Abstract
Specialized neurons throughout the developing central nervous system secrete Reelin, which binds to ApoE receptor 2 (ApoER2) and very low density lipoprotein receptor (VLDLR), triggering a signal cascade that guides neurons to their correct position. Binding of Reelin to ApoER2 and VLDLR induces phosphorylation of Dab1, which binds to the intracellular domains of both receptors. Due to differential splicing, several isoforms of ApoER2 differing in their ligand-binding and intracellular domains exist. One isoform harbors four binding repeats plus an adjacent short 13 amino acid insertion containing a furin cleavage site. It is not known whether furin processing of this ApoER2 variant actually takes place and, if so, whether the produced fragment is secreted. Here we demonstrate that cleavage of this ApoER2 variant does indeed take place, and that the resulting receptor fragment consisting of the entire ligand-binding domain is secreted as soluble polypeptide. This receptor fragment inhibits Reelin signaling in primary neurons, indicating that it can act in a dominant-negative fashion in the regulation of Reelin signaling during embryonic brain development.
Keywords: ApoE receptor 2/furin processing/Reelin signaling
Introduction
ApoE receptor 2 (ApoER2) and very low density lipoprotein receptor (VLDLR) are members of the LDL receptor family involved in Reelin signaling and thus in embryonic brain development (for review, see Herz, 2001; Rice and Curran, 2001). Specialized neurons throughout the developing central nervous system (CNS) secrete Reelin, which binds to cell surface receptors of target neurons, triggering a signal cascade that guides these neurons to their correct position within the developing brain. Disruption of this pathway by a mutation in the murine reelin gene produces the ‘reeler’ phenotype, characterized by ataxic gait due to a severe malformation of the cerebellum. Genetic evidence (Trommsdorff et al., 1999) and biochemical studies (D’Arcangelo et al., 1999; Hiesberger et al., 1999; Brandes et al., 2001) demonstrated that ApoER2 and VLDLR bind directly to extracellular Reelin and transduce the signal into the target neuron. Co-receptors, such as members of the family of cadherin-related neuronal receptors (Senzaki et al., 1999) and α3β1 integrin (Dulabon et al., 2000), are also likely to be involved in this process. However, ectopic expression of Reelin in reeler mice demonstrated that Reelin does not simply act as a cue for the correct positioning of migrating neurons, but is involved in a variety of cell–cell interactions necessary during brain development (Magdaleno et al., 2002). Binding of Reelin to ApoER2 and VLDLR induces the phosphorylation of Dab1 (Hiesberger et al., 1999; Howell et al., 2000), which binds to the intracellular domains of both receptors (Trommsdorff et al., 1998, 1999). Dab1 plays an indispensable role in this signaling pathway, since disruption of the Dab1 gene produces a phenotype indistinguishable from the reeler mouse (Howell et al., 1997; Sheldon et al., 1997). Further downstream events of the cascade are not yet established, but may involve cdk5 and its modulator p35 (Ohshima et al., 1996; Chae et al., 1997). However, the binding of many different adapter proteins, such as JIPs (Stockinger et al., 2000) and others (Gotthardt et al., 2000), to the tails of ApoER2 and VLDLR suggests that a network of pathways might participate in Reelin signaling.
Due to the presence of differentially spliced transcripts, the gene for ApoER2 codes for a receptor family on its own (Brandes et al., 1997, 2001; Kim et al., 1997). These structural variations concerning the ligand-binding and the intracellular domain of the receptor are species- and tissue-specific. In embryonic mouse brain, transcripts are expressed coding for receptor variants with either four or five ligand-binding repeats and for a variant with four binding repeats followed by a short 13 amino acid insertion containing a consensus furin cleavage site at its C-terminus (Brandes et al., 2001). A similar furin site is present in low density lipoprotein receptor-related protein (LRP), another member of the LDLR family, which is proteolytically processed by furin on its way through the secretory pathway (Herz et al., 1990). The two fragments, however, stay non-covalently associated with each other. Mutation at the processing site impairs efficient exit from the endoplasmic reticulum, but cleavage is not essential for LRP’s endocytic function (Ko et al., 1998). In the case of ApoER2, it is not known whether a similar proteolytic processing actually takes place and, if so, whether the produced fragment is secreted as a soluble receptor fragment or stays attached to the remaining receptor, as is the case for LRP. Here we demonstrate that cleavage of the ApoER2 variant carrying the furin consensus site does indeed take place and that the produced receptor fragment, which consists of the entire ligand-binding domain, is secreted as soluble peptide. The soluble receptor fragment inhibits Reelin signaling in primary neurons, indicating that it might act in dominant-negative fashion in the regulation of Reelin signaling during embryonic brain development.
Results
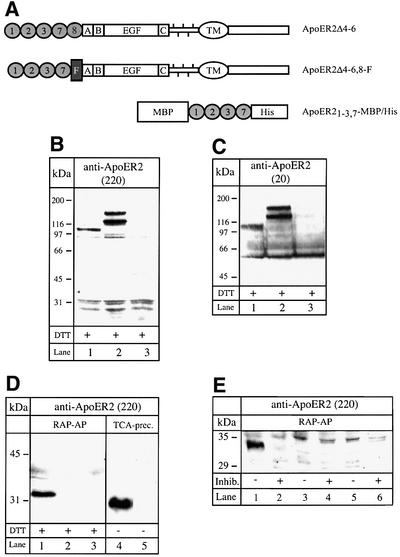
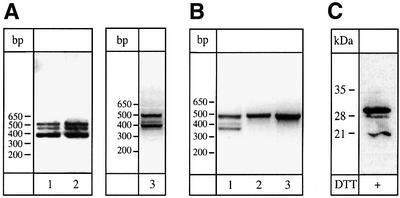
As demonstrated previously, ApoER2 transcripts are subject to complex differential splicing, which is species- and tissue-specific (Brandes et al., 1997, 2001). The mouse gene for ApoER2 contains six exons potentially coding for eight type A ligand-binding repeats. Exon 5, however, coding for repeats 4–6, is constitutively spliced out in all transcripts. The presence of a short exon, which is also subject to differential splicing, between that for repeat 8 and repeat A of the EGF precursor homology domain can give rise to a transcript for a receptor variant containing four type A repeats (1–3 and 7) and an insertion of 13 amino acids with a furin-consensus cleavage site. To test whether this variant undergoes proteolytic cleavage, we transfected 293 cells with an expression vector carrying the corresponding cDNA (ApoER2Δ4-6,8-F). As a control, we used a plasmid coding for ApoER2 containing repeat 8 instead of the insertion carrying the furin cleavage site (ApoER2Δ4-6). The structures of the corresponding receptor variants are shown in Figure 1A. Expression of the receptor variants was verified by western blotting using two different antibodies: the first (Ab 220) directed against the first 14 amino acids of the ligand-binding domain (Figure 1B) and the second (Ab 20) directed against the intracellular domain of the receptor (Figure 1C). The double bands probably originate from differential glycosylation of the receptors in these cells [compare with figure 6 in Brandes et al. (2001)]. If the receptor variant carrying the furin site is indeed processed in the secretory pathway by furin, the resulting soluble receptor fragment should accumulate in the culture medium. Initial attempts to detect such a fragment in conditioned media by western blotting using the antibody against the first ligand-binding repeat of ApoER2 failed. However, enrichment of the putative receptor fragment on a matrix containing receptor-associated protein (RAP), a high-affinity ligand for the LDL receptor family, yielded a peptide migrating with an apparent relative molecular mass of slightly >30 kDa under reducing conditions (dithiothreitol), which reacted with the antibody against the first ligand-binding repeat of the receptor (Figure 1D, lane 1). In conditioned medium derived from cells expressing the control plasmid (ApoER2Δ4-6) or from cells transfected with the empty vector, no such protein could be detected (Figure 1D, lanes 2 and 3). To further characterize the protein present in the supernatant, we concentrated conditioned medium 20-fold by precipitation with trichloroacetic acid (TCA) and performed a western blot under non-reducing conditions using the same antibody as described above. As seen in Figure 1D (lanes 4 and 5), the immunoreactive protein secreted by cells transfected with ApoER2Δ4-6,8-F migrates significantly faster under non-reducing conditions. The difference in migration under reducing versus non-reducing conditions is characteristic for members of the LDL receptor family and is explained by the fact that under reducing conditions the rigid secondary structure of the ligand-binding domain becomes unfolded and the protein migrates with an apparent higher relative molecular mass (Schneider et al., 1982). From these results, i.e. binding to RAP, recognition by a specific antibody against the first ligand-binding repeat of ApoER2, and different electrophoretic mobility under reducing versus non-reducing conditions, we conclude that 293 cells transfected with ApoER2Δ4-6,8-F secrete a soluble fragment of ApoER2. Deduced from the cDNA sequence, this peptide comprises binding repeats 1, 2, 3 and 7, plus 13 amino acids encoded by an additional exon. Interestingly, proteolytic processing of ApoER2 in 293 cells does not lead to significant accumulation of the remaining C-terminal part of the receptor. Such a product should have a relative molecular mass of ∼80 kDa and should be detected by the antibody against the cytoplasmic domain of the receptor. As demonstrated in Figure 1C, this antibody does not visualize such a product present in extracts derived from cells transfected with ApoER2Δ4-6,8-F, suggesting that under these conditions the remaining part of the receptor might be degraded rapidly. To test whether proteolytic cleavage is indeed mediated by furin, we cultured the transfected cells in the presence of the furin inhibitor decanoyl-RVKR-chloromethylketone. As demonstrated in Figure 1E (lanes 1 and 2), when added to cells expressing ApoER2Δ4-6,8-F, the inhibitor abolishes the production of the soluble receptor fragment.
Fig. 1. Western blot analysis of cell extracts and supernatants of 293 cells expressing distinct ApoER2 variants. (A) Cartoon of the ApoER2 variants expressed in 293 cells and the recombinant soluble receptor fragment produced in E.coli. Numbered circles represent distinct ligand-binding repeats. F represents the domain carrying the furin cleavage site. A, B and C describe the cysteine-rich repeats of the EGF-homology domain. TM marks the transmembrane domain. MBP, maltose-binding protein; His, His6 tag. (B) 293 cells were transfected with ApoER2Δ4-6,8-F (lane 1), ApoER2Δ4-6 (lane 2) or empty vector (lane 3). Ten micrograms of the corresponding cell extracts were separated on a 10% SDS–PAGE gel under reducing conditions and transferred to a nitrocellulose membrane. ApoER2 was detected by western blotting using an antibody recognizing the first ligand-binding repeat (220). Binding of the primary antibody was visualized with HRP–goat anti-rabbit and a chemiluminescence system. (C) As (B), but western blotting was performed using an antibody against the cytoplasmic domain of the receptor (20). (D) 293 cells were transfected with ApoER2Δ4-6,8-F (lanes 1 and 4), ApoER2Δ4-6 (lane 2) or empty vector (lanes 3 and 5). The respective cell culture supernatants (1.5 ml) were incubated with 50 µl of RAP–Sepharose beads (lanes 1–3) or directly precipitated with TCA (lanes 4 and 5). The precipitated material was separated on a 15% SDS–PAGE gel under reducing (lanes 1–3) or non-reducing (lanes 4 and 5) conditions. For detection of the receptor fragment, Ab 220 was used in a western blot as described for (B). (E) 293 cells were transfected with ApoER2Δ4-6,8-F (lanes 1 and 2), ApoER2Δ4-6 (lanes 3 and 4) or empty vector (lanes 5 and 6) and incubated in the presence (+) or absence (–) of the furin inhibitor decanoyl-RVKR-chloromethylketone. The respective cell culture supernatants were used for detection of the soluble receptor fragment as described for (D).
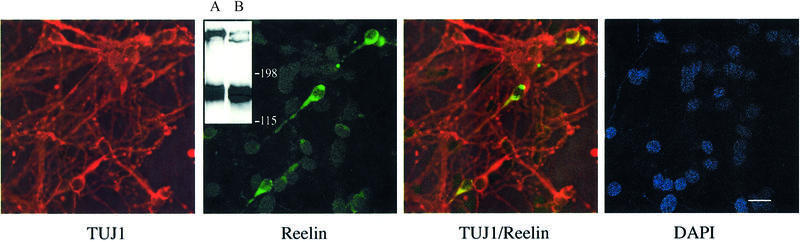
Fig. 6. Primary neurons in culture express and secrete Reelin. Primary mouse neuronal cultures (E15–16) were stained for the neuronal marker class III β-tubulin (TUJ1; red) and for Reelin (G10; green) as described in Materials and methods. Nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI; Roche). Bar: 30 µm. Inset: western blot analysis for the presence of Reelin in conditioned medium of primary mouse neurons. Supernatant (0.2 µl) from 293T cells expressing Reelin (lane A) and supernatant (15 µl) of a primary mouse E15–16 neuronal culture (lane B) were resolved on a 6% SDS–PAGE gel and tested for the presence of Reelin by western blotting using G10 as described in Materials and methods.
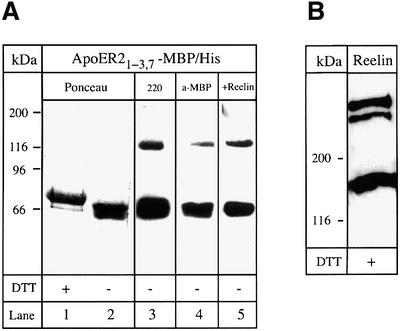
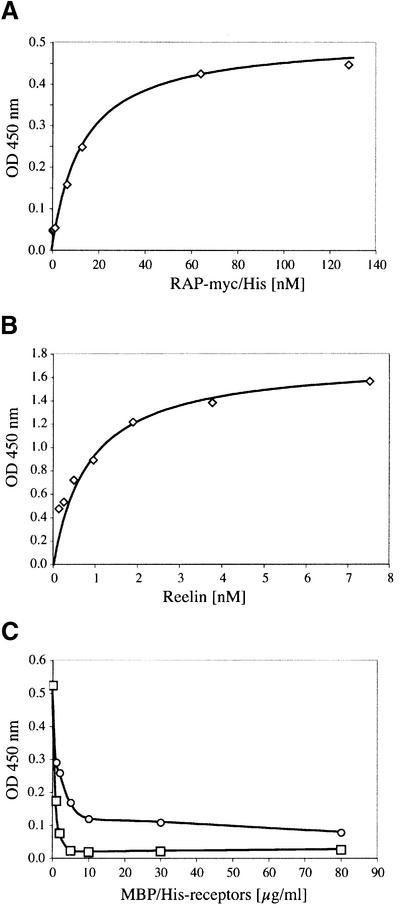
To study the interaction of Reelin with the soluble receptor fragment, we expressed the ligand-binding domain of ApoER2 fused to maltose-binding protein carrying a His tag at its C-terminus in Escherichia coli (ApoER21–3,7–MBP/His, see Figure 1A). In a recent publication, it was demonstrated that a fusion protein containing the ligand-binding domain of VLDLR and MBP spontaneously folds into a ligand binding-competent form in the presence of RAP (Ronacher et al., 2000). The recombinant purified ApoER21–3,7–MBP/His protein is shown in Figure 2A (lanes 1 and 2). As discussed above, the recombinant receptor fragment again migrates faster under non-reducing conditions. It reacts with the antibody against the first binding repeat of ApoER2 (Figure 2A, lane 3) and with an antibody recognizing MBP (Figure 2A, lane 4). A ligand blot performed with Reelin-conditioned medium from 293T cells expressing full-length Reelin (Figure 2B) (D’Arcangelo et al., 1999) demonstrates that the recombinant receptor fragment is able to bind Reelin (Figure 2A, lane 5). The bands present on the blot migrating above 116 kDa most likely represent dimers of the recombinant fusion protein. To characterize the interaction of Reelin with the soluble receptor fragment in more detail, we employed an ELISA-based solid phase binding assay using the recombinant receptor fragment. As demonstrated in Figure 3A, binding of RAP, which was used as a control for the assay, to the receptor fragment is saturated at a concentration of 80 nM with a Kd of 13 nM, indicating high affinity binding of RAP and thus proper folding of the receptor fragment. Interestingly, the affinity of RAP for the soluble ApoER2 fragment is very similar to those for LRP (14 nM) (Williams et al., 1992), megalin (8 nM) (Kounnas et al., 1992) and the VLDL receptor or LR8, the avian homolog of the VLDLR (0.3 and 0.7 nM) (Battey et al., 1994; Hiesberger et al., 1995). The binding of Reelin was similar to that of RAP, showing high affinity binding of Reelin to the soluble receptor fragment (Figure 3B). Since a purified Reelin preparation is not available to date, we used conditioned medium of 293T cells expressing and secreting Reelin (see above), and estimated the amount of Reelin present as described previously (D’Arcangelo et al., 1999). The estimated Kd of 0.85 nM for Reelin binding was in the range of that for Reelin binding to cells expressing full-length ApoER2 (D’Arcangelo et al., 1999) or to the recombinant receptor ectodomain of ApoER2 fused to a V5 epitope (Hiesberger et al., 1999).
Fig. 2. Recombinant ApoER21–3,7–MBP/His binds Reelin. (A) Five micrograms (lanes 1 and 2) or 0.5 µg (lanes 3–5) of recombinant ApoER21–3,7–MBP/His were separated on a 10% SDS–PAGE gel under reducing (lane 1) or non-reducing conditions (lanes 2–5). After transfer to a nitrocellulose membrane, lanes 1 and 2 were stained with Ponceau S, lanes 3 and 4 were processed for western blot analysis using the antibody against the first ligand-binding repeat of ApoER2 (220) (lane 3) or anti-MBP (lane 4), respectively. Lane 5 was incubated with Reelin-containing 293 medium and binding of Reelin was detected by western blotting using the antibody G10. Bound G10 was visualized with HRP–goat anti-mouse (1:15 000) and a chemiluminescence system. (B) Ten microliters of Reelin-conditioned medium were subjected to SDS–PAGE on a 6% gel, followed by a western blot using G10 as described in (A).
Fig. 3. (A and B) Binding of RAP-myc/His and Reelin to ApoER21–3,7–MBP/His. Ninety-six-well plates were coated with 10 µg/ml ApoER21–3,7–MBP/His. After incubation with the indicated amounts of either RAP-myc/His (A) or Reelin-containing 293T cell supernatant (B), bound RAP or Reelin was detected with anti-myc or anti-Reelin antibody and HRP-conjugated secondary antibody. (C) Ninety-six-well plates were coated with 10 µg/ml ApoER21–3,7–MBP/His (circles) or VLDLR1–8–MBP/His (squares) and incubated with Reelin (1 nM) in the presence of increasing amounts of recombinant ApoER21–3,7–MBP/His (0–80 µg/ml). Bound Reelin was detected with anti-Reelin antibody and HRP-conjugated secondary antibody.
Next, we investigated whether the soluble ApoER2 fragment would inhibit the binding of Reelin to ApoER2 as well as to VLDLR. Thus, ELISA plates were coated either with 10 µg/ml ApoER21–3,7–MBP/His or with an MBP fusion protein containing the entire ligand-binding domain of VLDLR (Ronacher et al., 2000). As expected, binding of Reelin to ApoER2 at a concentration of 1 nM (corresponding to half-maximal binding; Figure 3B) was inhibited by the soluble ApoER2 fragment in a dose-dependent manner (Figure 3C). Interestingly, half-maximal inhibition of Reelin binding to VLDLR by the soluble ApoER2 fragment was achieved at a 10-fold lower concentration (1 µg/ml versus 10 µg/ml), indicating a 10 times higher affinity of Reelin for ApoER2 than for VLDLR.
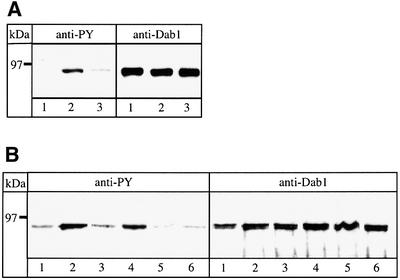
Having established that a soluble ApoER2 receptor fragment that binds Reelin with high affinity can be produced by cells expressing the corresponding ApoER2 transcript, we next tested whether such a fragment would act as a dominant-negative receptor inhibiting the Reelin signaling pathway. First, we used the recombinant MBP fusion protein in a Dab1 phosphorylation assay on primary embryonic mouse neuronal cultures using an anti- phosphotyrosine (PY) antibody (Hiesberger et al., 1999; Howell et al., 1999). Addition of Reelin-containing conditioned medium to primary mouse neurons induces Dab1 phosphorylation (Figure 4A, compare lanes 1 and 2, left panel). Simultaneous addition of the recombinant soluble receptor–MBP fusion protein (40 µg/ml incubation medium) significantly reduces the Reelin-induced phosphorylation of Dab1 (Figure 4A, lane 3, left panel). The same amount of an unrelated MBP fusion protein did not interfere with the action of Reelin (not shown). In addition, we performed the assay using conditioned medium of 293 cells secreting the native soluble receptor fragment (see above). As demonstrated in Figure 4B (lanes 1–3, left panel), simultaneous addition of medium containing the native receptor fragment and of Reelin-containing medium abolishes the Reelin-induced signal. Furthermore, the receptor fragment even reduces the basal Dab1 phosphorylation seen in unstimulated (i.e. without addition of Reelin) primary neurons (Figure 4B, lane 5, left panel). Replacing the medium containing the receptor fragment by medium from mock-transfected cells had no effect on Reelin-stimulated or non-stimulated neurons (Figure 4B, lanes 4 and 6, left panel).
Fig. 4. Reelin-induced Dab1 phosphorylation is inhibited by the soluble ApoER2 fragment. Reelin-induced Dab1 phosphorylation was measured in primary mouse neurons after immunoprecipitation of Dab1 from cell lysates, followed by separation on an 8% SDS–PAGE gel and western blotting using anti-phosphotyrosine (PY) antibody and anti-Dab1 as described in Materials and methods. (A) Neurons were stimulated with control medium (lane 1), Reelin-conditioned medium (lane 2) or Reelin-conditioned medium in the presence of 40 µg/ml recombinant ApoER21–3,7–MBP/His (lane 3). Cells were processed for immunoprecipitation with anti-Dab1 and subsequent western blots were performed using anti-PY and anti-Dab1 as indicated. (B) Neurons were incubated with control medium (lane 1), Reelin-conditioned medium (lane 2), Reelin-conditioned medium in the presence of medium from 293 cells expressing ApoER2Δ4-6,8-F (lane 3) or in the presence of supernatant from 293 cells transfected with the empty plasmid (lane 4), medium from 293 cells expressing ApoER2Δ4-6,8-F (lane 5) or the empty plasmid (lane 6). Immunoprecipitation and western blot analysis as in (A).
Finally, we tested whether primary neurons, as used for the Reelin-induced Dab1 phosphorylation assay, express the transcript coding for the cleavable receptor variant and produce the soluble receptor fragment. We evaluated the presence of the corresponding transcript in these cells by RT–PCR experiments using mRNA from primary neurons and a primer set flanking the region between repeat 7 and the 5′-end of repeat A. The amplicons obtained were compared with those seen in embryonic brain (E15) of normal and reeler mice (Takahara et al., 1996). As shown in Figure 5A (lane 2), cultured primary neurons express the same set of transcripts as seen in the embryonic brain [Figure 5A, lane 1; compare with figure 2 in Brandes et al. (2001)]. Absence of functional Reelin during embryo development does not change the splicing pattern of ApoER2 mRNA (Figure 5A, lane 3). In addition, we performed a spatial analysis of the receptor transcripts in the embryonic brain (E15). As demonstrated in Figure 5B, the transcript coding for the soluble receptor is only present in the cerebrum (lane 1), and is absent from the cerebellum (lane 2) and the olfactory bulbs (lane 3). The cerebellum and the olfactory bulbs produce only one transcript coding for a receptor with five ligand-binding repeats.
Fig. 5. Analysis of splice variants of ApoER2 and detection of soluble ApoER2 in primary mouse neuronal cultures. (A) mRNA from embryonic brain from wild-type mice (lane 1), reeler mice (lane 3) and primary neuronal cultures (E15–16) (lane 2) was used for cDNA synthesis with reverse transcriptase and the resulting cDNA was used for PCR amplification as described in Materials and methods. Amplified products were separated on a 1.5% agarose gel. (B) Embryonic brains (E15–16) were dissected and the cerebrum (lane 1), the cerebellum (lane 2) and the olfactory bulbs (lane 3) were used for cDNA synthesis and PCR was performed as in (A). (C) Supernatant from a primary neuronal culture (E15–16) was immunoprecipitated with anti-Reelin antibody (G10). Immunocomplexes were precipitated with a mixture of protein A– and protein G–Sepharose and resolved on a 4.5–18% gradient polyacrylamide gel and transferred to nitrocellulose as described in Materials and methods. The soluble ApoER2 fragment was detected by western blotting using an antibody against the first ligand-binding repeat (220). Binding of the primary antibody was visualized with HRP–goat anti-rabbit (1:10 000) and a chemiluminescence system.
To test whether the presence of the transcript coding for the cleavable receptor does indeed lead to the production of the receptor fragment, we first tried to enrich the supernatant of cultured neurons by affinity precipitation with RAP–Sepharose as described above, and analyzed the bound proteins by western blot analysis using the ligand-binding domain-specific antibody. With this approach we were not able to detect the putative receptor fragment. However, we reasoned that the presence of Reelin in these cultures might complex the soluble receptor fragment, thereby abolishing RAP binding, as suggested by the results presented in Figure 3. Therefore, we tested conditioned medium from primary neurons by western blotting and, indeed, could demonstrate the presence of Reelin in the medium of these cultures (Figure 6, inset, lane B). As a control (lane A), we used 100×-diluted conditioned medium from 293T cells expressing Reelin (see Figure 2B). Since the medium used for the primary neuronal cultures does not contain any Reelin (data not shown), the Reelin must presumably be secreted by at least some of the cells present in these cultures. In order to confirm this result, expression of Reelin was visualized by immunofluorescence using antibodies against the neuronal marker class III β-tubulin and Reelin, respectively. As seen in Figure 6, ∼10% of the neurons present express Reelin. Lack of co-staining with an antibody against calbindin demonstrated that these cells are not GABAergic cells which are also present in layer I of the marginal zone (Alcantara et al., 1998). Double staining for calretinin, a marker for murine Cajal–Retzius cells (Del Rio et al., 1996), indicates that these cells are bona fide Cajal–Retzius cells (data not shown). Based on this finding, we immunoprecipitated Reelin from primary neuronal culture medium and tested the precipitate for the presence of the soluble receptor fragment by western blotting. With this co-precipitation approach, the soluble receptor fragment could clearly be demonstrated in the medium of primary mouse neurons (Figure 5C).
Discussion
ApoER2 is a member of the LDL receptor family with unusual features. First, it acts as signal transducer in the CNS (Herz, 2001). Whether it is also engaged in endocytosis of macromolecules in vivo, as most other members of the family, is currently not resolved (Li et al., 2001; Riddell et al., 2001). The second feature is that, due to differential splicing, numerous transcripts exist, coding for receptors with potentially different functions (Brandes et al., 1997, 2001; Kim et al., 1997). Another level of complexity arises from the evolution of the ApoER2 gene, i.e. the murine gene contains two additional exons not present in the chicken (Brandes et al., 1997, 2001). One of these exons codes for an insertion in the cytoplasmic tail of the receptor, representing an additional binding site for adapter proteins (Stockinger et al., 2000). The other exon specifies a short insertion of 13 amino acids providing a consensus site for proteolytic cleavage by furin and is located between that for ligand-binding repeat 8 and EGF-type repeat A. This is reminiscent of LRP (Herz et al., 1988), which is proteolytically processed by furin in the trans-Golgi compartment, resulting in 515 and 85 kDa subunits which remain non-covalently linked on the cell surface (Herz et al., 1990; Willnow et al., 1996). We first evaluated whether an ApoER2 variant carrying the furin cleavage site is indeed processed. As demonstrated here, 293 cells expressing this ApoER2 variant proteolytically process the receptor in a furine-dependent manner and secrete a soluble fragment containing the entire ligand-binding domain. Using a recombinant MBP/His-tagged receptor fragment covering the four ligand-binding repeats of the soluble ApoER2 fragment, we were able to show that the bacterially expressed protein has the same affinity for RAP as the native receptor, and concluded that this protein can be used to evaluate the function of the native receptor fragment. Ligand blots and solid phase binding assays showed that the recombinant fragment also binds Reelin with the same affinity as native ApoER2 (D’Arcangelo et al., 1999; Hiesberger et al., 1999; Brandes et al., 2001). Inhibition experiments demonstrated that the soluble ApoER2 fragment not only inhibits Reelin binding to ApoER2, but also to VLDLR. Interestingly, a 10× lower concentration of the ApoER2 fragment was sufficient to abolish Reelin binding to VLDLR compared with ApoER2, indicating a significantly higher affinity of Reelin to ApoER2 than to VLDLR.
Functional studies on primary embryonic mouse neurons revealed that the receptor fragment inhibits Reelin-induced Dab1 phosphorylation. Thus, the soluble ApoER2 fragment can act as dominant-negative receptor by blocking the interaction of Reelin with ApoER2 and VLDLR on the surface of neurons. Interestingly, soluble ApoER2 also abolishes the background phosphorylation of Dab1 in non-stimulated neurons, indicating that this phosphorylation is induced by an external stimulus present in the primary neuronal cultures. In fact, as demonstrated here by western blotting and immunocytochemistry, some of these neurons produce and secrete detectable amounts of Reelin. Double staining for calretinin suggests that these cells are bona fide Cajal–Retzius cells present in the culture. Precipitation of Reelin present in the culture medium demonstrated that neurons also secrete the soluble ApoER2 fragment complexed to Reelin. In the case of cultured neurons, the amount of Reelin present appears to be in excess of that of the receptor fragment, since attempts to isolate the fragment using RAP–Sepharose were unsuccessful. This is in agreement with the observation that, despite endogenous production of the receptor fragment, a low but significant Dab1 phosphorylation in these cultures can be detected.
In recent years, it has become evident that for a number of signaling receptors, such as cytokine receptors, soluble counterparts exist (for review, see Ehlers and Riordan, 1991). An instructive example is the TNFR superfamily of membrane proteins, which serve as signaling receptors to mediate apoptosis, cell survival, proliferation and differentiation (Smith et al., 1994). Soluble forms of these receptors modulate or inhibit the effects of cognate ligands by preventing their interaction with their cellular targets. Interestingly, several mechanisms exist to produce these soluble receptor fragments. The so-called ‘receptor-shedding’ is not only known to result in soluble TNFRs (Kohno et al., 1990), but seems to be a common mechanism also known for the human growth hormone receptor (Trivedi and Daughaday, 1988) and NGFR (DiStefano and Johnson, 1988). This probably occurs by a proteolytic process, which takes place at the cell surface. The second mechanism is the expression of a related gene that codes for a soluble protein resembling the extracellular domain of TNFRs, as is the case for osteoprotegerin, which regulates bone density by inhibition of osteoclast differentiation from precursor cells (Simonet et al., 1997). The third known mechanism to produce a soluble form of a TNFR is the production of soluble murine Fas generated by alternative splicing (Hughes and Crispe, 1995). This splicing event creates a Fas transcript that codes for a truncated soluble form of the receptor.
As described here, production of a soluble biologically active ApoER2 fragment is achieved by a novel mechanism. Differential splicing generates a transcript harboring an additional exon encoding a short stretch containing a furin-processing site, which leads to the secretion of a soluble receptor fragment from primary neurons in culture. However, attempts to isolate the receptor fragment from total brain failed. The reason for this could be rapid degradation of the fragment, a very low concentration which is below the detection limit of our tools, or both. If the fragment is indeed present in the CNS, it might have a variety of consequences. First, the fragment might abolish Reelin-mediated Dab1 phosphorylation, thereby negatively regulating Reelin signaling. However, it is still too early to postulate a negative feedback regulation, since we do not know yet whether the amount of the secreted soluble receptor is itself regulated. Secondly, the secreted receptor fragment might bind to other ligands of the receptor family present in the CNS, e.g. apoE or thrombospondin, thus modulating their turnover. These findings suggest that ApoER2 might play a far more complex role in Reelin signaling than merely being one of the cell surface receptors for Reelin.
Materials and methods
cDNA constructs and production of recombinant proteins
For ApoER2Δ4-6,8-F, a construct containing a splice variant of ApoER2 coding for repeats 1–3 and 7, and for a consensus site for furin cleavage (Brandes et al., 2001) was cloned into the expression vector pCI-neo. The 5′-part of the corresponding transcript was amplified with two sets of primers (set 1: 5′-GGAGCCCCGGGCCCGCATGG; 5′-CCCTGGGA TGCCTCCTGTTTCCCGG; set 2: 5′-GCTGCCTTCAGGTTGCACCA TCATTCC; 5′-TCAATGAGGACCACCTGCTCG; 35 cycles at 58°C). The two PCR products were combined via an internal XmaI site and cloned into pCR2.1 in such a way that a NotI site was located at each site of the insert. The insert was then cut out with NotI and cloned into pCI-neo. In order to produce the full-length cDNA, a BglII–NotI fragment was taken from a partial cDNA clone described previously (Novak et al., 1996) and cloned into the BglII–NotI site of the pCI-Neo-construct containing the 5′-part of the cDNA. The construct for ApoER2Δ4-6 expression has been described previously (Brandes et al., 2001).
The construct for expression of an MBP fusion protein containing the ligand-binding domain of ApoER2Δ4-6,8-F was cloned by inserting a cDNA coding for repeats 1–3 and 7 of ApoER2 into pMalc2x (New England Biolabs) as described previously (Ronacher et al., 2000). The construct ApoER21–3,7–MBP/His or a construct coding for an unrelated MBP/His fusion protein was expressed in E.coli TOP 10 F′ cells. Cells were grown until they reached mid-log phase and 0.5 mM isopropyl-1-thio-β-galactopyranoside was added to induce expression for 4 h at 30°C. The cells were harvested, resuspended in TBS-C (TBS pH 7.4, 2 mM CaCl2) with protease inhibitors (Complete™) and lysed by sonication. After centrifugation, the supernatant was applied to a nickel-NTA–Sepharose column (Qiagen) and rotated overnight at 4°C. After washing, the column was eluted with 250 mM imidazole in TBS-C. For isolation of correctly folded ApoER21–3,7–MBP/His, the Ni-NTA eluate was subjected to RAP affinity chromatography overnight at 4°C and the bound protein was eluted with 1 M NH3 in 0.5× TBS-C. This eluate was concentrated 2-fold in the Speedvac to remove NH3 and the protein was stored at –20°C. The MBP fusion protein containing the entire ligand-binding domain of VLDL receptor (VLDLR1–8–MBP/His) was obtained as described previously (Ronacher et al., 2000).
Recombinant human RAP was produced as a glutathione S-transferase (GST) fusion protein using a pGEX2T-derived (Pharmacia) expression plasmid in DH5α bacteria (Herz et al., 1991). Recombinant myc-tagged RAP was prepared as follows. A plasmid containing the entire cDNA of rat RAP (kindly provided by Dr Joachim Herz, University of Texas Southwestern Medical Center, Dallas, TX) was used as a PCR template. The following oligonucleotides containing the restriction sites for NdeI and BamHI were used for PCR amplification: 5′-CACCATATGG CCGCCAAGCGTGAGTCC-3′ and 5′-CACGGATCCTCATAGATCT TCTTCTGAGGATCAGCTTTTGTTCGAGCTCATTGTGCCGAGC-3′. The 3′ primer sequence contained the c-myc tag sequence and a stop codon in order to generate RAPmyc/His. The resulting product was subcloned into pET-15b (Novagen) and the protein was produced in BL21 E.coli (Invitrogen). Purification of hisRAPmyc was achieved by Ni-NTA purification as described above. Reelin-conditioned media were prepared as described previously (Brandes et al., 2001).
Antibodies
Rabbit anti-ApoER2 (Ab 220) was raised in rabbits against a peptide corresponding to the first 14 amino acids of the first ligand-binding repeat of ApoER2. Rabbit anti-ApoER2 (Ab 20) was raised against a GST fusion protein containing the intracellular domain of ApoER2 and is described in Stockinger et al. (1998). Both antisera were used in western blots at a dilution of 1:500. Rabbit anti-MBP was obtained from New England Biolabs and used in western blots at a dilution of 1:10 000.
Rabbit anti-Dab1 was obtained from Joachim Herz (Dallas, TX) and monoclonal mouse anti-Dab1 (D4; used 1:400) and mouse anti-Reelin (G10; used 1:1000) were a kind gift of Andre Goffinet (University of Louvain, Brussels, Belgium). Mouse anti-phosphotyrosine (K130; 1:1000) was purchased from Santa Cruz. Mouse anti-myc (9E10) was used as supernatant of the hybridoma cell line 9E10 from ATCC at a dilution of 1:100.
Expression of mouse ApoER2Δ4-6,8-F and ApoER2Δ4-6
For expression of ApoER2Δ4-6,8-F and ApoER2Δ4-6, the human embryonic kidney cell line 293 was transfected with the constructs or empty vector as control using Lipofectin Reagent (Life Technologies, Inc.) according to the manufacturer’s protocol. Twenty-four hours after transfection, the medium was changed to serum-free Optimem (Gibco-BRL) supplemented with glutamate and antibiotics; in the case of the furin inhibition experiment, decanoyl-RVKR-chloromethylketone (100 µM; Bachem, Switzerland) was added. For detection of ApoER2 expression, cells and medium were used after an additional 24 h.
Preparation of cell extracts, electrophoresis, western blotting and ligand blotting
Total cell extracts from 293 cells expressing ApoER2Δ4-6,8-F or ApoER2Δ4-6 were obtained after washing the cells twice with phosphate-buffered saline (PBS), scraping them into lysis buffer [50 mM Tris pH 8.0, 140 mM NaCl, 1% Triton X-100, proteinase inhibitor mix Complete™ (Roche)] and centrifugation for 30 min at 15 000 g. SDS–PAGE was carried out according to Laemmli (1970) and proteins were transferred to nitrocellulose membranes by semi-dry blotting. For western blot analysis with Ab 220, α-MBP, G10, 9E10 and D4 nitrocellulose membranes were blocked overnight at 4°C in TBS-T (TBS pH 7.4, 0.1% Tween) containing 5% milk powder. For western blots using K130 and ligand blots, 5% bovine serum albumin (BSA) instead of milk powder was used. Appropriate horseradish peroxidase (HRP)-conjugated secondary antibodies from goat (Jackson ImmunoResearch; 1:20 000) were used for detection with enhanced chemiluminescence (Pierce). For ligand blots, membranes were incubated with Reelin-containing medium supplemented with 2 mM CaCl2. Bound Reelin was detected using G10 as described above for western blotting.
Precipitation of the soluble receptor fragment
Supernatant (1.5 ml) of 293 cells (10 cm dish) transfected with ApoER2Δ4-6,8-F, ApoER2Δ4-6 or empty pCI-neo-vector were incubated with 25 µl of RAP–GST bound to glutathione–Sepharose (Amersham) for 2 h at 4°C. The beads were collected by centrifugation (1000 r.p.m., 2 min), washed twice with TBS-C and boiled in reducing Laemmli sample buffer before electrophoresis. Alternatively, 1.5 ml of supernatant were mixed with 300 µl of ice-cold TCA and 150 µl of 10% Triton X-100 and kept on ice for 30 min to precipitate the receptor fragment. After centrifugation (20 min at 15 000 g), the pellet was washed with ice-cold acetone, dried, dissolved in non-reducing Laemmli sample buffer and subjected to SDS–PAGE.
Solid phase binding assay
ApoER21–3,7–MBP/His or VLDLR1–8–MBP/His (both at 10 µg/ml) in TBS-C were coated on a 96-well plate overnight at 4°C. All further incubations were carried out at room temperature for 1 h and ligands or antibodies were diluted in blocking solution (2% BSA in TBS-C, 0.05% Tween). After blocking and binding of the ligand (RAP-myc/His or Reelin-conditioned medium), anti-myc or anti-Reelin followed by HRP-conjugated secondary antibodies were used for detection of the bound ligands. For the color reaction, 0.1 mg/ml 3,3′,5,5′-tetramethylbenzidine was used in 0.1 M natrium acetate pH 6.0 containing 10 mM H2O2. The reaction was stopped after 5 min by addition of 0.3 M H2SO4 and the resulting yellow product was measured at 450 nm.
Dab1 phosphorylation assay
The Dab1 phosphorylation assay was essentially performed as described previously (D’Arcangelo et al., 1999; Hiesberger et al., 1999). Briefly, brains from E15 mouse embryos (Balb/c) were homogenized in HBSS, centrifuged (1200 r.p.m., 4 min), resuspended in medium [DMEM/Nutmix F-12 (HAM) containing B27 supplement (Gibco-BRL), 10 mM glutamate and antibiotics] and plated on poly-l-ornithine-coated 10 cm dishes. After 3 days in culture, the cells were washed with HBSS and incubated with different media containing the indicated ligands (see figures). After 25 min at 37°C, cells were washed again, scraped into 500 µl of RIPA buffer [10 mM sodium phosphate pH 7.4, 150 mM NaCl, 2 mM EDTA, 50 mM NaF, 2 mM Na3VO4, 1% β-mercaptoethanol, 1% Triton X-100, 1% sodium deoxycholate, 0.1% SDS and Complete™ protease inhibitor cocktail (Roche)] and lysed for 25 min on ice. The lysates were centrifuged at 15 000 r.p.m. for 30 min and the supernatants were immediately used for immunoprecipitation of Dab1 with 5 µl of 2720 antiserum. After 2 h at 4°C, 20 µl of protein A beads (Amersham) were added for 2 h at 4°C. The beads were washed with RIPA buffer and boiled in reducing Laemmli buffer prior to SDS–PAGE and western blotting. One half of the precipitate was used to quantify precipitated Dab1 by western blotting with D4 (anti-Dab1 in Figure 4) and the other half was used to visualize Dab1 phosphorylation by western blotting with K130 (anti-PY in Figure 4).
Immunocytochemistry
Mouse primary neurons were prepared as described for the Dab1 phosphorylation assay, but plated on slides coated with 0.15 mg/ml poly-l-ornithine (Sigma) and 0.01 mg/ml fibronectin (Sigma). After 3 days on the slides, cells were washed with PBS, fixed for 10 min at –20°C with pre-cooled MeOH:acetone (1:1), washed three times with PBS, blocked (PBS; 4% goat serum; 2% BSA) for 1 h at room temperature, incubated with primary antibody in blocking solution for 2 h, washed three times with PBS, incubated with secondary antibody for 2 h at room temperature, and washed twice with PBS and once with H2O. Primary antibodies and dilutions: anti-Reelin (G10; monoclonal, 1:300), anti-class III β-tubulin (TUJ1; polyclonal, 1:2000; BAbCO), anti-calbindin (polyclonal, 1:300; Chemicon), anti-calretinin (N18; polyclonal, 1:1000; Santa Cruz); secondary antibodies and dilutions: Alexa Fluor 488 goat anti-mouse IgG (1:500; Molecular Probes), Alexa Fluor 594 goat anti-rabbit IgG (1:500; Molecular Probes).
RT–PCR analysis
Mouse primary neurons were cultured for 3 days on poly-l-ornithine-coated plates. Poly(A)+ RNA from primary neurons and mouse embryonic brain, respectively, was prepared using the Micro-Fast Track mRNA Isolation Kit (Invitrogen) according to the manufacturer’s protocol. First strand cDNA synthesis was performed using SuperScript Reverse Transcriptase (Life Technologies, Inc.). The cDNA was used for subsequent PCR in the presence of 0.2 mM dNTPs, 2 U of DynazymeII DNA polymerase (Finnzymes) and the following primers at 1 µM: mouse 1, 5′-CGAGAATGAGTTCCAGTGTGG-3′; mouse 2, 5′-CGTGAAG ATCAGAGATGGGC-3′. PCR conditions were as follows: 1 min initial denaturation at 94°C, 1 min denaturation at 94°C, 30 s annealing at 60°C and 1 min extension at 72°C for 35 cycles. PCR products were analyzed by agarose gel electrophoresis.
Co-immunoprecipitation of Reelin and the soluble ApoER2 fragment
Four hundred microliters of supernatant from primary mouse neuronal cultures (E15–16) were mixed with 1.1 ml of Hunt buffer (20 mM Tris pH 8.0, 100 mM NaCl, 0.5% NP-40) containing a Complete™ (EDTA-free) protease inhibitor cocktail and incubated overnight with 5 µl of anti-Reelin antibody (G10). Immunocomplexes were precipitated by incubating the reaction mixture with 30 µl of a 50% slurry of a mixture (1:1) of protein A– and protein G–Sepharose for 2 h. After four washing steps with 1 ml of Hunt buffer each, the beads were incubated for 5 min at 95°C with 30 µl of reducing Laemmli sample buffer. Subsequently, the beads were removed by centrifugation and the supernatant subjected to western blotting using the antibody against the first ligand-binding repeat of ApoER2 (220) as described above.
Acknowledgments
Acknowledgements
This work was supported by Austrian Science Foundation Grants P13931-MOB and F606 (to J.N.), and F-608 and P13940-MOB (to W.J.S.). Antibodies against Reelin and Dab1 were generous gifts from Andre Goffinet (University of Louvain Medical School, Developmental Genetics Unit, Brussels, Belgium) and Joachim Herz (Department of Molecular Genetics, University of Texas Southwestern Medical Center, Dallas, TX 75390-9046). We appreciate the assistance of the Group of Dieter Blaas (Department of Medical Biochemistry, BioCenter and University of Vienna) with the preparation of ApoER21–3,7–MBP/His and VLDLR1–8–MBP/His.
References
- Alcantara S., Ruiz,M., D’Arcangelo,G., Ezan,F., de Lecea,L., Curran,T., Sotelo,C. and Soriano,E. (1998) Regional and cellular patterns of reelin mRNA expression in the forebrain of the developing and adult mouse. J. Neurosci., 18, 7779–7799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battey F.D., Gåfvels,M.E., FitzGerald,D.J., Argraves,W.S., Chappell, D.A., Strauss,J.F.,III and Strickland,D.K. (1994) The 39-kDa receptor-associated protein regulates ligand binding by the very low density lipoprotein receptor. J. Biol. Chem., 269, 23268–23273. [PubMed] [Google Scholar]
- Brandes C., Novak,S., Stockinger,W., Herz,J., Schneider,W.J. and Nimpf,J. (1997) Avian and murine LR8B and human apolipoprotein E receptor 2: differentially spliced products from corresponding genes. Genomics, 42, 185–191. [DOI] [PubMed] [Google Scholar]
- Brandes C., Kahr,L., Stockinger,W., Hiesberger,T., Schneider,W.J. and Nimpf,J. (2001) Alternative splicing in the ligand binding domain of mouse ApoE receptor-2 produces receptor variants binding reelin but not α2-macroglobulin. J. Biol. Chem., 276, 22160–22169. [DOI] [PubMed] [Google Scholar]
- Chae T., Kwon,Y.T., Bronson,R., Dikkes,P., Li,E. and Tsai,L.H. (1997) Mice lacking p35, a neuronal specific activator of Cdk5, display cortical lamination defects, seizures and adult lethality. Neuron, 18, 29–42. [DOI] [PubMed] [Google Scholar]
- D’Arcangelo G., Homayoundi,R., Keshvara,L., Rice,D.S., Sheldon,M. and Curran,T. (1999) Reelin is a ligand for lipoprotein receptors. Neuron, 24, 471–479. [DOI] [PubMed] [Google Scholar]
- Del Rio J.A., Heimrich,B., Super,H., Borrell,V., Frotscher,M. and Soriano,E. (1996) Differential survival of Cajal–Retzius cells in organotypic cultures of hippocampus and neocortex. J. Neurosci., 16, 6896–6907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiStefano P.S. and Johnson,E.M.,Jr (1988) Identification of a truncated form of the nerve growth factor receptor. Proc. Natl Acad. Sci. USA, 85, 270–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulabon L., Olson,E.C., Taglienti,M.G., Eisenhuth,S., McGrath,B., Walsh,C.A., Kreidberg,J.A. and Anton,E.S. (2000) Reelin binds α3β1 integrin and inhibits neuronal migration. Neuron, 27, 33–44. [DOI] [PubMed] [Google Scholar]
- Ehlers M.R. and Riordan,J.F. (1991) Membrane proteins with soluble counterparts: role of proteolysis in the release of transmembrane proteins. Biochemistry, 30, 10065–10074. [DOI] [PubMed] [Google Scholar]
- Gotthardt M., Trommsdorff,M., Nevitt,M.F., Shelton,J., Richardson,J.A., Stockinger,W., Nimpf,J. and Herz,J. (2000) Interactions of the low density lipoprotein receptor gene family with cytosolic adaptor and scaffold proteins suggest diverse biological functions in cellular communication and signal transduction. J. Biol. Chem., 275, 25616–25624. [DOI] [PubMed] [Google Scholar]
- Herz J. (2001) The LDL receptor gene family: (un)expected signal transducers in the brain. Neuron, 29, 571–581. [DOI] [PubMed] [Google Scholar]
- Herz J., Hamann,U., Rogne,S., Myklebost,O., Gausepohl,H. and Stanley,K.K. (1988) Surface location and high affinity for calcium of a 500-kDa liver membrane protein closely related to the LDL-receptor suggest a physiological role as lipoprotein receptor. EMBO J., 7, 4119–4127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herz J., Kowal,R.C., Goldstein,J.L. and Brown,M.S. (1990) Proteolytic processing of the 600 kd low density lipoprotein receptor-related protein (LRP) occurs in a trans-Golgi compartment. EMBO J., 9, 1769–1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herz J., Goldstein,J.L., Strickland,D.K., Ho,Y.K. and Brown,M.S. (1991) 39-kDa protein modulates binding of ligands to low density lipoprotein receptor-related protein/α2-macroglobulin receptor. J. Biol. Chem., 266, 21232–21238. [PubMed] [Google Scholar]
- Hiesberger T. et al. (1995) The chicken oocyte receptor for yolk precursors as a model to study the action of receptor associated protein and lactoferrin. J. Biol. Chem., 270, 18219–18226. [DOI] [PubMed] [Google Scholar]
- Hiesberger T., Trommsdorff,M., Howell,B.W., Goffinet,A., Mumby, M.C., Cooper,J.A. and Herz,J. (1999) Direct binding of reelin to VLDL receptor and ApoE receptor 2 induces tyrosin phosphorylation of the adaptor protein disabled-1 and modulates tau phosphorylation. Neuron, 24, 481–489. [DOI] [PubMed] [Google Scholar]
- Howell B.W., Hawkes,R., Soriano,P. and Cooper,J.A. (1997) Neuronal position in the developing brain is regulated by mouse disabled-1. Nature, 389, 733–737. [DOI] [PubMed] [Google Scholar]
- Howell B.W., Herrick,T.M. and Cooper,J.A. (1999) Reelin-induced tyrosine phosphorylation of disabled 1 during neuronal positioning. Genes Dev., 13, 643–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell B.W., Herrick,T.M., Hildebrand,J.D., Zhang,Y. and Cooper,J.A. (2000) Dab1 tyrosine phosphorylation sites relay positional signals during mouse brain development. Curr. Biol., 10, 877–885. [DOI] [PubMed] [Google Scholar]
- Hughes D.P. and Crispe,I.N. (1995) A naturally occurring soluble isoform of murine Fas generated by alternative splicing. J. Exp. Med., 182, 1395–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D.-H. et al. (1997) Exon/intron organization, chromosome localization, alternative splicing and transcription units of the human apolipoprotein E receptor 2. J. Biol. Chem., 272, 8498–8504. [DOI] [PubMed] [Google Scholar]
- Ko K.W.S., McLeod,R.S., Avramoglu,R.K., Nimpf,J., FitzGerald,D.J., Vukmirica,J. and Yao,Z.M. (1998) Mutation at the processing site of chicken low density lipoprotein receptor-related protein impairs efficient endoplasmic reticulum exit, but proteolytic cleavage is not essential for its endocytic functions. J. Biol. Chem., 273, 27779–27785. [DOI] [PubMed] [Google Scholar]
- Kohno T., Brewer,M.T., Baker,S.L., Schwartz,P.E., King,M.W., Hale,K.K., Squires,C.H., Thompson,R.C. and Vannice,J.L. (1990) A second tumor necrosis factor receptor gene product can shed a naturally occurring tumor necrosis factor inhibitor. Proc. Natl Acad. Sci. USA, 87, 8331–8335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kounnas M.Z., Argraves,W.S. and Strickland,D.K. (1992) The 39-kDa receptor-associated protein interacts with two members of the low density lipoprotein receptor family, α2-macroglobulin receptor and glycoprotein 330. J. Biol. Chem., 267, 21162–21166. [PubMed] [Google Scholar]
- Laemmli U.K. (1970) Cleavage of structural proteins during assembly of the head of the bacteriophage T4. Nature, 227, 680–685. [DOI] [PubMed] [Google Scholar]
- Li Y., Lu,W., Marzolo,M.P. and Bu,G. (2001) Differential functions of members of the low density lipoprotein receptor family suggested by their distinct endocytosis rates. J. Biol. Chem., 276, 18000–18006. [DOI] [PubMed] [Google Scholar]
- Magdaleno S., Keshvara,L. and Curran,T. (2002) Rescue of ataxia and preplate splitting by ectopic expression of reelin in reeler mice. Neuron, 33, 573–586. [DOI] [PubMed] [Google Scholar]
- Novak S., Hiesberger,T., Schneider,W.J. and Nimpf,J. (1996) A new low density lipoprotein receptor homologue with 8 ligand binding repeats in brain of chicken and mouse. J. Biol. Chem., 271, 11732–11736. [DOI] [PubMed] [Google Scholar]
- Ohshima T., Ward,J.M., Huh,C.G., Longenecker,G., Veeranna, Pant, H.C., Brady,R.O., Martin,L.J. and Kulkarni,A.B. (1996) Targeted disruption of the cyclin-dependent kinase 5 gene results in abnormal corticogenesis, neuronal pathology and perinatal death. Proc. Natl Acad. Sci. USA, 93, 11173–11178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice D.S. and Curran,T. (2001) Role of the reelin signaling pathway in central nervous development. Annu. Rev. Neurosci., 24, 1005–1039. [DOI] [PubMed] [Google Scholar]
- Riddell D.R., Sun,X.M., Stannard,A.K., Soutar,A.K. and Owen,J.S. (2001) Localization of apolipoprotein E receptor 2 to caveolae in the plasma membrane. J. Lipid Res., 42, 998–1002. [PubMed] [Google Scholar]
- Ronacher B., Marlovits,T.C., Moser,R. and Blaas,D. (2000) Expression and folding of human very-low-density lipoprotein receptor fragments: neutralization capacity toward human rhinovirus HRV2. Virology, 278, 541–550. [DOI] [PubMed] [Google Scholar]
- Schneider W.J., Beisiegel,U., Goldstein,J.L. and Brown,M.S. (1982) Purification of the low density lipoprotein receptor, an acidic glycoprotein of 164 000 molecular weight. J. Biol. Chem., 257, 2664–2673. [PubMed] [Google Scholar]
- Senzaki K., Ogawa,M. and Yagi,T. (1999) Proteins of the CNR family are multiple receptors for Reelin. Cell, 99, 635–647. [DOI] [PubMed] [Google Scholar]
- Sheldon M. et al. (1997) Scrambler and yotari disrupt the disabled gene and produce a reeler-like phenotype in mice. Nature, 389, 730–733. [DOI] [PubMed] [Google Scholar]
- Simonet W.S. et al. (1997) Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell, 89, 309–319. [DOI] [PubMed] [Google Scholar]
- Smith C.A., Farrah,T. and Goodwin,R.G. (1994) The TNF receptor superfamily of cellular and viral proteins: activation, costimulation and death. Cell, 76, 959–962. [DOI] [PubMed] [Google Scholar]
- Stockinger W., Hengstschläger-Ottnad,E., Novak,S., Matus,A., Hüttinger,M., Bauer,J., Lassmann,H., Schneider,W.J. and Nimpf,J. (1998) The LDL receptor gene family: differential expression of two α2-macroglobulin receptors in the brain. J. Biol. Chem., 273, 32213–32221. [DOI] [PubMed] [Google Scholar]
- Stockinger W., Brandes,C., Fasching,D., Hermann,M., Gotthardt,M., Herz,J., Schneider,W.J. and Nimpf,J. (2000) The Reelin receptor ApoER2 recruits JNK-interacting proteins-1 and 2. J. Biol. Chem., 275, 25625–25632. [DOI] [PubMed] [Google Scholar]
- Takahara T. et al. (1996) Dysfunction of the Orleans reeler gene arising from exon skipping due to transposition of a full-length copy of an active L1 sequence into the skipped exon. Hum. Mol. Genet., 5, 989–993. [DOI] [PubMed] [Google Scholar]
- Trivedi B. and Daughaday,W.H. (1988) Release of growth hormone binding protein from IM-9 lymphocytes by endopeptidase is dependent on sulfhydryl group inactivation. Endocrinology, 123, 2201–2206. [DOI] [PubMed] [Google Scholar]
- Trommsdorff M., Borg,J.-P., Margolis,B. and Herz,J. (1998) Interaction of cytosolic adaptor proteins with neuronal apoE receptors and the amyloid precursor proteins. J. Biol. Chem., 273, 33556–33565. [DOI] [PubMed] [Google Scholar]
- Trommsdorff M., Gotthardt,M., Hiesberger,T., Shelton,J., Stockinger, W., Nimpf,J., Hammer,R., Richardson,J.A. and Herz,J. (1999) Reeler/Disabled-like disruption of neuronal migration in knock out mice lacking the VLDL receptor and apoE receptor-2. Cell, 97, 689–701. [DOI] [PubMed] [Google Scholar]
- Williams S.E., Ashcom,J.D., Argraves,W.S. and Strickland,D.K. (1992) A novel mechanism for controlling the activity of α2-macroglobulin receptor/low density lipoprotein receptor-related protein. J. Biol. Chem., 267, 9035–9040. [PubMed] [Google Scholar]
- Willnow T.E., Moehring,J.M., Inocencio,N.M., Moehring,T.J. and Herz,J. (1996) The low-density-lipoprotein receptor-related protein (LRP) is processed by furin in vivo and in vitro. Biochem. J., 313, 71–76. [DOI] [PMC free article] [PubMed] [Google Scholar]