promoter fused to a luciferase reporter gene was co-transfected into S2 cells with plasmids expressing GAGA and/or TTK. As shown in Figure 4A, overexpression of GAGA results in a significant activation of the eve stripe 2 promoter. Luciferase activity increases upon increasing GAGA overexpression reaching a maximum activation of ∼15-fold when 4–6 µg of the GAGA expressing vector is co-transfected. Activation of the eve stripe 2 promoter by GAGA is efficiently repressed by overexpressing TTK (Fig. 4B). Luciferase activity is drastically reduced in the presence of 0.5 µg of the TTK-expressing plasmid, reaching background levels with just 2 µg of vector. As judged by western analysis (data not shown), the level of GAGA overexpression is not significantly reduced by overexpressing TTK, indicating that the decrease in luciferase activity observed in the presence of TTK reflects repression of the eve stripe 2 promoter rather than a reduced expression of the activator, GAGA. TTK overexpression by itself does not affect the basal activity of the eve stripe 2 promoter since no significant changes in luciferase activity are detected when TTK is overexpressed in the absence of GAGA overexpression (Fig. 4C). Note that, in Figure 4C, luciferase activities are normalised with respect to the basal activity of the eve stripe 2 promoter determined in the absence of any overexpressed protein. Actually, in S2 cells, the eve stripe 2 promoter shows significant basal transcription activity. Luciferase activity produced by the pGL3 reporter plasmid is 250-fold higher in the presence of the eve stripe 2 promoter than in its absence being, therefore, sufficiently high to eventually detect repression.
Figure 4.
TTK represses GAGA-dependent activation of the eve stripe 2 promoter element. S2 cells were co-transfected with a pGL3 reporter plasmid carrying a luciferase gene fused to the eve stripe 2 promoter element and the amounts indicated of pAct5CPPA plasmids overexpressing GAGA (A) or TTK (C), or with the same reporter plasmid and 4 µg of the plasmid overexpressing GAGA and the amounts indicated of a plasmid overexpressing TTK (B) or ΔPOZTTK (D). Luciferase activities were measured as indicated in Materials and Methods, and normalised with respect to the basal activity of the eve stripe 2 promoter determined when only empty pAct5CPPA overexpression plasmids were co-transfected and, therefore, no proteins were overexpressed. The fold of activation is presented as a function of increasing amounts of the relevant overexpression plasmid. The data correspond to the average of four independent experiments.
Repression by TTK of GAGA-dependent activation requires the contribution of the POZ/BTB domains of TTK and GAGA
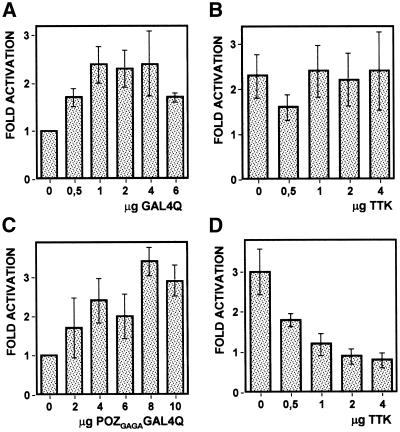
The results reported above indicate that, in the experimental system described here, repression by TTK of the eve promoter depends on its activation by GAGA, suggesting that it actually involves direct interaction between the two proteins. In good agreement with this hypothesis, TTK repression of the GAGA-activated eve stripe 2 promoter depends on the presence of the POZ/BTB domain that, as shown above, mediates GAGA–TTK interaction. As shown in Figure 4D, overexpression of ΔPOZTTK does not interfere with GAGA-dependent activation. In these experiments, ΔPOZTTK was expressed at equivalent levels as TTK, as judged by western blotting (data not shown). Reciprocal experiments were performed to analyse the contribution of the POZ/BTB domain of GAGA but it turned out that ΔPOZGAGA was not efficiently expressed in S2 cells (data not shown). The contribution of the POZ/BTB domain of GAGA to repression by TTK was, however, demonstrated in a heterologous system. The C-terminal glutamine-rich domain of GAGA (Q domain) is known to be required for transcription activation both in vitro as well as in vivo (22). Moreover, a heterologous protein, GAL4Q, carrying the DNA-binding domain of the yeast activator GAL4 fused to the Q domain of GAGA, was found to drive transcription from a 5×GAL4-hsp70 promoter that contains five GAL4 binding sites inserted upstream of the minimal hsp70 promoter of Drosophila (22). As shown in Figure 5A, maximal activation is obtained in the presence of 1–4 µg of the GAL4Q-expressing vector. A similar fusion protein, POZGAGAGAL4Q, carrying the POZ/BTB domain of GAGA, activates transcription from the 5×GAL4-hsp70 promoter to equivalent levels as GAL4Q, though, in this case, maximal activation occurs at a higher concentration of vector (Fig. 5C). In this context, TTK represses transcription from the 5×GAL4-hsp70 promoter when it is activated by POZGAGAGAL4Q (Fig. 5D). On the contrary, no repression is observed when the promoter is activated by GAL4Q (Fig. 5B). TTK represses POZGAGAGAL4Q as efficiently as GAGA; full repression is observed in the presence of 1–2 µg of the TTK-expressing vector but a significant effect is detected with just 0.5 µg of vector (Fig. 5D). Similar results were obtained when the ability of TTK to repress activation by GAL4VP16, which carries the acidic transactivation domain of VP16, was determined. Also in this case, TTK was unable to efficiently repress activation of the 5×GAL4-hsp70 promoter by GAL4VP16 in the absence of the POZ/BTB domain (data not shown).
Figure 5.
Repression by TTK of GAGA-dependent activation requires the POZ/BTB domain of GAGA. S2 cells were co-transfected with a reporter plasmid carrying a luciferase gene fused to the 5×GAL4-hsp70 promoter (see Materials and Methods) and the amounts indicated of plasmids overexpressing GAL4Q (A) or POZGAGAGAL4Q (C), or with the same reporter plasmid and either 2 µg of the plasmid overexpressing GAL4Q (B) or 8 µg of the plasmid overexpressing POZGAGAGAL4Q (D) and the amounts indicated of the plasmid overexpressing TTK. The data correspond to the average of six independent experiments.
Altogether these results indicate that repression by TTK of GAGA-dependent activation requires the contribution of the POZ/BTB domain of both TTK as well as GAGA and, therefore, strongly argue in favour of mechanisms involving direct GAGA–TTK interaction.
DISCUSSION
Here, we have reported the interaction of the Drosophila transcription factors GAGA and TTK. Based on in vitro co-translation, performed in a cell-free system, and co-immunoprecipitation experiments, it was shown earlier (4) that a truncated form of GAGA, carrying the first 245 residues, was capable of interacting in vitro with an epitope-tagged form of TTK, carrying the first 153 residues. The two truncated proteins tested in these experiments contain, in addition to the corresponding POZ/BTB domain, regions of significant length (127 residues for GAGA and 38 residues for TTK) that could also contribute to the interaction. Here, we have shown that GAGA and TTK actually interact in vivo, in yeast as well as in Drosophila S2 cells. Moreover, our results unequivocally demonstrated, both in vivo as well as in vitro, the essential contribution of the POZ/BTB domain to GAGA–TTK interaction.
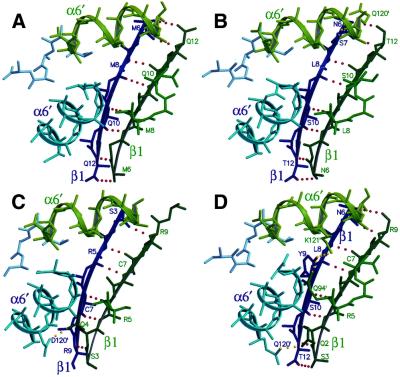
What sort of molecular interactions could account for the formation of GAGA–TTK hetero-oligomers? The POZ/BTB domain, that mediates GAGA–TTK interaction, is also responsible for the formation of GAGA–GAGA (6,11) and TTK–TTK homo-oligomers (Fig. 2B). Since POZ/BTB is a highly conserved domain, structural comparative models of those present in GAGA and TTK could be built from the crystal structure of the POZ/BTB dimer of PLZF (15,16). Actually, the modelled structures of putative GAGA–GAGA and TTK–TTK homo-dimers bear a similar hydrophobic dimerisation interface as in the PLZF dimer, with a slightly lower complementarity (Table 1). All the contacts involved in dimerisation are conserved and the central cavity is even more hydrophobic than in PLZF. These results strongly suggest that homomeric GAGA–GAGA and TTK–TTK interactions are likely to involve similar molecular interactions as those observed in the PLZF dimer. Moreover, similar models could also be built for heteromeric GAGA–TTK interaction (Table 1), indicating that a putative GAGA–TTK hetero-dimer would be stabilised by the same interactions involved in homomeric GAGA–GAGA and TTK–TTK interactions. The extensive dimerisation interface suggests, however, that POZ/BTB-containing proteins are obligated homo-dimers. Actually, there is no experimental evidence indicating that either GAGA or TTK could ever exist as monomers in solution (see below). A putative GAGA–TTK hetero-dimer could, therefore, arise from the respective homo-dimers by swapping of the POZ/BTB domains. However, the extension and high hydrophobic character of the contacts involved in these interactions raise the question of whether such a domain-swapping model could actually account for heteromeric GAGA–TTK interaction. In this respect, it must be noted that the crystal structure of the POZ/BTB domain of PLZF was solved independently by two different groups (15,16) and, in spite of belonging to different crystallographic space groups, both structures showed conserved dimer–dimer interactions, involving the extension of the β1/β5′-sheet of one dimer towards the same region of the next one, keeping an antiparallel orientation (Fig. 6A). Eight hydrogen bonds connect main-chain atoms of the β1 chains of each dimer. Each dimer buries 700 Å2 in this interface, with a high complementarity score (Sc = 0.723) (Table 1). To address the question of whether these dimer–dimer interactions are also conserved in the POZ/BTB domains of GAGA and TTK, we modelled GAGA and TTK homo-tetramers (Fig. 6B and C), as well as a GAGA–TTK hetero-tetramer (Fig. 6D). The modelled tetramers have an interacting surface and complementarity similar to those of the PLZF homo-tetramer (Table 1). The GAGA homo-tetramer and the GAGA–TTK hetero-tetramer keep all the eight hydrogen bonds between the β1/β5′-sheets, but the TTK homo-tetramer may lack those at the ends. These considerations raise the possibility that, rather than the formation of GAGA–TTK hetero-dimers, GAGA– TTK interaction would actually involve dimer–dimer interactions through the β1/β5′-sheets. GAGA–TTK interaction appears to be specific since, as it was found earlier (4), TTK does not interact in vitro with the POZ/BTB domains of either ZID or ZF5. Interestingly, the β5-strand of the β1/β5′ motif is fully identical in GAGA and TTK, a feature not shared by any of the other POZ/BTB-containing proteins analysed. In the crystal of the POZ/BTB domain of PLZF, this dimer–dimer interaction propagates through the lattice, suggesting that it could give rise to the formation of multimers of higher stoichiometry. Actually, bacterially expressed TTK and GAGA, but not ΔPOZGAGA, form in vitro oligomers of high apparent M (6). On the other hand, dimer stability itself strongly relies on the β1/β5′-sheets which, in PLZF, involve the formation of five main chain H-bonds between the β1 chain of one monomer and the β5′ chain of the second (15,16). The only other contact that contributes to dimer formation involves the symmetry related α1/α1′ helices of each monomer (15,16). Therefore, the β1 residues that could mediate dimer–dimer interactions are themselves essential for dimerisation as it was found by mutational analysis of the POZ/BTB domain of PLZF (33).
Table 1. Accessible solvent area (D-asa) and surface complementarity (Sc) of the homo-dimers and homo-tetramers of the POZ/BTB domains of PLZF, GAGA and TTK, and of the GAGA/TTK hetero-dimer and hetero-tetramer.
| Dimers D-asa (Å2)a | Sc | Tetramers D-asa (Å2)b | Sc | |
|---|---|---|---|---|
| PLZF | 1972 | 0.690 | 700 | 0.723 |
| GAGA | 1847 | 0.519 | 759 | 0.591 |
| TTK | 1847 | 0.596 | 594 | 0.463 |
| GAGA/TTK | 1899 | 0.502 | 653 | 0.604 |
aBy monomer.
bBy dimer.
Figure 6.
Dimer–dimer interaction as observed in the crystal structure of the POZ/BTB domain of PLZF (A) and in the modelled homo-tetramers of the POZ/BTB domains of GAGA (B) and TTK (C), and of a GAGA–TTK hetero-tetramer (D). Secondary structures from each dimer are shown in blue and green, respectively. Shown as dotted lines are the main-chain contacts between the β1/β5′-sheets (red) as well as some possible side-chain contacts (yellow). The positions of relevant residues are indicated.
GAGA–TTK interaction might play a role in the regulation of transcription. In the early Drosophila embryo, TTK was proposed to act as a maternally provided repressor of several pair-rule genes (23). Ectopic expression of TTK69 in blastoderm-stage embryos results in repression of several pair-rule genes, such as eve and fushi tarazu (ftz) (24,25). GAGA, which is also maternally provided, activates the expression of ftz (34) and, as indicated by our results, it could also activate eve expression. We have shown here that TTK represses GAGA-dependent activation, but not basal activity, of the eve stripe 2 promoter. Repression by TTK of GAGA-dependent activation requires the contribution of the POZ/BTB domain of TTK as well as of GAGA, indicating that it actually involves direct GAGA–TTK interaction. Several models could account for these results; GAGA–TTK hetero-oligomers might not be capable of binding DNA or, alternatively, they might either actively participate in the recruitment of co-repressor complexes or interfere with loading, and/or assembly, of the activation complex at the promoter. Additional experiments are required to discriminate between these possibilities. Our results, however, indicate that neutralising the action of transcription activators, such as GAGA, might be a major determinant of the contribution of TTK to transcription repression.
Acknowledgments
ACKNOWLEDGEMENTS
TTK69 cDNA and eve stripe 2 promoter were kindly provided by Drs C. Montell and M. Levine, respectively. We are thankful to Drs I. García-Bassets, M. Martínez-Balbás and J. C. Pizarro for technical assistance and helpful discussions. This work was supported by grants from the Ministerio de Ciencia y Tecnología (BMC2000-878 and BMC2000-898) and the CIRIT (SGR99-185). S.P. and M.O.-L. were recipients of a doctoral fellowship from the CIRIT and a long-term postdoctoral fellowship from FEBS, respectively. This work was carried out within the framework of the ‘Centre de Referència en Biotecnologia’ of the Generalitat de Catalunya.
REFERENCES
- 1.Harrison S.D. and Travers,A.A. (1990) The tramtrack gene encodes a Drosophila finger protein that interacts with the ftz transcriptional regulatory region and shows a novel embryonic expression pattern. EMBO J., 9, 207–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Soeller W.C., Oh,C.E. and Kornberg,T.B. (1993) Isolation of cDNAs encoding the Drosophila GAGA transcription factor. Mol. Cell. Biol., 13, 7961–7970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aravind L. and Koonin,E.V. (1999) Fold prediction and evolutionary analysis of the POZ domain: structural and evolutionary relationship with the potassium channel tetramerization domain. J. Mol. Biol., 285, 1353–1361. [DOI] [PubMed] [Google Scholar]
- 4.Bardwell V.J. and Treisman,R. (1994) The POZ domain: a conserved protein–protein interaction motif. Genes Dev., 8, 1664–1677. [DOI] [PubMed] [Google Scholar]
- 5.Dhordain P., Albagli,O., Ansieau,S., Koken,M.H., Deweindt,C., Quief,S., Lantoine,D., Leutz,A., Kerckaert,J.-P. and Leprince,D. (1995) The BTB/POZ domain targets the LAZ3/BCL6 oncoprotein to nuclear dots and mediates homomerisation in vivo. Oncogene, 11, 2689–2697. [PubMed] [Google Scholar]
- 6.Espinás M.L., Jiménez-García,E., Vaquero,A., Canudas,S., Bernués,J. and Azorín,F. (1999) The N-terminal POZ domain of GAGA mediates the formation of oligomers that bind DNA with high affinity and specificity. J. Biol. Chem., 274, 16461–16469. [DOI] [PubMed] [Google Scholar]
- 7.Li X., Lopez-Guisa,J.M., Ninan,N., Weiner,E.J., Rauscher,F.J.I. and Marmorstein,R. (1997) Overexpression, purification, characterization and crystallization of the BTB/POZ domain from the PLZF oncoprotein. J. Biol. Chem., 272, 27324–27329. [DOI] [PubMed] [Google Scholar]
- 8.Hong S.H., David,G., Wong,C.W., Dejean,A. and Privalski,M.L. (1997) SMRT corepressor interacts with PLZF and with PML-retinoic acid receptor alpha (RARalpha) and PLZF-RARalpha oncoproteins associated with acute promyelocytic leukaemia. Proc. Natl Acad. Sci. USA, 94, 9028–9033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.David G., Alland,L., Hong,S.H., Wong,C.W., DePinho,R.A. and Dejean,A. (1998) Histone deacetylase associated with mSin3A mediates repression by the acute promyelocytic leukemia-associated PLZF protein. Oncogene, 16, 2549–2556. [DOI] [PubMed] [Google Scholar]
- 10.Grignani F., DeMatteis,S., Nervi,C., Tomassoni,L., Gelmetti,V., Cioce,M., Fanelli,M., Ruthardt,M., Ferrara,F.F., Zamir,I., Seiser,C., Lazar,M.A., Minucci,S. and Pelicci,P.G. (1998) Fusion proteins of the retinoic acid receptor-α recruit histone deacetylase in promyelocytic leukaemia. Nature, 391, 815–818. [DOI] [PubMed] [Google Scholar]
- 11.Huynh K.D. and Bardwell,V.J. (1998) The BCL-6 POZ domain and other POZ domains interact with the co-represors N-CoR and SMRT. Oncogene, 17, 2473–2484. [DOI] [PubMed] [Google Scholar]
- 12.Lin R.J., Nagy,L., Inoue,S., Shao,W., Miller,W.H.J. and Evans,R.M. (1998) Role of histone deacetylase complex in acute promyelocytic leukaemia. Nature, 391, 811–814. [DOI] [PubMed] [Google Scholar]
- 13.Espinás M.L., Canudas,S., Fanti,L., Pimpinelli,S., Casanova,J. and Azorín,F. (2000) The GAGA factor of Drosophila interacts with SAP18, a Sin3-associated polypeptide. EMBO Rep., 1, 253–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wong C.-W. and Privalsky,M.L. (1998) Components of the SMRT corepressor complex exhibit distinctive interactions with the POZ domain oncoproteins PLZF, PLFZ-RARα. J. Biol. Chem., 273, 27695–27702. [DOI] [PubMed] [Google Scholar]
- 15.Li X., Peng,H., Schultz,D.C., Lopez-Guisa,J.M., Rauscher,F.J.,III and Marmonstein,R. (1999) Structure–function studies of the POZ/BTB transcriptional repression domain from the promyelocytic leukemia zinc finger oncoprotein. Cancer Res., 59, 5275–5282. [PubMed] [Google Scholar]
- 16.Ahmad K.F., Engel,C.K. and Privé,G.G. (1998) Crystal structure of the BTB domain from PLZF. Proc. Natl Acad. Sci. USA, 95, 12123–12128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Read D. and Manley,J.L. (1992) Alternatively spliced transcripts of the Drosophila tramtrack gene encode zinc finger proteins with distinct DNA binding specificities. EMBO J., 11, 1035–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Benyajati C., Mueller,L., Xu,N., Pappano,M., Gao,J., Mosammaparast,M., Conklin,D., Granok,H., Craig,C. and Elgin,S. (1997) Multiple isoforms of GAGA factor, a critical component of chromatin structure. Nucleic Acids Res., 25, 3345–3353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Small S., Blair,A. and Levine,M. (1992) Regulation of even-skipped stripe 2 in the Drosophila embryo. EMBO J., 11, 4047–4057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Read D., Nishigaki,T. and Manley,J.L. (1990) The Drosophila even-skipped promoter is transcribed in a stage-specific manner in vitro and contains multiple, overlapping factor-binding sites. Mol. Cell. Biol., 10, 4334–4344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilkins R.C. and Lis,J.T. (1997) Dynamics of potentiation and activation: GAGA factor and its role in heat shock gene regulation. Nucleic Acids Res., 25, 3963–3968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vaquero A., Espinás,M.L., Azorín,F. and Bernués,J. (2000) Functional mapping of the GAGA factor assigns its transcriptional activity to the C-terminal glutamine-rich domain. J. Biol. Chem., 275, 19461–19468. [DOI] [PubMed] [Google Scholar]
- 23.Brown J.L., Sonoda,S., Ueda,H., Scott,M.P. and Wu,C. (1991) Repression of the Drosophila fushi tarazu (ftz) segmentation gene. EMBO J., 10, 665–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brown J.L. and Wu,C. (1993) Repression of Drosophila pair-rule segmentation genes by ectopic expression of tramtrack. Development, 117, 45–58. [DOI] [PubMed] [Google Scholar]
- 25.Read D., Levine,M. and Manley,J.L. (1992) Ectopic expression of the Drosophila tramtrack gene results in multiple embryonic defects, including repression of even-skipped and fushi tarazu. Mech. Dev., 38, 183–196. [DOI] [PubMed] [Google Scholar]
- 26.Xiong W.-C. and Montell,C. (1993) tramtrack is a transcriptional repressor required for cell fate determination in the Drosophila eye. Genes Dev., 7, 1085–1096. [DOI] [PubMed] [Google Scholar]
- 27.Sali A. and Blundell,T.L. (1993) Comparative protein modelling by satisfaction of spatial restraints. J. Mol. Biol., 234, 779–815. [DOI] [PubMed] [Google Scholar]
- 28.Sippl M.J. (1993) Recognition of errors in three-dimensional structures of proteins. Proteins, 17, 779–815. [DOI] [PubMed] [Google Scholar]
- 29.Hooft R.W.W., Vriend,G., Sanders,C. and Abola,E.E. (1996) Errors in protein structures. Nature, 381, 272. [DOI] [PubMed] [Google Scholar]
- 30.Lawrence M.C. and Colman,P.M. (1993) Shape complementarity at protein/protein interfaces. J. Mol. Biol., 234, 946–950. [DOI] [PubMed] [Google Scholar]
- 31.Nicholls A., Sharp,K.A. and Honig,B. (1991) Protein folding and associations: insights from the interfacial and thermodynamic properties of hydrocarbons. Proteins, 11, 281–296. [DOI] [PubMed] [Google Scholar]
- 32.Katsani K.R., Hajibagheri,M.A.N. and Verrijzer,C.P. (1999) Co-operative DNA binding by GAGA transcription factor requires the conserved BTB/POZ domain and reorganizes promoter topology. EMBO J., 18, 698–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Melnick A., Ahmad,K.F., Arai,S., Polinger,A., Ball,H., Borden,K.L., Carlile,G.W., Prive,G.G. and Licht,J.D. (2000) In-depth mutational analysis of the promyelocytic leukemia zinc finger BTB/POZ domain reveals motifs and residues required for biological and transcriptional functions. Mol. Cell. Biol., 20, 6550–6567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bhat K.M., Farkas,G., Karch,F., Gyurkovics,H., Gausz,J. and Schedl,P. (1996) The GAGA factor is required in the early Drosophila embryo not only for transcriptional regulation but also for nuclear division. Development, 122, 1113–1124. [DOI] [PubMed] [Google Scholar]