Abstract
Translation initiation in Coxsackievirus B3 (CVB3) occurs via ribosome binding to an internal ribosome entry site (IRES) located in the 5′-untranslated region (UTR) of the viral RNA. This unique mechanism of translation initiation requires various trans-acting factors from the host. We show that human La autoantigen (La) binds to the CVB3 5′-UTR and also demonstrate the dose-dependent effect of exogenously added La protein in stimulating CVB3 IRES-mediated translation. The requirement of La for CVB3 IRES mediated translation has been further demonstrated by inhibition of translation as a result of sequestering La and its restoration by exogenous addition of recombinant La protein. The abundance of La protein in various mouse tissue extracts has been probed using anti-La antibody. Pancreatic tissue, a target organ for CVB3 infection, was found to have a large abundance of La protein which was demonstrated to interact with the CVB3 5′-UTR. Furthermore, exogenous addition of pancreas extract to in vitro translation reactions resulted in a dose dependent stimulation of CVB3 IRES-mediated translation. These observations indicate the role of La in CVB3 IRES-mediated translation, and suggest its possible involvement in the efficient translation of the viral RNA in the pancreas.
INTRODUCTION
Internal initiation of translation, also referred to as cap-independent translation, is fundamentally different from the general cap-dependent scanning mechanism present in eukaryotes (1). In the former mechanism the ribosome is directly recruited to a site within the 5′-untranslated region (5′-UTR) of the RNA and can initiate translation at the next downstream AUG codon (2–4). The cis-acting RNA element responsible for this internal ribosome binding is referred to as the internal ribosome entry site (IRES). IRES-mediated translation was first discovered in the naturally uncapped picornavirus RNAs (5,6), and later extended to some other viruses like the Hepatitis C Virus (HCV) (7) and a number of cellular mRNAs (8).
Coxsackievirus B3 (CVB3) is an important human pathogen and has been implicated as the leading cause of virus induced myocarditis and dilated cardiomyopathy (9). Myo carditis induced by CVB3 in humans is a biphasic disease in that an initial acute response occurs away from the heart (mainly in the pancreas), and later a more chronic disease spreads to the heart due to an autoimmune response against cardiac proteins (10,11). The 741 nt 5′-UTR of the CVB3 RNA is highly structured and contains eight AUG codons upstream of the initiator AUG. The 5′-UTR contains an IRES that directs internal initiation of translation of the coding region of the viral genome (12).
Ribosome binding to the IRES is mediated by an unknown number of proteins, the majority of these being cellular cytoplasmic proteins, as the infecting viral RNA is translated prior to the production of any viral proteins. It is generally believed that internal initiation by picornavirus IRESs requires most of the canonical eukaryotic translation initiation factors (eIFs) (13,14). However, as many picornaviruses (e.g. Poliovirus and Coxsackievirus) induce the cleavage of the eIF4G component of the tri-molecular eIF4F cap-binding complex (15), it is clear that the requirement of IRES-mediated translation for initiation factors is distinct from that of general cap-dependent protein synthesis. In addition to the canonical initiation factors, picornavirus IRES elements need additional trans-acting factors for efficient translation initiation. The proteins that have been found to interact with IRES elements and stimulate translation are the human La autoantigen (La), polypyrimidine-tract-binding protein (PTB) (16,17), poly (rC)-binding protein 2 (PCBP2) (18), unr and unrip (19).
The La protein was originally identified as an autoantigen that was recognized by sera from patients with systemic lupus erythematosus and Sjögren syndrome (20). It is predominantly localized within the nucleus and functions in the maturation of RNA polymerase III transcripts (21,22) and the unwinding of double-stranded RNA (23). It is present as 52 or 45 kDa isoforms in different cell types (24). La antigen binds to a variety of RNA structures via its RNA binding motif (25). The viral targets of the La protein binding include the 5′-UTR of some picornaviruses (26), HCV (27), influenza virus (28), sindbis virus (29) and the HIV TAR element (30). In the case of poliovirus, the La protein plays a functional role in internal initiation of translation where addition of purified La to rabbit reticulocyte lysate (RRL) inhibits the accumulation of aberrant translation products in in vitro translation assays and also causes a modest stimulation of translation (31,32). La protein has also been demonstrated to stimulate translation directed by the IRES elements of Encephalomyocarditis virus (EMCV) (33) and HCV (34). Sequestration of La protein by a small yeast RNA (I-RNA) has been shown to inhibit poliovirus and HCV IRES mediated translation, which could be rescued by exogenous addition of purified La protein (35–38). A similar inhibition of HCV IRES-directed translation in HeLa lysates has been demonstrated by sequestering La using a SELEX RNA specific for La (34).
The present study was designed to investigate the involvement of La protein in CVB3 IRES-mediated translation. We show that human La protein binds to the CVB3 5′-UTR and demonstrate the dose-dependent effect of exogenously added recombinant La in stimulating CVB3 IRES-mediated translation in vitro. A 60 nt yeast RNA (I-RNA) which has been shown to bind to La protein (37) was used to sequester La in in vitro translation assays to inhibit CVB3 IRES-mediated translation which could be rescued by exogenous addition of La protein. In order to investigate the activity of La from a cellular source, abundance of La in various target tissues was probed using La antibodies. The highest abundance of La protein was found in the pancreas, a primary target organ of CVB3. Therefore, exogenous addition of pancreatic extract to in vitro translation reactions was carried out to explore its ability to stimulate CVB3 IRES mediated translation. These results constitute the first report of the role of La in Coxsackievirus B3 IRES-mediated translation and suggest the possibility of its involvement in the efficient translation of the viral RNA in the pancreas.
MATERIALS AND METHODS
Plasmid constructs
The 5′-UTR of CVB3 was amplified by PCR from the CVB3 full-length infectious cDNA (a generous gift from Prof. Nora Chapman, UNMC) using 5′ and 3′ primers containing HindIII and EcoRI sites, respectively, and cloned into the corresponding sites of vector pCDNA3 (Invitrogen). The monocistronic construct pCD-CVB 5′-UTR–green fluorescent protein (GFP) was constructed by cloning the GFP gene downstream of the CVB3 5′-UTR cloned in pCDNA3. The construct pCD–GFP contained the GFP gene cloned in pCDNA3. The plasmid pCDIR contains the 60 nt I-RNA coding synthetic oligonucleotide sequence cloned in pCDNA3 under T7/CMV promoters (a generous gift from Prof. Asim Dasgupta, UCLA; 36).
Preparation of HeLa S10 cell extract and mouse tissue extracts
HeLa cells used for preparing cell extract were grown in minimal essential medium (GIBCO BRL) pH 7.0, supplemented with 10% fetal calf serum. A monolayer of HeLa cells was harvested, pelleted down and washed three times with cold isotonic buffer (35 mM HEPES pH 7.4, 146 mM NaCl, 11 mM glucose), resuspended in 1.5× packed cell volume of hypotonic buffer (10 mM HEPES pH 7.4, 15 mM KCl, 1.5 mM Mg-acetate and 6 mM β-ME) and then incubated on ice for 10 min for swelling. Cells were then transferred to a Down’s Homogeniser and disrupted by 50 strokes on ice. The lysate was incubated in 1× incubation buffer (10×: 200 mM HEPES, 1.2 M KCl, 50 mM Mg-acetate and 60 mM β-ME) for 10 min. Cytoplasmic extract (S10 supernatant) was isolated by centrifuging the lysate at 10 000 g for 30 min at 4°C. The supernatant was dialyzed for 2–4 h against 100 vol dialysis buffer (10 mM HEPES, 90 mM KCl, 1.5 mM Mg-acetate, 7 mM β-ME, 20% glycerol).Various organs were carefully dissected from 2-month-old Balb/c mice, minced and resuspended in isotonic buffer. The samples were then processed as mentioned above. The protein contents of the tissue S10 extracts were estimated by Bradford’s method using a BSA standard curve.
Purification of recombinant La protein
The cDNA encoding the human La antigen was PCR amplified from the plasmid pET-La (a generous gift from Prof. Jack Keene, Duke University) and cloned into pRSET A vector between BamHI and EcoRI sites and was used for the expression of the recombinant La protein. The expression of La was induced by 0.6 mM IPTG in Escherichia coli (BL21-DE3) cells and the His-tagged protein was purified using Ni2+-nitrilotriacetic acid-agarose (Qiagen) under non-denaturing conditions and eluted with 250 mM imidazole. The purified His-tagged La protein has an approximate molecular mass of 56.6 kDa.
In vitro transcription
mRNAs were transcribed in vitro from different linearized plasmid constructs in run-off transcription reactions. The clone pCD-CVB 5′-UTR–GFP was linearized downstream of GFP, eluted from agarose gels and then transcribed using T7 RNA polymerase to generate the monocistronic CVB 5′-UTR-GFP mRNA. The construct pCD–GFP was linearized and transcribed in the presence of m7G(5′)ppp(5′)G RNA capping analog (Gibco BRL) to generate capped GFP mRNA. The construct pCDIR was linearized using EcoRI and transcribed using T7 RNA polymerase to generate the 60 nt I-RNA. All in vitro transcription reactions were carried out under standard conditions using reagents from either Promega Corporation or New England Biolabs, Inc. The pCD-CVB 5′-UTR clone was linearized, gel eluted and transcribed in vitro using T7 RNA polymerase and [α-32P]UTP (NEN Co.) to generate the 32P-labeled CVB full-length 5′-UTR RNA. pGEM-3Z vector DNA (Promega) was linearized with HindIII and transcribed using T7 RNA polymerase to generate a 72 nt non-specific RNA corresponding to the polylinker sequence.
In vitro translation
In vitro translation of the CVB 5′-UTR–GFP mRNA was carried out using 8.75 µl (200 µg) of micrococcal nuclease-treated RRL medium (Promega), 0.5 µl of amino acid mixture minus methionine, 20 µCi of 35S-methionine (NEN) and supplemented with HeLa S10 where indicated. Purified recombinant La protein and pancreas S10 extract was added to the reaction mixtures at various concentrations as indicated in the results. In vitro transcribed I-RNA was added in calculated molar quantities to reaction mixtures as indicated in the results of the inhibition and rescue experiments. The reaction mixtures were incubated at 30°C for 90 min, and the products were analyzed on SDS–12.5% polyacrylamide gels followed by autoradiography or phosphorimaging (Fuji Imaging).
UV crosslinking of proteins with RNA
Forty femtomoles (∼10 ng) of in vitro transcribed 32P-labeled CVB full-length 5′-UTR RNA (741 nt) was incubated with protein samples (HeLa cell extract, purified La and Balb/c mouse tissue extracts as indicated in Results) in 2× RNA binding buffer [5 mM HEPES, 25 mM KCl, 2 mM MgCl2, 2 mM dithiothreitol (DTT), 0.1 mM EDTA, 3.8% glycerol, 1.5 mM ATP and 2 mM GTP] having 2 µg tRNA, for 30 min and UV crosslinked by irradiation from a hand-held UV lamp. For all the competition assays, competitor RNAs were added along with the components of the reaction mixture before UV cross linking. Unbound RNAs were digested by treatment with 30 µg of RNase A at 37°C for 30 min. The protein– nucleotidyl complexes were run on SDS–12.5% polyacrylamide gels followed by autoradiography or phosphorimaging.
Immunoblotting
Various tissue extracts from Balb/c mice, containing equivalent amounts of proteins (estimated by Bradford’s method), were suspended in equal volumes of 2× SDS gel-loading buffer (100 mM Tris–Cl, 200 mM DTT, 4% SDS, 0.2% bromophenol blue, 20% glycerol) and run on SDS–10% polyacrylamide gel. Electro-transfer of proteins to nitrocellulose membranes was carried out at 40 mA (constant) for 1.5 h followed by immunoblotting using anti-La polyclonal antibodies raised in rabbit (1:3000 titre). The membranes were probed with horseradish peroxidase conjugated anti-rabbit secondary antibodies (Bangalore Genei). Peroxidase activity detection was carried out using Diaminobenzidine (Sigma-Aldrich) and 3% H2O2 in PBS. A replica blot was probed with mouse anti-actin antibodies (Santa Cruz Biotech) and anti-mouse secondary antibodies followed by enhanced chemiluminescence (ECL) detection (Amersham-Pharmacia).
Immunoprecipitation
Pancreas and liver S10 extracts (50 µg of protein; 10 µg in each of five reactions) of Balb/c mice or HeLa S10 fraction (72 µg of protein; 18 µg in each of four reactions) were mixed with 32P-labeled CVB 5′-UTR RNA in RNA binding buffer. After UV crosslinking and RNase digestion, the reactions were pooled together and the volume made up to 200 µl using 1× RIPA buffer (5 mM Tris–Cl pH 7.4, 150 mM NaCl, 1% Triton-X100, 0.1% SDS, 1% sodium deoxycholate). Anti-La polyclonal antibody or rabbit pre-immune serum was added and incubated on ice for 4 h. The immunocomplexes were precipitated by Protein A–CL Sepharose beads (Sigma-Aldrich) for 2 h at 4°C. The beads were washed three times with RIPA buffer. The bound proteins were analyzed by SDS–10% polyacrylamide gel electrophoresis (PAGE) followed by phosphorimaging.
RESULTS
CVB3 5′-UTR binds to the La protein
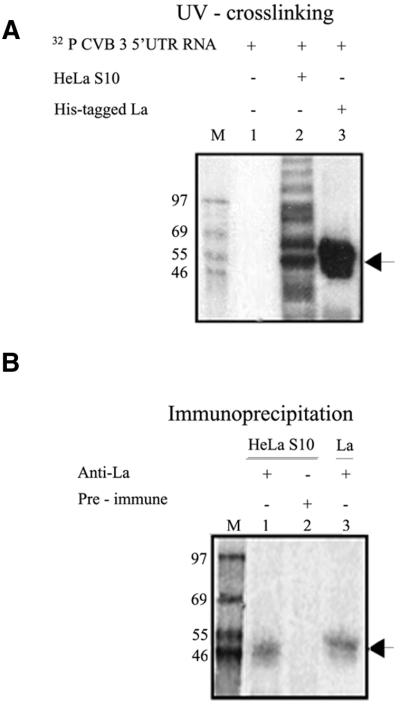
The human La autoantigen has been reported to interact with the IRES elements of poliovirus, EMCV and HCV. In order to determine the binding of cellular proteins to the CVB3 5′-UTR, UV-crosslinking assay using 32P-labeled CVB full-length 5′-UTR RNA was performed with 9 µg HeLa S10 cellular extract. The CVB3 5′-UTR showed strong interaction with a number of cellular proteins from HeLa cytoplasmic extract (Fig. 1A, lane 2) including a protein corresponding to ∼52 kDa. This band may be that of the La antigen, which is known to be abundant in HeLa cells. UV crosslinking between full-length CVB3 5′-UTR RNA and purified, recombinant His-tagged La protein demonstrated a strong interaction (Fig. 1A, lane 3). To further confirm the specific interaction of La protein from HeLa cells with the CVB3 5′-UTR, immunoprecipitation of the nucleo–protein complexes formed after UV crosslinking was performed using anti-La polyclonal antibodies. The immunoprecipitation showed the presence of a CVB3 5′-UTR–La complex of ∼50–52 kDa from HeLa extract (Fig. 1B, lane 1), corresponding in size to the protein band observed in the UV crosslinking (Fig. 1A, lane 2). The imunoprecipitated complex between CVB3 5′-UTR RNA and the His-tagged La protein (Fig. 1B, lane 3) showed slightly higher migration than the endogenous La as also seen in the UV-crosslinking experiment (Fig. 1A, lane 3). However, no bands were observed upon immunoprecipitation using rabbit pre-immune serum (Fig. 1B, lane 2). Therefore, CVB3 5′-UTR is found to interact specifically with both the HeLa cellular and recombinant La proteins.
Figure 1.
Specific interaction of La protein with the CVB3 5′-UTR. (A) UV crosslinking of 32P-labeled CVB3 5′-UTR with 9 µg of HeLa S10 extract (lane 2) and 400 ng of purified His-La protein (lane 3). Lane 1 contains the probe in absence of the added proteins. (B) CVB3 5′-UTR–La UV-crosslinked complexes with HeLa S10 (lanes 1 and 2) and purified His-La protein (lane 3) were immunoprecipitated using anti-La antibodies (lanes 1 and 3) and pre-immune rabbit serum (lane 2). The samples were resolved by SDS–10% PAGE and the gels were exposed for phosphorimaging. Lane M represents 14C-labeled protein markers. (A and B) The position of the La–CVB3 RNA complex is indicated by arrows.
Dose-dependent effect of purified La on CVB3 IRES-mediated translation in vitro
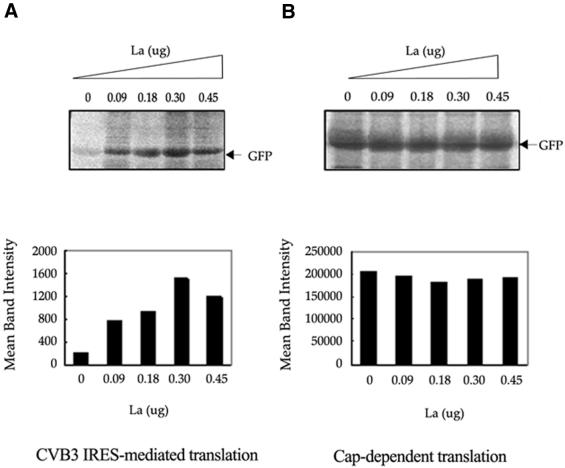
La protein has been demonstrated to stimulate translation directed by the IRES elements of poliovirus, EMCV and HCV. In order to examine the functional involvement of La in CVB3 IRES-mediated translation, an in vitro synthesized uncapped CVB3 5′-UTR–GFP RNA, in which the CVB3 IRES drives the translation of the reporter gene GFP, was used as the template. Increasing doses of purified, recombinant La (90, 180, 300 and 450 ng) were exogenously added to the in vitro translation reactions of CVB3 5′-UTR–GFP. Similar quantities of the recombinant protein were also added to translation reactions having capped GFP RNA as the template. The results showed that exogenous addition of La to RRL was able to significantly stimulate the efficiency of CVB3 IRES-mediated translation (Fig. 2A). The effect of La was quite pronounced (∼7-fold increase in reporter gene translation) as seen from quantification of the intensities of the translated protein bands. However, the level of translation was slightly reduced from the maximum level upon addition of the highest dose (450 ng) of La protein. No significant increase was observed upon addition of similar concentrations of La on the cap-dependent translation of GFP (Fig. 2B). These observations indicate the functional involvement of the La protein in CVB3 IRES-mediated translation.
Figure 2.
Stimulation of CVB3 IRES mediated translation of GFP in RRL by His-tagged La protein. (A) Representative translation of in vitro transcribed uncapped CVB3 5′-UTR–GFP RNA in RRL supplemented with increasing amounts of purified La protein (as indicated above the panel). (B) Translation of in vitro transcribed, capped GFP RNA in presence of the same increasing concentrations of La protein. The translation reactions were carried out using ∼1 µg of RNA template. The gels were treated with 1 M sodium salicylate, dried and exposed for phosphorimaging. Intensities of GFP bands in the translation reactions were quantified by Fuji Imagegauge and are graphically represented below each panel.
La protein binding to the CVB3 5′-UTR can be competed out by a small RNA (I-RNA)
A 60 nt RNA originally isolated from the yeast Saccharo myces cerevisae (I-RNA) has been shown to bind to La protein (37) and also to compete efficiently with the poliovirus 5′-UTR for binding to HeLa cellular La antigen. In order to determine whether the I-RNA can compete with the CVB3 IRES for binding to purified La protein, competition UV-crosslinking assays were performed using radiolabeled CVB3 5′-UTR RNA and increasing concentrations of unlabeled I-RNA as the competitor RNA.
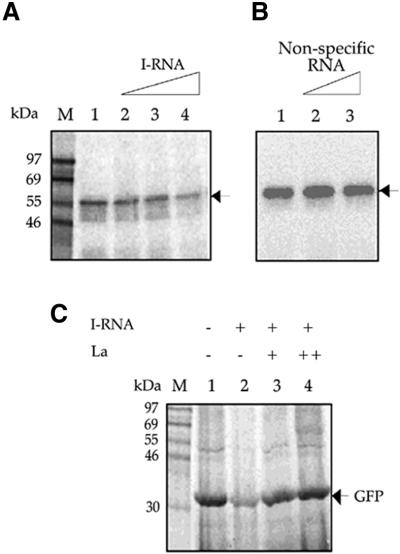
The result showed that the amount of La protein bound to the CVB3 5′-UTR reduced in a gradual manner on addition of increasing molar concentrations of in vitro transcribed I-RNA (200-, 400- and 600-fold excess) as indicated by the reduction in the intensity of the La protein–nucleotidyl complex band (Fig. 3A, lanes 1–4). This points to the fact that I-RNA is able to successfully compete out the CVB3 5′-UTR from its binding with La protein. However, 400- and 600-fold molar excess of a non-specific RNA (72 nt long), when added to the binding reaction, was unable to compete with the CVB3 5′-UTR for La binding (Fig. 3B, lanes 1–3).
Figure 3.
Competition of I-RNA with CVB3 5′-UTR for binding to La and rescue of CVB3 IRES-mediated translation by recombinant La protein. (A) [32P]UTP labeled CVB3 5′-UTR RNA probe was UV crosslinked to His-tagged La protein in the absence (lane 1) and presence (lanes 2–4) of 200-, 400- and 600-fold molar excess of unlabeled I-RNA. (B) [32P]UTP labeled CVB3 5′-UTR RNA probe was UV crosslinked to His-tagged La protein in the absence (lane 1) and presence (lanes 2 and 3) of 400- and 600-fold molar excess of a non-specific RNA. (C) CVB3 5′-UTR–GFP RNA was translated in vitro in RRL supplemented with 0.5 µg HeLa S10 (lanes 1–4), 50-fold excess of I-RNA (lanes 2–4) and two concentrations (200 and 400 ng) of La protein (lanes 3 and 4). The positions of the La–CVB3 RNA complex (A and B) and the translation product GFP (C) are indicated by arrows.
Inhibition of CVB3 IRES-mediated translation by I-RNA and rescue by La protein
I-RNA, which can bind to the La protein, has been shown to be able to selectively block translation initiation directed by poliovirus (35,36) and HCV IRESs (38). Moreover, I-RNA-mediated inhibition of translation directed by poliovirus and HCV IRESs in HeLa S10 extract was rescued by exogenous addition of purified La protein (35,37). In order to investigate the necessity of La protein for CVB3 IRES-mediated translation, 2 µg (50-fold excess) of I-RNA was added to the in vitro translation reaction of CVB3 5′-UTR–GFP in RRL supplemented with 500 ng of HeLa S10 extract. The supplementation of the reaction medium with HeLa cytoplasmic extract was necessitated by the very low basal levels of translation directed by the CVB3 5′-UTR in RRL which is enhanced by addition of HeLa extract. It was observed that the translation of GFP directed by the CVB3 IRES was drastically reduced by the addition of I-RNA (Fig. 3C, lane 2). In order to examine the ability of La protein to rescue the I-RNA-mediated inhibition of translation, increasing concentrations (200 and 400 ng) of recombinant La protein were added to the in vitro translation reactions in presence of the same quantities of I-RNA. It was observed that the CVB3 IRES-mediated translation was restored by La protein in a dose-dependent manner (Fig. 3C, lanes 3 and 4) to the original level. The data suggest that inhibition of CVB3 IRES-mediated translation by I-RNA was primarily due to the sequestration of La protein and La is necessary for CVB3 IRES-mediated translation. Moreover, addition of La protein is sufficient to rescue the I-RNA-mediated inhibition of the CVB3 IRES-directed translation in RRL.
La protein is present at variable levels in different tissues
La is a cellular autoantigen that has been shown to be present in different tissues predominantly as 52 or 45 kDa isoforms. La antigen-encoding mRNA transcripts also exhibit a tissue-specific expression pattern, with a 1.9 kb transcript being ubiquitously expressed in human tissues and a 2.3 kb transcript which is expressed at high levels in spleen, PBL, brain, kidney and pancreas (39). Although the extent of difference between the protein products of these transcripts is not known, it has been suggested that the 1.9 kb transcript is predominantly translated by a cap-dependent scanning mechanism while the 2.3 kb transcript undergoes internal initiation (39). In order to detect the presence and relative abundance of La protein in various mouse tissues, western blotting of equal quantities of total cytoplasmic protein from these tissues was performed using polyclonal anti-La antibodies. Mouse tissue cytoplasmic extracts were used for the experiment because the course of CVB3 infection in the murine disease model is same as that in humans (10). The immunoblot showed that the relative abundance of the La protein, corresponding to a band of ∼45 kDa, was highest in pancreas, followed by spleen, kidney and brain tissue extracts (Fig. 4). A corresponding faint band was detectable in the heart extract but was undetected in liver. A replica blot was also probed with anti-β actin antibody as internal control. These observations suggest that the abundance of the La protein is different in the different tissues tested and is relatively high in the pancreas.
Figure 4.
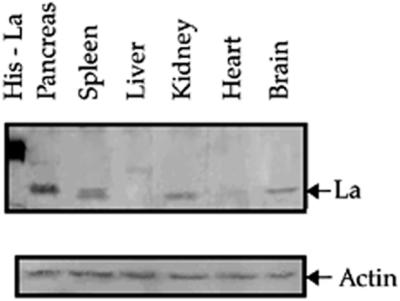
Immunoblot of mouse tissue extracts using anti-La polyclonal antibodies. Various mouse tissue extracts (as indicated) containing equivalent amounts of total protein were resolved on SDS–10% PAGE and probed with polyclonal anti-La antibody. After probing with HRP-conjugated secondary antibody, immunoreactive bands were visualized by peroxidase reaction using diaminobenzidine as substrate. A replica blot containing the same amounts of protein per lane was probed with anti-β actin monoclonal antibody and anti-mouse secondary antibody followed by ECL.
CVB3 5′-UTR specifically interacts with La protein from the mouse pancreas
CVB3, like other enteroviruses, first enters through the small intestine and then infects adjacent organs, mainly the pancreas, and later spreads to other sites like the heart. The pancreas serves as the viral reservoir. The efficient propagation of CVB3 in the pancreas may be due to the presence of tissue-specific factors, like the La protein, which enhances virus translation/replication in vivo. As the La protein was found to be abundant in the pancreatic tissue, we investigated the ability of the cellular La protein to interact with the CVB3 5′-UTR RNA by UV crosslinking assay followed by immunoprecipitation.
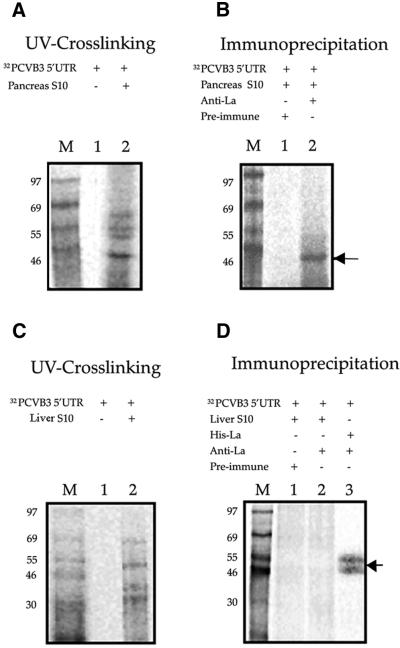
The UV crosslinking showed a number of proteins from the pancreas cytoplasmic extract binding to the CVB3 5′-UTR. A prominent RNA–protein complex, corresponding to ∼45 kDa was observed (Fig. 5A, lane 2). The other protein–nucleotidyl complexes may correspond to various trans-acting factors including PTB (p57/p60) and PCBP2 (39 kDa), which may also interact with the CVB3 5′-UTR.
Figure 5.
Interaction of CVB3 5′-UTR RNA with La protein from mouse pancreas. (A) 32P-labeled CVB3 5′-UTR RNA was UV crosslinked to pancreas cytoplasmic extract of Balb/c mice (lane 2). Lane 1 contains the free probe in the absence of any added proteins. (B) 32P-labeled CVB3 5′-UTR RNA–protein complexes from the pancreas extract were immunoprecipitated using anti-La antibodies (lane 2) and pre-immune serum (lane 1). (C and D) UV crosslinking and immunoprecipitation of 32P-labeled CVB3 5′-UTR RNA–protein complexes from liver cytoplasmic extract of Balb/c mice. Lanes 1 and 2 correspond to those in (A) and (B). Lane 3 in (D) contains purified His-tagged La protein immunoprecipitated with anti-La antibodies. The samples were resolved by SDS–10% PAGE, the gels were dried and exposed for phosphorimaging. Lane M represents 14C-labeled protein markers. The arrow indicates CVB3 5′-UTR RNA–La complex.
To confirm the interaction of the cellular La protein with the CVB3 5′-UTR, the UV-crosslinked CVB3 5′-UTR RNA– protein complex from pancreas was immunoprecipitated using anti-La antibodies. A complex of CVB3 5′-UTR RNA and La was observed (Fig. 5B, lane 2) upon UV crosslinking followed by immunoprecipitation with anti-La antibodies but not with pre-immune rabbit serum (Fig. 5B, lane 1). However, immunoprecipitation of CVB3 5′-UTR RNA–protein complexes from liver extract of mouse, using anti-La antibodies, did not show any specific band (Fig. 5D, lane 2). These results were consistent with the observation in the western blot and suggest a specific interaction between the CVB3 IRES and La protein from mouse pancreas. Similar La protein–nucleotidyl complexes were observed upon immunoprecipitation of spleen and brain tissue extracts after UV crosslinking with CVB3 5′-UTR RNA (data not shown).
Effect of exogenous addition of mouse pancreas cytoplasmic extract on CVB3 IRES-mediated translation
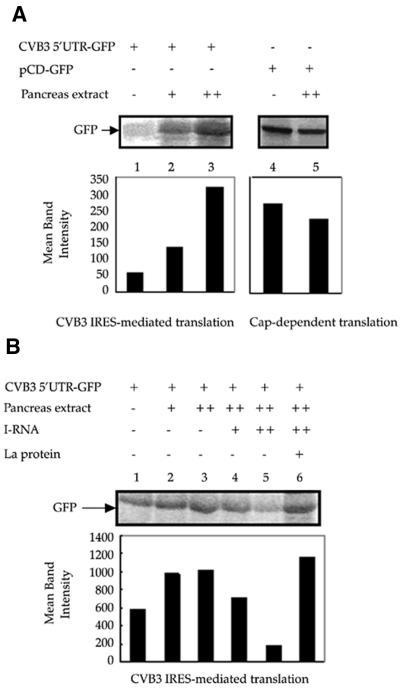
The pancreas serves as the primary site of infection by CVB3 and is also a major target organ for CVB3-mediated pathogenesis. As we found that the La protein was required for translation directed by the CVB3 IRES, and the abundance of La was greatest in the pancreatic tissue, we wanted to examine the ability of pancreatic tissue extract to stimulate the translation directed by the CVB3 IRES. Therefore, in vitro translation reactions in RRL using CVB3 5′-UTR–GFP RNA as the template were supplemented by S10 extracts of the pancreas of Balb/c mice at two increasing concentrations. The results showed that addition of pancreas extract stimulated the translation of the reporter gene GFP in a concentration-dependent manner above the basal level (Fig. 6A). The higher concentration of the tissue extract was also added to translation reactions having capped-GFP RNA as the template. However, addition of the pancreatic extract did not exhibit an enhancement of cap-dependent translation (Fig. 6A, right), rather a slight reduction in the translation efficiency was observed. These observations suggest that the efficiency of the CVB3 IRES-mediated translation in pancreatic tissue is high and this may be correlated to the high concentration of La protein in the tissue.
Figure 6.
Effect of exogenously added mouse pancreas extract on CVB3 IRES-mediated translation. (A) Two increasing doses of pancreas extract from Balb/c mice were exogenously added to in vitro translation reactions of CVB3 5′-UTR–GFP RNA in RRL (lanes 2 and 3). Approximately 1 µg of RNA template was used in each reaction. The higher dose of the pancreas extract was added to in vitro translation reaction using capped GFP RNA as template (lane 5). Control lanes (lanes 1 and 4) indicate translation of RNA templates in the absence of any supplementation. (B) Two increasing doses of pancreas extract (lanes 2 and 3) were added to in vitro translation reactions of CVB3 5′-UTR–GFP in RRL. Approximately 2 µg of RNA template was used in each reaction. In the presence of the higher dose of pancreas extract, 20- and 40-fold excess of I-RNA (lanes 4 and 5) was added to the reaction medium. In the presence of the higher dose of the pancreas extract and the 40-fold excess of I-RNA, 600 ng of purified La protein was added to the reaction mixture (lane 6). The GFP bands are indicated by arrows. The GFP band intensities were quantified and are represented graphically below each panel.
In order to determine whether the stimulation in translation observed upon addition of pancreas extract could be abrogated by sequestering the La protein, 20- and 40-fold excess of I-RNA was added to the in vitro translation reactions supplemented with pancreas cytoplasmic extract (Fig. 6B, lanes 2–6). A significant dose-dependent reduction in the translation efficiency was observed (Fig. 6B, lanes 4 and 5), suggesting that I-RNA can inhibit the translation mediated by the CVB3 IRES in presence of pancreas extract. Furthermore, to investigate whether the I-RNA mediated inhibition was due to the sequestration of the La protein from pancreas extract, 600 ng of recombinant La protein was added to the translation reaction supplemented with pancreas extract in presence of 40-fold excess of I-RNA. It was observed that the addition of La protein could restore the translation efficiency to original levels (Fig. 6B, lane 6). Taken together, these observations suggest that La protein is required for CVB3 IRES-mediated translation and may be an important trans-acting factor present in the pancreas, which enhances the efficiency of Coxsackievirus IRES-mediated translation in the organ.
DISCUSSION
A growing body of evidence suggests that internal initiation of translation by picornaviruses and cellular mRNAs is a phenomenon mediated by specific interactions between cis-elements on the RNA (the IRES) and various trans-acting factors. These factors required for internal initiation are thought to be proteins from the host cell cytoplasm. However, the functional role of these proteins in IRES-mediated translation is not known although a function as RNA chaperones has been proposed. Studies on these IRES-interacting cellular proteins hold the promise of explaining the differential translation efficiency of the viral RNA in different tissues. It has been seen that in transient expression assays, the efficiency of different IRES elements varies greatly in different cell types (40,41). Hence, the translational efficiency of these IRES elements must be influenced by cellular trans-acting factors. Indeed, the neurovirulence of wild-type poliovirus is dependent on recognition of poliovirus IRES within neuronal cells, whereas the IRES of vaccine strains function poorly in these cells (13). It has also been reported that tissue-specific expression of PTB and nPTB (neuronal isoform) is an important determinant of cell-specific translational control and neurovirulence of Theiler’s murine encephalitis virus (42).
The CVB3 receptor (coxsackie and adenovirus receptor) is known to be present on most tissues in the body (43) but the virus infects specific organs like the heart, pancreas, brain and spleen. Therefore, phenomena like host tissue specificity may be explained as a complex interplay of cellular surface receptors and intracellular trans-acting factors influencing translation/replication of the virus (44). The only protein that has been reported to interact with the CVB3 5′-UTR and influence the IRES-mediated translation is PCBP2 (45).
Our observations show that the human La antigen specifically interacts with the CVB3 5′-UTR containing the IRES element and stimulates CVB3 IRES-mediated translation, as is also seen in the case of poliovirus and HCV. The basal level of translation directed by the CVB3 IRES in RRL, which has limiting quantities of La, is low. Therefore, the stimulatory effect of La is quite pronounced. The slight reduction in the translation level on adding higher quantities of La may be due to the non-specific RNA–protein interactions at high concentrations of added protein, which may prevent the binding of other canonical and non-canonical translation factors.
Our experiments with I-RNA, which has been reported to bind to the La protein, demonstrated that I-RNA can compete with the CVB3 5′-UTR for binding to La and can also inhibit CVB3 IRES-directed translation. This suggests that I-RNA sequesters the La protein from the HeLa S10 supplemented RRL and, thus, prevents its interaction with the CVB3 IRES. This inhibition could be abolished by exogenous addition of La protein, which restored the translation efficiency to the original levels. This indicates that La protein is necessary for efficient translation initiation directed by the CVB3 IRES. Although the I-RNA is known to bind to other cellular proteins and may also sequester them, addition of La protein alone was sufficient to rescue the I-RNA-mediated inhibition of translation directed by the CVB3 IRES. It is possible that the other proteins, which bind to the I-RNA (46), may not be as critical for the CVB3 IRES-mediated translation. Alternatively, the binding of La to the CVB3 IRES facilitates its interaction with other trans-acting factors. This might also be the reason why a lower concentration of I-RNA (50-fold excess) is able to inhibit CVB3 IRES-mediated translation by sequestering La and preventing these interactions, although a larger amount (600-fold excess) is needed to compete out the binding of La with the CVB3 5′-UTR. This observation is consistent with earlier reports where I-RNA-mediated inhibition of poliovirus and HCV IRES-driven translation was reversed by the exogenous addition of purified La protein alone. Moreover, the amount of La protein required to rescue the CVB3 IRES mediated translation is comparable with the levels required for rescuing the translation directed by the poliovirus IRES (35).
In an attempt to identify the major cellular sources of La protein, immunblotting of various tissue cytoplasmic extracts from Balb/c mouse was performed using anti-La antibodies which demonstrated the presence of the protein at variable levels in various tissues. Interestingly, the maximum concentration of the La protein was found in the pancreas extract. La mRNA has been shown to be present at different levels in various organs and some of the organs exhibiting high levels of the La mRNA, like the pancreas, spleen and the brain, were also found to have high levels of the protein. These organs, most importantly the pancreas, also serve as target organs of CVB3. Moreover, it has been suggested that a specific spliced form of the La mRNA (La 1′) is translated more efficiently in a cap-independent manner (39) and will, therefore, continue to be translated upon CVB3 infection, when cap-dependent translation has been shut down due to the cleavage of cellular eIF4G. Surprisingly, the level of La protein was found to be very low in the heart tissue cytoplasmic extract in the immunoblot. However, it should be mentioned that La is predominantly a nuclear protein and, in the case of poliovirus infection, major amounts of the protein are localized to the cytoplasm only on cleavage of the nuclear localization signal by viral 3C-protease (47). The recruitment of the nuclear-localized La protein in heart tissue after infection by CVB3 may be of major importance to the propagation of the virus in the heart. On the other hand, the cytoplasmic levels of La would normally be crucial for viral translation because the viral genome has to be initially translated post-infection, in order to synthesize the virus-encoded proteins, and this translation would be dependent on the cytoplasmic pool of the La protein. It has also been reported that cells stably expressing the La 1′ mRNA showed a predominantly cytoplasmic localization of the La protein, whereas those expressing the alternately spliced La 1 mRNA showed a nuclear localization of the protein (48).
The La protein, demonstrated to be present in the pancreatic extract, was also found to interact specifically with the CVB3 5′-UTR RNA. The pancreas is now thought to be the primary site of CVB3 infection and the viral reservoir. Therefore, the efficiency of translation of the viral RNA in the pancreas, post infection, would play a crucial role in the ability of the virus to propagate in the tissue. It has been demonstrated that transgenic mice expressing IFN-γ under the influence of the insulin promoter in the pancreatic β cells does not allow CVB3 replication in the pancreas. These mice failed to develop myocarditis after being infected by the virus although there was no expression of IFN-γ in the heart tissue (10). Therefore, the translation/replication of CVB3 RNA within the pancreas will be of great importance to the survival and propagation of the virus. The translation efficiency in pancreatic tissue may be influenced by the presence of high amounts of La protein, which was found to be crucial for the enhancement of CVB3 IRES-mediated translation in vitro. Accordingly, it was observed that cytoplasmic extract from the pancreas was able to enhance the CVB3 IRES-mediated translation in a dose-dependent manner. Previously, it has been reported that the exogenous addition of liver cytoplasmic extract was able to enhance the translation mediated by the Hepatitis A virus IRES in RRL, although the stimulation was not attributed to any specific trans-acting factor (49). However, the stimulation in CVB3 IRES-mediated translation, which is observed on addition of pancreas extract, is abrogated on addition of excess of I-RNA, which is known to bind to La protein and which can inhibit the translation driven by the CVB3 IRES in RRL medium supplemented with HeLa cytoplasmic extract. Moreover, the translation efficiency can be restored on addition of excess purified La protein to the reaction medium. This seems to reinforce the idea that La protein plays an important role in the stimulation of CVB3 IRES-mediated translation by pancreatic tissue extract and possibly in the virus proliferation in the tissue.
This study constitutes the first report of the involvement of La in CVB3 IRES-mediated translation. It demonstrates the necessity of La protein for the process of internal initiation by the CVB3 IRES and also forwards the possibility that the presence of an abundance of La protein is correlated to efficient viral translation in a major target organ, namely the pancreas. It would be of interest to see how the presence of specific trans-acting factors is able to regulate CVB3 IRES-mediated translation and virus proliferation in various tissues. Further studies involving identification of such tissue-specific factors will help elucidate the mechanism of IRES-mediated translation of the CVB3 RNA and may provide new targets for anti-viral therapeutics.
Acknowledgments
ACKNOWLEDGEMENTS
We thank Dr Nora M. Chapman for the CVB3 cDNA clone, Dr Jack Keene for the pET-La plasmid and Dr Asim Dasgupta for the pCDIR clone. We gratefully acknowledge our laboratory members for their help and discussion. We are also grateful to Dr Umesh Varshney and Dr M. S. Shaila for their comments on the manuscript. The work is supported by a research grant to S.D. from The Council of Scientific and Industrial Research, India.
REFERENCES
- 1.Jackson R.J. and Kaminski,A. (1995) Internal initiation of translation in eukaryotes: the picornavirus paradigm and beyond. RNA, 1, 985–1000. [PMC free article] [PubMed] [Google Scholar]
- 2.Iizuka N., Chen,C., Yang,O., Johannes,G. and Sarnow,P. (1995) Cap-independent translation and internal initiation of translation in eukaryotic cellular mRNA molecules. Curr. Top. Microbiol. Immunol., 203, 155–177. [DOI] [PubMed] [Google Scholar]
- 3.Jackson R.J., Hunt,S.L., Reynolds,J.E. and Kaminski,A. (1995) Cap-dependent and cap-independent translation: operational distinctions and mechanistic interpretations. Curr. Top. Microbiol. Immunol. 203, 1–29. [DOI] [PubMed] [Google Scholar]
- 4.Vagner S., Galy,B. and Pyronnet,S. (2001) Irresistible IRES. Attracting the translation machinery to internal ribosome entry sites. EMBO Rep., 2, 893–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pelletier J. and Sonenberg,N. (1988) Internal initiation of translation of eukaryotic mRNA directed by a sequence derived from poliovirus RNA. Nature, 334, 320–325. [DOI] [PubMed] [Google Scholar]
- 6.Meerovitch K. and Sonenberg,N. (1993) Internal initiation of picornavirus RNA translation. Semin. Virol., 4, 217–227. [Google Scholar]
- 7.Tsukiyama-Kohara K., Iizuka,N., Kohara,M. and Nomoto,A. (1992) Internal ribosome entry site within hepatitis C virus RNA. J. Virol., 66, 1476–1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holcik M., Sonenberg,N. and Korneluk,R.G. (2000) Internal ribosome initiation of translation and cell death. Trends Genet., 16, 469–473. [DOI] [PubMed] [Google Scholar]
- 9.Baboonian C., Davies,M.J., Booth,J.C. and McKenna,W.J. (1997) Coxsackie B viruses and human heart disease. Curr. Top. Microbiol. Immunol., 223, 31–52. [DOI] [PubMed] [Google Scholar]
- 10.Horwitz M.S., La Cava,A., Fine,C., Rodriguez,E., Ilic,A. and Sarvetnick,N. (2000) Pancreatic expression of interferon-γ protects mice from lethal coxsackievirus B3 infection and subsequent myocarditis. Nature Med., 6, 693–697. [DOI] [PubMed] [Google Scholar]
- 11.Wolfgram L.J., Beisel,K.W. and Rose,N.R. (1985) Heart specific antibodies following murine coxsackievirus B3 myocarditis. J. Exp. Med., 161, 1112–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang D., Wilson,J.E., Anderson,D.R., Bohunek,L., Cordeiro,C., Kandolf,R. and McManus,B.M. (1997) In vitro mutational and inhibitory analysis of the cis-acting translational elements within the 5′ untranslated region of coxsackievirus B3: potential targets for antiviral action of antisense oligomers. Virology, 228, 63–73. [DOI] [PubMed] [Google Scholar]
- 13.Belsham G.J. and Sonenberg,N. (2000) Picornavirus RNA-translation: role for cellular proteins. Trends Microbiol., 8, 330–335. [DOI] [PubMed] [Google Scholar]
- 14.Pestova T.V., Hellen,C.U. and Shatsky,I.N. (1996) Canonical eukaryotic initiation factors determine initiation of translation by internal ribosome entry. Mol. Cell. Biol., 16, 6859–6869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ventoso I., MacMillan,S.E., Hershey,J.W. and Carrasco,L. (1998) Poliovirus 2A proteinase cleaves directly the eIF-4G subunit of eIF-4F complex. FEBS Lett., 435, 79–83. [DOI] [PubMed] [Google Scholar]
- 16.Belsham G.J. and Sonenberg,N. (1996) RNA-protein interactions in regulation of picornavirus RNA translation. Microbiol. Rev., 60, 499–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hunt S.L. and Jackson,R.J. (1999) Polypyrimidine-tract-binding protein (PTB) is necessary, but not sufficient, for efficient internal initiation of translation of human rhinovirus-2 RNA. RNA, 5, 344–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blyn L.B., Towner,J.S., Semler,B.L. and Ehrenfeld,E. (1997) Requirement of poly (rC) binding protein 2 for translation of poliovirus RNA. J. Virol., 71, 6243–6246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hunt S.L., Hsuan,J.J., Totty,N. and Jackson,R.J. (1999) unr, a cellular cytoplasmic RNA-binding protein with five cold-shock domains, is required for internal initiation of translation of human rhinovirus RNA. Genes Dev., 13, 437–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tan E.M. (1989) Antinuclear antibodies: diagnostic markers for autoimmune diseases and probes for cell biology. Adv. Immunol., 44, 93–151. [DOI] [PubMed] [Google Scholar]
- 21.Gottlieb E. and Steitz,J.A. (1989) The RNA binding protein La influences both the accuracy and the efficiency of RNA polymerase III transcription in vitro. EMBO J., 8, 841–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fan H., Sakulich,A.L., Goodier,J.L., Zhang,X., Qin,J. and Maraia,R.J. (1997) Phosphorylation of the human La antigen on serine 366 can regulate recycling of RNA polymerase III transcription complexes. Cell, 88, 707–715. [DOI] [PubMed] [Google Scholar]
- 23.Huhn P., Pruijn,G.J.M., van Venrooij,W.J. and Bachmann,M. (1997) Characterization of the autoantigen La (SS-B) as a dsRNA unwinding enzyme. Nucleic Acids Res., 25, 410–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pruijn G.J.M. (1994) The SS-B/La antigen. In van Venrooij,W.J. and Maini,R.N. (eds), Manual of Biological Markers of Disease, B4.2. Kluwer Academic Press, Dordrecht, The Netherlands, pp. 1–14.
- 25.Kenan D.J., Query,C.C. and Keene,J.D. (1991) RNA-recognition: towards identifying determinants of specificity. Trends Biochem. Sci., 16, 214–220. [DOI] [PubMed] [Google Scholar]
- 26.Meerovitch K., Pelletier,J. and Sonenberg,N. (1989) A cellular protein that binds to the 5′-noncoding region of poliovirus RNA: implications for internal translation initiation. Genes Dev., 3, 1026–1034. [DOI] [PubMed] [Google Scholar]
- 27.Ali N. and Siddiqui,A. (1997) The La antigen binds 5′ noncoding region of the hepatitis C virus RNA in context of the initiator AUG codon and stimulates internal ribosome entry site-mediated translation. Proc. Natl Acad. Sci. USA, 94, 2249–2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park Y.W. and Katze,M.G. (1995) Translational control by influenza virus: identification of cis-acting sequences and trans-acting factors which may regulate selective viral mRNA translation. J. Biol. Chem., 270, 28433–28439. [DOI] [PubMed] [Google Scholar]
- 29.Pardigon N. and Strauss,J.H. (1996) Mosquito homolog of the La autoantigen binds to sindbis virus RNA. J. Virol., 70, 1173–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chang Y., Kenan,D.J., Keene,J.D., Gatignol,A. and Jeang,K. (1994) Direct interactions between autoantigen La and human immunodeficiency virus leader RNA. J. Virol., 68, 7008–7020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meerovitch K., Svitkin,Y.V., Lee,H.S., Lejbkowicz,F., Chan,E.K., Agol,V.I., Keene,J.D. and Sonenberg,N. (1993) La autoantigen enhances and corrects aberrant translation of poliovirus RNA in reticulocyte lysate. J. Virol., 67, 3798–3807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Svitkin Y.V., Meerovitch,K., Lee,H.S., Dholakia,J.N., Kenan,D.J., Agol,V.I. and Sonenberg,N. (1994) Internal translation initiation on poliovirus RNA: further characterization of La function in poliovirus translation in vitro. J. Virol., 68, 1544–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim Y.K. and Jang,S.K. (1999) La protein is required for efficient translation driven by encephalomyocarditis virus internal ribosomal entry site. J. Gen. Virol., 80, 3159–3166. [DOI] [PubMed] [Google Scholar]
- 34.Ali N., Pruijn,G.J.M., Kenan,D.J., Keene,J.D. and Siddiqui,A. (2000) Human La antigen is required for the hepatitis C virus internal ribosome entry site-mediated translation. J. Biol. Chem., 275, 27531–27540. [DOI] [PubMed] [Google Scholar]
- 35.Isoyama T., Kamoshita,N., Yasui,K., Iwai,A., Shiroki,K., Toyoda,H., Yamada,A., Takasaki,Y. and Nomoto,A. (1999) Lower concentration of La protein required for internal ribosome entry on hepatitis C virus RNA than on poliovirus RNA. J. Gen. Virol., 80, 2319–2327. [DOI] [PubMed] [Google Scholar]
- 36.Das S., Coward,P. and Dasgupta,A. (1994) A small yeast RNA selectively inhibits internal initiation of translation programmed by poliovirus RNA: specific interaction with cellular proteins that bind to the viral 5′-untranslated region. J. Virol., 68, 7200–7211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Das S., Kenan,D.J., Bocskai,D., Keene,J.D. and Dasgupta,A. (1996) Sequences within a small yeast RNA required for inhibition of internal initiation of translation: interaction with La and other cellular proteins influences its inhibitory activity. J. Virol., 70, 1624–1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Das S., Ott,M., Yamane,A., Tsai,W., Gromeier,M., Lahser,F., Gupta,S. and Dasgupta,A. (1998) A small yeast RNA blocks hepatitis C virus internal ribosome entry site (HCV IRES)-mediated translation and inhibits replication of a chimeric poliovirus under translational control of the HCV IRES element. J. Virol., 72, 5638–5647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carter M.S. and Sarnow,P. (2000) Distinct mRNAs that encode La autoantigen are differentially expressed and contain internal ribosome entry sites. J. Biol. Chem., 275, 28301–28307. [DOI] [PubMed] [Google Scholar]
- 40.Borman A.M., Le Mercier,P., Girard,M. and Kean,K.M. (1997) Comparison of picornaviral IRES-driven internal initiation of translation in cultured cells of different origins. Nucleic Acids Res., 25, 925–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roberts L.O., Seamons,R.A. and Belsham,G.J. (1998) Recognition of picornavirus internal ribosome entry sites within cells; influence of cellular and viral proteins. RNA, 4, 520–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pilipenko E.V., Viktorova,E.G., Guest,S.T., Agol,V.I. and Roos,R.P. (2001) Cell-specific proteins regulate viral RNA translation and virus-induced disease. EMBO J., 20, 6899–7908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bergelson J.M., Cunningham,J.A., Droguett,G., Kurt-Jones,E.A., Krithivas,A., Hong,J.S., Horwitz,M.S., Crowell,R.L. and Finberg,R.W. (1997) Isolation of a common receptor for Coxsackie B viruses and adenoviruses 2 and 5. Science, 275, 1320–1323. [DOI] [PubMed] [Google Scholar]
- 44.Zell R., Klingel,K., Sauter,M., Fortmuller,U. and Kandolf,R. (1995) Coxsackieviral proteins functionally recognize the polioviral cloverleaf structure of the 5′-NTR of a chimeric enterovirus RNA: influence of species-specific host cell factors on virus growth. Virus Res., 39, 87–103. [DOI] [PubMed] [Google Scholar]
- 45.Walter B.L., Nguyen,J.H.C., Ehrenfeld,E. and Semler,B.L. (1999) Differential utilization of poly (rC) binding protein 2 in translation directed by picornavirus IRES elements. RNA, 5, 1570–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Izumi R.E., Valdez,B., Banerjee,R., Srivastava,M. and Dasgupta,A. (2001) Nucleolin stimulates viral internal ribosome entry site-mediated translation. Virus Res., 76, 17–29. [DOI] [PubMed] [Google Scholar]
- 47.Shiroki K., Isoyama,T., Kuge,S., Ishii,T., Ohmi,S., Hata,S., Suzuki,K., Takasaki,Y. and Nomoto,A. (1999) Intracellular redistribution of truncated La protein produced by poliovirus 3Cpro-mediated cleavage. J. Virol., 73, 2193–2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Grolz D. and Bachmann,M. (1997) An altered intracellular distribution of the autoantigen La/SS-B when translated from a La mRNA isoform. Exp. Cell Res., 234, 329–335. [DOI] [PubMed] [Google Scholar]
- 49.Glass M.J. and Summers,D.F. (1993) Identification of a trans-acting activity from liver that stimulates hepatitis A virus translation in vitro. Virology, 193, 1047–1050. [DOI] [PubMed] [Google Scholar]