Abstract
The Rab-specific αGDP-dissociation inhibitor (αGDI) regulates the recycling of Rab GTPases. We have now identified a novel αGDI complex from synaptic membranes that contains three chaperone components: Hsp90, Hsc70 and cysteine string protein (CSP). We find that the αGDI–chaperone complex is dissociated in response to Ca2+-induced neurotransmitter release, that chaperone complex dissociation is sensitive to the Hsp90 inhibitor geldanamycin (GA) and that GA inhibits the ability of αGDI to recycle Rab3A during neurotransmitter release. We propose that αGDI interacts with a specialized membrane-associated Rab recycling Hsp90 chaperone system on the vesicle membrane to coordinate the Ca2+-dependent events triggering Rab-GTP hydrolysis with retrieval of Rab-GDP to the cytosol.
Keywords: CSP/GDI/Hsp90/Rab3A/synaptic vesicle
Introduction
The entry of Ca2+ into nerve terminals initiates a series of molecular events that culminates with the fusion of the synaptic vesicle and the release of neurotransmitter into the synaptic cleft (Calakos and Scheller, 1996). Rab3A is a small G protein belonging to the ubiquitous Rab GTPase family now recognized to contain >60 members that facilitate the targeting/fusion of vesicle carriers between all compartments of the exocytic and endocytic pathways (Pereira-Leal and Seabra, 2001; Zerial and McBride, 2001). Rab3A is likely to be a key molecule involved in Ca2+-dependent exocytosis at the synapse through regulation of the assembly and disassembly of tethering/fusion complexes (Geppert and Sudhof, 1998).
A number of regulators of Rab3A have now been identified (Takai et al., 2001). However, an understanding of the physiological role of Rab3A and its effectors in synaptic function has remained elusive. Rab family-specific GDP-dissociation inhibitors (GDI) comprise a group that regulates the recycling of Rab proteins (Wu et al., 1996). The GDI superfamily (Alory and Balch, 2001) is now recognized to contain at least three GDI isoforms that interact with prenylated forms of Rab, an essential post-translational modification required for membrane association (Desnoyers et al., 1996). GDI binds membrane-associated Rab-GDP and extracts the targeted protein to the cytosol, where the complex functions as a reservoir for recruitment of Rab GTPases in subsequent rounds of vesicle formation. The importance of the αGDI isoform, found principally in the brain (Takai et al., 2001), in Rab recycling is illustrated by the fact that inherited mutant forms of αGDI leading to human X-linked mental retardation are associated with epileptic seizures (D’Adamo et al., 1998). Furthermore, studies using αGDI-deficient mice suggest that it plays a specialized role in Rab3A recycling to suppress hyper-excitability via modulation of presynaptic forms of plasticity (Ishizaki et al., 2000).
To understand the molecular basis of GDI function, we have elucidated the structure of bovine αGDI at 1.04 Å resolution, revealing a two-domain organization (Schalk et al., 1996; Luan et al., 2000). Sequence conserved regions (SCRs) in domain I form the Rab-binding platform. In contrast, a highly conserved flexible loop, referred to as the mobile effector loop or MEL (found in SCRs contributing to the structural organization of domain II), are not involved in Rab binding; rather, it directs GDI to the membrane and regulates the ability of GDI to retrieve Rab to the cytosol (Luan et al., 2000). Mutation of MEL leads to a pronounced defect in vesicular traffic and inhibition of cell growth (Luan et al., 1999, 2000).
The requirement for MEL in GDI function suggests that a membrane-associated factor(s) is involved in Rab-GDP retrieval following vesicle fusion. This factor, referred to as Rab recycling factor (RRF), has been postulated to be required to initiate Rab extraction from membranes (Luan et al., 1999, 2000). We have now identified key components comprising RRF in the synapse using synaptosomes, an enriched membrane fraction specialized in Rab3A recycling. While the major pool comprising the cytosolic form of αGDI in the brain is largely found in a complex with Rab3A (Takai et al., 2001), we find that a minor pool of membrane-associated αGDI forms a complex with chaperones present on the synaptic vesicle membrane. These include Hsp90, Hsc70 and cysteine string protein (CSP). Interaction of αGDI with the Hsp90-containing chaperone complex is required for efficient Rab3A retrieval from membrane and for Ca2+-dependent neurotransmitter release as these events are sensitive to the Hsp90 inhibitor geldanamycin (GA). Because chaperones such as Hsp90 are ideally suited to participate in recyc ling processes, we propose that the Hsp90-containing chaperone system fulfills an essential role that modulates αGDI function to retrieve prenylated Rab3A from the lipid bilayer during neurotransmitter release.
Results
Identification of an αGDI–Hsp90 chaperone complex
In order to determine the basis for association of αGDI with synaptic membranes, we isolated synaptosomes from rat brain, treated them with the non-cleavable cross-linker disuccinimidyl suberate and subfractionated the synaptosomes into a synaptic soluble fraction (SS), a crude synaptic membrane fraction (CSM) and a crude synaptic vesicle fraction (CSV). The distribution of αGDI in each fraction was resolved by SDS–PAGE and detected by immunoblotting using an αGDI-specific antibody. As expected, αGDI was recovered in the SS as an 85 kDa cytosolic complex that contains both Rab3A (25 kDa) and αGDI (55 kDa) (Takai et al., 2001) (Figure 1A, lane b, arrow). In contrast, in the CSV and CSM we did not detect the 85 kDa complex; rather, we observed a substantial loss of recovery of αGDI (Figure 1A, compare lanes c and d, e and f, arrowhead), presumably due to the formation of higher order molecular weight complex(es) that could not be solubilized, or were excluded from the gel.
Fig. 1. Identification of an αGDI–Hsp90 chaperone complex. (A) Synaptosomes were incubated in the absence or presence of a non-cleavable cross-linker (NCL). Subsequently, a synaptic soluble (SS) fraction, a crude synaptic vesicle (CSV) fraction and a crude synaptic membrane (CSM) fraction were analyzed for the presence of αGDI by immunoblotting using an anti-αGDI mouse monoclonal antibody. (B) Silver-stained gel of proteins co-immunoprecipitated with αGDI (lanes b and c) or control antibody (a). DPS were treated in the absence or presence of the cleavable cross-linker (CL). Bands denoted by asterisks are unidentified components. (C) Components recovered in anti-αGDI immunoprecipitates (B) were identified by immunoblotting with the indicated antibodies. Results shown are representative of at least three independent experiments.
To identify novel αGDI interacting proteins, we immunoprecipitated αGDI from the synaptic membrane fraction in the absence or presence of the reversible cross-linker dithiobis-succinimidylpropionate. To deplete the abundant cytosolic pool containing the soluble αGDI– Rab3A complex that may interfere with the identification of membrane-associated binding partners, synaptosomes were first permeabilized on ice with digitonin, a detergent that favors selective permeabilization of the plasma membrane (Bittner and Holz, 1992). Under these conditions, ∼90% of the total αGDI–Rab3A pool found in intact synaptosomes could be depleted following washing (data not shown). The digitonin-perforated synaptosomes (referred to as DPS) were subsequently incubated for 15 min at 30°C in either the absence or presence of cleavable cross-linker. After quenching, DPS were solubilized with a detergent-containing lysis buffer, immunoprecipitated with an αGDI antibody and proteins separated using SDS–PAGE in the presence of reducing agent.
Strikingly, in the presence of cross-linker, we observed a prominent ∼90 kDa protein that co-immunoprecipitated with near stoichiometry with the membrane-associated αGDI (Figure 1B, lane c, Hsp90 arrowhead). A significant, but reduced, recovery was observed in the absence of cross-linker. In addition to the ∼90 kDa band, we also observed additional bands in the presence of cross-linker (Figure 1B, lane c, labeled arrowheads) and several that appeared in the immunoprecipitate only in the absence of cross-linker (Figure 1B, lane b). MALDI-TOF mass spectrometry of the 90 kDa band (Figure 1B) and immunoblotting (Figure 1C) revealed that the major binding ∼90 kDa protein was the molecular chaperone Hsp90 (Richter and Buchner, 2001; Young et al., 2001). In the presence of cross-linker, we also recovered the molecular chaperones Hsc70 and CSP (Figure 1B, lane c, and C) and several as yet unidentified bands (Figure 1B, lane c, asterisks). CSP is an abundant synaptic vesicle membrane-associated protein that contains a DnaJ chaperone homology domain involved in recognition of Hsc70 (Chamberlain and Burgoyne, 2000). These results suggest that a novel membrane-associated chaperone complex may facilitate the functional interaction of αGDI with the synaptic membrane.
To gain insight into the distribution of the αGDI– chaperone complex in synaptosomes, we utilized both subcellular fractionation and immunoelectron microscopy. Biochemically, we found that Hsp90, Hsc70 and αGDI showed a similar distribution pattern, primarily localized in the soluble fraction, but with significant pools in the CSV containing synaptophysin and CSP (Supplementary figure 1A available at The EMBO Journal Online). Using ultra-thin cryosections of washed DPS (Supplementary figure 1B), we found that the Hsp90 in washed DPS was localized throughout the synapse in a pattern similar to the CSP-containing synaptic vesicle pool, suggesting that the distribution of Hsp90 was not simply limited to membranes forming the active zone, the site of vesicle fusion and Rab recycling.
Dissociation of αGDI from the Hsp90 chaperone complex is dependent on Ca2+-induced neurotransmitter release
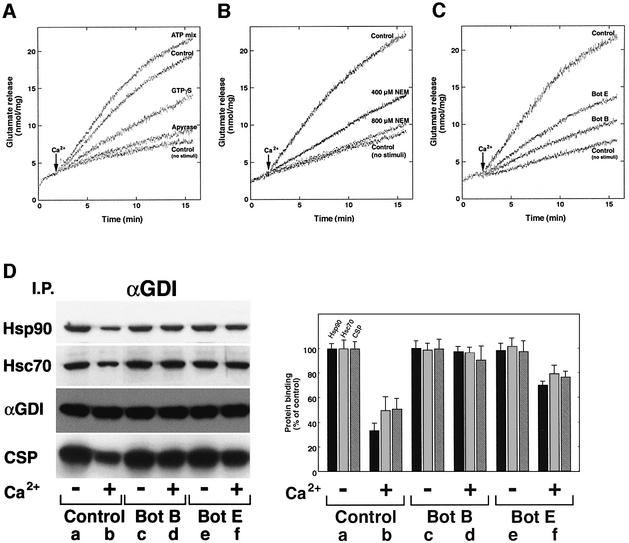
To begin to address the physiological role of the Hsp90-containing chaperone complex in αGDI activity and Rab recycling, we examined the relationship between complex formation and Ca2+-induced neurotransmitter release in DPS. Like streptolysin-permeabilized synaptosomes (Tandon et al., 1998), incubation of washed DPS in vitro resulted in the efficient (>50%) release of the neurotransmitter glutamate in a reaction that was dependent on the addition of exogenous Ca2+ and required ATP, as apyrase potently inhibited release (Figure 2A). Furthermore, release required GTP, as the presence of the non-hydrolyzable analog GTPγS (1 mM) inhibited Ca2+-dependent glutamate release from DPS by ∼40% compared with the control (Figure 2A). We also examined whether Ca2+-dependent transmitter release required SNAp-REceptors (SNAREs) involved in vesicle fusion (Sollner et al., 1993). Glutamate release was sensitive to N-ethylmaleimide (NEM), an inhibitor of SNARE function (Matveeva and Whiteheart, 1998) (Figure 2B). Moreover, when DPS were pretreated with recombinant light chain of either botulinum (Bot) toxin B or E at 30°C for 15 min, washed and assayed for glutamate release stimulated by Ca2+, the Bot B toxin inhibited glutamate release >80%, whereas Bot E inhibited release by ∼50%, similar to values reported previously for streptolysin-permeabilized synaptosomes (Tandon et al., 1998) (Figure 2C).
Fig. 2. Dissociation of αGDI from the Hsp90 chaperone complex is dependent on Ca2+-induced neurotransmitter release. (A–C) Requirement for energy and SNARE function for Ca2+-mediated glutamate release from DPS as measured spectrofluorometrically. (A) DPS were incubated in the absence or presence of the ATP-regenerating system (ATP mix), or in the presence of GTPγS or treated with 20 U of apyrase for 15 min at 30°C, washed and assayed for glutamate release in the absence of the ATP mix. (B) Where indicated, synaptosomes were pretreated with NEM before permeabilization. (C) DPS were treated with recombinant light chains of Bot B and E toxins for 15 min at 30°C, and washed prior to Ca2+-stimulated glutamate release. (D) Effects of Bot B and E on dissociation of αGDI from the Hsp90-containing chaperone complex. Untreated (lanes a and b), Bot B-treated (lanes c and d) or Bot E-treated DPS (lanes e and f) were incubated in the absence (a, c and e) or presence (b, d and f) of 10 µM Ca2+ and incubated for 15 min at 30°C. After Ca2+ stimulation, membranes were solubilized and αGDI-containing complexes were immunoprecipitated using the anti-αGDI mouse monoclonal antibody (left panel) and quantitated (right panel) by immunoblotting. Results shown in all panels are representative of at least three independent experiments.
To examine whether the assembly of the αGDI–Hsp90 complex was sensitive to Ca2+-induced neurotransmitter release, synaptosomes were incubated at 30°C for 15 min in the absence or presence of Ca2+, DPS were treated with the cleavable cross-linker, solubilized with lysis buffer and immunoprecipitated with αGDI-specific antibody. In the presence of Ca2+, we observed an ∼70% dissociation of Hsp90 from the αGDI–chaperone complex and an ∼50% reduction in the amount of Hsc70 and CSP recovered in the αGDI immunoprecipitate (Figure 2D, lanes a and b). The latter result was confirmed by immunoprecipitation with CSP-specific antibody (data not shown). While treatment with Bot E had only a small effect on complex dissociation (Figure 2D, lanes e and f), consistent with its modest effect on transmitter release, pretreatment with Bot B reduced the ability of αGDI to dissociate from the chaperone complex ∼30% compared with controls (Figure 2D, lanes c and d). These results suggest that Ca2+ stimulation triggers the dissociation of αGDI from the chaperone complex, a condition that favors Rab3A GTP hydrolysis, synaptic vesicle fusion and recycling of Rab3A by αGDI. Thus, function of the SNARE fusion machinery correlates with the ability of the αGDI– chaperone complex to disassemble.
The Hsp90 inhibitors GA and radicicol block both neurotransmitter release and αGDI release from the chaperone complex
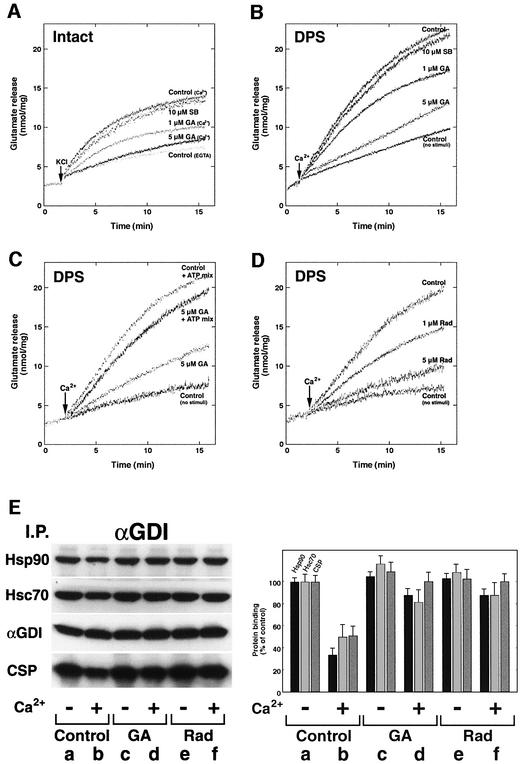
To further explore the role of Hsp90 in the αGDI/Rab3A cycle, we examined the effect of the Hsp90 inhibitor GA on Ca2+-induced neurotransmitter release using intact synaptosomes and DPS. GA has previously been shown to bind to the ATP site of Hsp90 and prevent interaction with its co-chaperone partners, thereby preventing activation of downstream signaling pathways (Richter and Buchner, 2001; Young et al., 2001). While pretreatment of synaptosomes with GA for 10 min at 30°C had no effect on their morphology, based on electron microscopy, and no effect on the total content of glutamate in synaptosomes (data not shown), it had a potent effect on Ca2+-induced neurotransmitter release. As shown in Figure 3, neurotransmitter release was blocked in a dose-dependent fashion from both intact synaptosomes (Figure 3A) and DPS (Figure 3B). As expected, inhibition could be competed by the addition of high levels of ATP (Figure 3C). Using morphometry (Supplementary figure 2), we observed a 40–60% reduction in the size of the synaptic vesicle pool in DPS in response to Ca2+, a result that was nearly completely inhibited in the presence of GA. To provide additional evidence that Hsp90 is involved in αGDI function, we used a structurally and chemically different Hsp90 inhibitor, the macrocyclic drug radicicol, known to bind to Hsp90 with high specificity and to inhibit its ATPase activity (Roe et al., 1999). We found that a 10 min pretreatment of synaptosomes with radicicol inhibited neurotransmitter release in a dose-dependent manner from both intact synaptosomes (data not shown) and DPS (Figure 3D).
Fig. 3. Dissociation of αGDI from the Hsp90-containing chaperone complex is prevented by GA and radicicol. (A–D) Sensitivity of glutamate release to Hsp90 inhibitors. (A) Intact synaptosomes were pretreated with GA, SB 203580 (SB) or DMSO (as a control) and then depolarized by the addition of KCl (30 mM). (B) Intact synaptosomes were pretreated with GA, SB or DMSO before permeabilization and then stimulated for glutamate release by the addition of Ca2+ (10 µM). (C) Intact synaptosomes were pretreated with GA or DMSO before permeabilization and in the absence or presence of an ATP-regenerating system (ATP mix) and stimulated for glutamate release by the addition of Ca2+. (D) Intact synaptosomes were pretreated with radicicol (Rad) or DMSO before permeabilization and then stimulated for glutamate release by the addition of Ca2+. (E) Effect of GA and radicicol on dissociation of αGDI from the Hsp90-containing chaperone complex. Untreated (lanes a and b), GA-treated (lanes c and d) or radicicol-treated DPS (lanes e and f) were incubated in the absence (a, c and e) or presence (b, d and f) of 10 µM Ca2+ and incubated for 15 min at 30°C. After stimulation, membranes were solubilized and αGDI-containing complexes were immunoprecipitated using the anti-αGDI mouse monoclonal antibody (left panel) and quantitated (right panel) by immunoblotting. The results shown for all panels are representative of at least three independent experiments.
To examine whether the effects of Hsp90 inhibitors on neurotransmitter release were associated with changes in the binding of αGDI with the chaperone complex, we examined the ability of αGDI to remain bound to Hsp90, Hsc70 and CSP in response to Ca2+-induced neurotransmitter release in the absence or presence of GA or radicicol. Both GA and radicicol significantly reduced the ability of αGDI to cycle through the chaperone system (Figure 3E), preventing normal αGDI function. These results suggest that Ca2+ may activate release of αGDI from the Hsp90-containing complex in a step coordinated with Rab3A recycling and neurotransmitter release.
Formation of the αGDI–Hsp90 complex is ATP dependent and requires the function of the αGDI mobile effector loop (MEL)
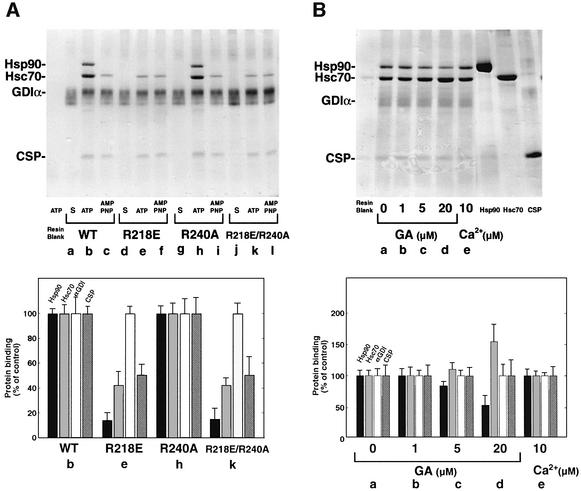
The assembly of steroid hormone receptor–Hsp90 complexes and activation of steroid binding activity by purified chaperones require ATP hydrolysis (Richter and Buchner, 2001; Young et al., 2001). Thus, we wanted to determine whether the assembly of αGDI with Hsp90, Hsc70 and CSP requires ATP. For this purpose, we attached recombinant His6-tagged αGDI protein to protein A– Sepharose beads (αGDI–beads). αGDI–beads were incubated with purified Hsp90, Hsc70 and recombinant CSP in the presence of an ATP-regenerating system. As shown in Figure 4A, αGDI incubated with Hsp90, Hsc70 and CSP in the presence of an ATP-regenerating system formed an αGDI–Hsp90 heterocomplex. In contrast, in the presence of the non-hydrolyzable analog AMP-PNP, heterocomplex formation was blocked. Given the ability of GA to interfere with normal αGDI function in DPS, the dose–response effect of GA on complex assembly was examined by SDS–PAGE followed by Coomassie Blue staining (Figure 4B). In a dose-dependent manner, GA reduced recovery of Hsp90 in a αGDI-containing complex by ∼50% and increased the recovery of Hsc70. Quantitative densitometry of stained protein bands suggested half-maximal inhibition of association with the complete complex at ∼7.5 µM GA. Thus, assembly in vitro of αGDI with Hsp90 requires the normal assembly pathway observed for steroid hormone receptors that may first involve recruitment to Hsc70/Hsp40 components (Richter and Buchner, 2001; Young et al., 2001). As Ca2+ did not affect the assembly of the complex in vitro (Figure 4B), we suggest that additional factors facilitate Ca2+-stimulated association events observed in DPS.
Fig. 4. Formation of αGDI–Hsp90 complexes is ATP dependent and requires the function of the αGDI MEL. (A) Requirement for MEL. Lanes a, d, g and j, His6-αGDI–beads (S) incubated with ATP-regenerating in buffer alone; lanes b, e, h and k, His6-αGDI–beads incubated with purified Hsp90, Hsc70 and recombinant CSP in the presence of the ATP-regenerating system; lanes c, f, i and l, His6-αGDI–beads incubated with purified Hsp90, Hsc70 and CSP in the presence of the ATP-regenerating system containing AMP-PNP instead of ATP. Bound proteins were separated by SDS–PAGE, stained with Coomassie Blue (upper panel) and quantitated (lower panel) by densitometry. (B) Dose-dependent effect of GA on the formation of the Hsp90–αGDI–chaperone complex. His6-αGDI–beads containing wild-type αGDI were incubated in the presence of the ATP-regenerating system with DMSO-treated chaperones (lane a), with the indicated concentration of GA (lanes b–d) treated chaperones (lane e) in the presence of Ca2+. Proteins used for reconstitution are indicated in the left three lanes. Bound proteins were separated by SDS–PAGE, stained with Coomassie Blue (upper panel) and quantitated (lower panel). The results shown in both panels are representative of at least three independent experiments.
To provide additional evidence for the specificity of the in vitro assembly reaction, we have previously demonstrated the importance of the Rab-binding domain (RBD) in domain I of αGDI for interaction with Rab (Schalk et al., 1996; Wu et al., 1998; Luan et al., 1999), and the domain II MEL for Rab recycling and interaction with RRF (Luan et al., 2000). To address the importance of Rab-binding and MEL function in αGDI–Hsp90 complex assembly in vitro, we generated αGDI mutants that inhibit separately the function of the key residues in the RBD (αGDI[R240A]) (Schalk et al., 1996; Wu et al., 1998; Luan et al., 1999) and MEL (αGDI[R218E]) (Luan et al., 2000), as well as a double mutant inhibiting the function of both domains (αGDI[R218E/R240A]). These αGDI mutants were bound to beads and incubated with Hsp90, Hsc70 and CSP in the presence of the ATP-regenerating system. Strikingly, we found that αGDI[R218E] and αGDI[R218E/R240A] mutants did not recruit Hsp90 and showed a marked reduction in binding of Hsc70 and CSP (Figure 4A). In contrast, wild-type αGDI and the αGDI[R240A] mutant showed equivalent interaction with Hsp90, Hsc70 and CSP. These results suggested that MEL is important for the specific association with the Hsp90 complex.
GA inhibits Rab3A extraction by endogenous αGDI
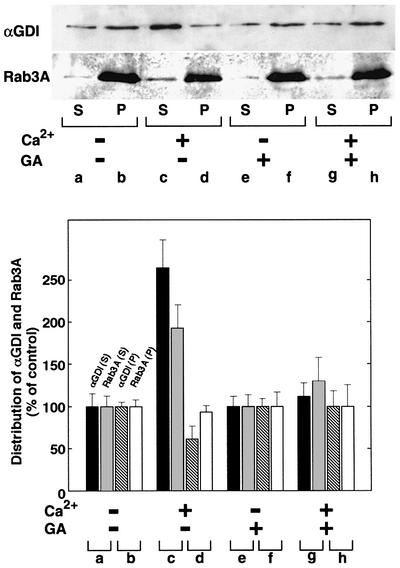
To determine whether the effects of GA directly affect the ability of endogenous αGDI to extract Rab3A from membranes during vesicle fusion, we examined the redistribution of Rab3A and αGDI in DPS in response to Ca2+ stimulation. In the absence of GA, we found that αGDI could efficiently retrieve Rab3A from the membrane in response to Ca2+-induced neurotransmitter release (Figure 5, lanes a–d). The stoichiometry of the recovered αGDI–Rab3A cytosolic complex was 1:1 based on quantitative immunoblotting (data not shown). In contrast, we found that GA potently inhibited Rab3A retrieval from membranes in response to Ca2+-induced neurotransmitter release (Figure 5, lanes e–h). These results suggest that GA may inhibit Ca2+-induced neurotransmitter release in response to a defect in the retrieval of Rab3A from membranes through the activity of an intermediate Hsp90-containing chaperone complex.
Fig. 5. GA inhibits Ca2+-induced Rab3A recycling. DPS preincubated in the absence (lanes a–d) or presence (lanes e–h) of GA (5 µM) were incubated for 15 min at 30°C in the absence (a, b, e and f) or presence (c, d, g and h) of Ca2+ (10 µM). Following incubation, membranes were collected by centrifugation, and the resulting soluble fraction was immunoprecipitated with anti-αGDI. The immunoprecipitate of the soluble fraction (S) (50% of total shown) and the solubilized membrane pellet fraction (P) (10% of total shown) (upper panel) were quantitated by immunoblotting with anti-αGDI and Rab3A antibodies as indicated (lower panel). The results shown are representative of at least three independent experiments.
GA inhibits the ability of exogenous GST–αGDI to extract Rab3A in vitro
To determine whether Hsp90 is directly required for αGDI function, we examined the ability of exogenous glutathione S-transferase (GST)-tagged αGDI to extract Rab3A from synaptic membranes (SM). Purified recombinant GST–αGDI was added to SM in the presence of GDP and Mg2+, conditions that facilitate physiological extraction of Rab proteins by αGDI. After incubation at 37°C for 20 min, the membranes were chilled on ice and recovered by centrifugation. Supernatant fractions were incubated with glutathione–Sepharose (GS) beads to recover GST–αGDI. As shown in Figure 6A, addition of increasing recombinant GST–αGDI extracted 80–90% of the membrane-bound Rab3A. We next tested the effects of GA and radicicol under conditions in which GST–αGDI would extract 50% of the Rab3A. After a 10 min preincubation of SM in the presence of an increasing concentration of GA/radicicol, the capacity of αGDI to extract Rab3A was reduced in a dose-dependent fashion (Figure 6B).

Fig. 6. Rab3A extraction is inhibited by Hsp90 inhibitors and mutation of MEL. (A) Rab3A extraction by GST–αGDI. GST–αGDI at the indicated concentrations was incubated with SM. Membranes were pelleted and the soluble fraction was incubated with GS–beads to recover the released GST–αGDI–Rab3A complex. The recovery of Rab3A associated with either GS–beads (50% of total) (S) (closed circles) or the membrane pellet (50% of total) (P) (closed triangles) was determined by quantitative immunoblotting. (B) Effects of GA and radicicol on Rab3A extraction by GST–αGDI. SM were pretreated with the indicated concentrations of drugs and subsequently incubated with 50 nM GST–αGDI. Recovery quantitated as in (A). (C) Effect of αGDI mutants on Rab3A extraction and binding to Rab3A. Upper panel: SM were incubated with the indicated concentration of the wild type or His6-αGDI mutants. Membranes were pelleted and the supernatant was incubated with cobalt beads to recover the His6-αGDI–Rab3A complex and recovery was determined by quantitative immunoblotting. Lower panel: CHAPS-extracted SM (to release prenylated Rab3A) were incubated with the indicated concentration of wild type or His6-αGDI mutants. Following centrifugation, His6-αGDI was recovered from the supernatant by incubation with cobalt beads and Rab3A binding determined by quantitative immunoblotting. (D) Effects of αGDI mutants on the glutamate release. DPS were preincubated with each of the various αGDI mutants (4 µM final concentration) for 15 min at 4°C, washed and assayed for Ca2+-stimulated glutamate release. The results shown in all panels are representative of at least three independent experiments.
Because GA has recently been shown to inhibit the MAP kinase cascade through its ability to disrupt interactions requiring Hsp90 chaperone activity (Richter and Buchner, 2001; Young et al., 2001) and also the fact that the MAP kinase p38 has been proposed to regulate GDI activity in the recycling of the small GTPase Rab5 found in the endocytic pathway (Cavalli et al., 2001), we tested the effects of the p38-specific inhibitor SB203580 on αGDI function. In contrast to the potent effects of SB203580 on Rab5 recycling (Cavalli et al., 2001), we observed no effect of SB203580 on the activity of GST– αGDI to extract Rab3A from SMs (see Supplementary figure 3), or as an inhibitor of neurotransmitter release (Figure 3A and B). We conclude that p38 is not a general regulator of αGDI function and that the Hsp90 chaperone complex found in the synapse may directly mediate an intermediate step in αGDI function in Rab retrieval to the cytosol.
To demonstrate the role of MEL in αGDI function in the synapse, we examined the effects of αGDI mutants on Rab3A retrieval and neurotransmitter release. We found that the αGDI[R218E], αGDI[R218E/R240A] and αGDI[R240A] mutants all had dramatically decreased ability to extract Rab3A compared with wild-type αGDI, as would be expected if a requirement for interaction with both Rab3A and the Hsp90 complex were necessary (Figure 6C, upper panel). In contrast, when we examined the ability of these proteins to bind Rab3A, we found that while αGDI[R218E] bound Rab3A in a comparable fashion to wild-type αGDI, the αGDI[R218E/R240A] and αGDI[R240A] mutants did not (Figure 6C, lower panel), consistent with previous results (Schalk et al., 1996; Wu et al., 1998; Luan et al., 1999, 2000). A requirement for both the RBD and MEL was also observed for the ability of αGDI to inhibit neurotransmitter release from DPS (Figure 6D). We found that inhibition of glutamate release by wild-type αGDI was saturable at 4 µM αGDI, resulting in 60–70% inhibition. As expected, the αGDI[R240A] mutant reduced inhibition by 60–70% (Figure 6D). Whereas the αGDI[R218E] MEL substitution reduced inhibition by wild-type αGDI by 25–30%, the αGDI[R218E/R240A] double mutant reduced inhibition by ∼90%. These results are consistent with the conclusion that the MEL mutant has reduced interaction with the Rab recycling machinery compared with wild-type αGDI, and that both RBD and MEL contribute to neurotransmitter release through Hsp90.
Discussion
The dynamic and sequential assembly, rearrangement and disassembly of protein complexes are now a defining hallmark of current models of vesicle traffic as the molecular events dictating membrane transport are cyclical in nature and highly regulated. It is, therefore, not surprising that molecular chaperones emerge as important factors. Several chaperone systems and their client substrates are essential for normal function of the synaptic vesicle cycle during neurotransmitter release (Zinsmaier and Bronk, 2001). The NSF/SNAP-chaperone machine mediates the activity of the general SNARE system modulating vesicle fusion (Sollner et al., 1993); the auxillin/Hsc70 complex regulates clathrin uncoating pathways involved in synaptic vesicle recycling (Ungewickell et al., 1995; Morgan et al., 2001). More recently, a CSP/Hsc70/SGT complex has been proposed to carry out an unknown function in neurotransmitter release (Bronk et al., 2001; Tobaben et al., 2001). We have now identified a fourth molecular chaperone system containing Hsp90, Hsc70 and CSP that modulates Rab3A GTPase recycling through the activity of αGDI. Hsp90 is a specialized chaperone that is now best recognized for its role in stabilizing intermediates of client molecules involved in signaling pathways (Richter and Buchner, 2001; Young et al., 2001). Our results lead us to suggest a novel role for Hsp90 in regulation of the client molecule αGDI in Rab3A function leading to vesicle targeting and fusion at the synapse.
A chaperone complex containing Hsp90 and CSP modulates αGDI function
We have provided four independent lines of evidence that Hsp90 is likely to play a critical role in the αGDI cycle of Rab3A in the synapse: (i) co-immunoprecipitation of endogenous αGDI with Hsp90; (ii) the ability of GA/radicicol, inhibitors of Hsp90 chaperone complex function (Richter and Buchner, 2001; Young et al., 2001), to prevent recycling of Rab3A by endogenous αGDI; (iii) the ability of GA/radicicol to prevent the ability of exogenous GST–αGDI to retrieve Rab3A from synaptic membranes under physiological conditions; and (iv) the specific role of MEL to facilitate direct interaction of αGDI with the Hsp90-containing complex. Moreover, we found substantial correlative results between the ability of GA/radicicol to disrupt Rab3A recycling, interfere with αGDI association with the Hsp90 complex and inhibit Ca2+-dependent neurotransmitter release.
In order to determine the molecular partners involved in αGDI function in Rab3A recycling, we utilized synaptosomes permeabilized with digitonin. In contrast to the variable activity of different preparations of streptolysin used to perforate synaptosomes in previous studies (Tandon et al., 1998), we have found that the digitonin permeabilization technique described herein is highly reproducible and allows the measurement of neurotransmitter release in vitro in real time with high efficiency. This technique should provide a useful new tool to study the role of other components mediating neurotransmitter release.
Using DPS, we identified a membrane-associated detergent soluble pool that, in the absence of cross-linker, showed efficient binding of αGDI to Hsp90. This interaction was strengthened in the presence of cross-linker to a near stoichiometric complex. Moreover, in the presence of cross-linker, we recovered Hsc70 and CSP, a synaptic vesicle-associated DnaJ domain-containing protein (Chamberlain and Burgoyne, 2000), as well as additional proteins of unknown identity. These results suggest that a major component of a membrane-associated complex containing αGDI is likely to include Hsp90 tethered to synaptic vesicles, through CSP/Hsc70 and other factors. The potential association of the αGDI with the Hsp90 chaperone complex and with synaptic vesicles was supported by subfractionation, and by biochemical studies in which these interactions were demonstrated using purified, recombinant proteins. This reaction specifically involved MEL, a key effector region in domain II of αGDI required for Rab retrieval through the RRF (Luan et al., 2000).
Hsp90 complexes are now recognized to contain a number of facilitating co-chaperones that modulate function (Buchner, 1999). The potential importance of both Hsc70 and CSP in a vesicle targeting/fusion complex is supported by separate studies in which CSP (Ranjan et al., 1998) and Hsc70 (Bronk et al., 2001) have been suggested to play important roles in exocytosis and modulation of Ca2+ sensitivity. In contrast, we have been unable to detect the co-chaperones Hop and Hip involved in steroid hormone signaling (Richter and Buchner, 2001; Young et al., 2001; T.Sakisaka and W.E.Balch, unpublished observations). These proteins contain tetratricopeptide repeat-containing domains (TPRs), a motif that interacts with Hsp90 and Hsp/Hsc70. The lack of Hop or Hip is consistent with the fact that TPR-containing proteins are generally specific for a particular Hsp90-regulated signaling pathway (Blatch and Lassle, 1999). Interestingly, CSP and Hsc70 have recently been shown to form a molecular complex of unknown function on synaptic vesicles that binds the TPR-containing protein SGT, a CSP-interacting protein that has been proposed to function as a co-chaperone like Hip (Hohfeld et al., 1995; Tobaben et al., 2001). Thus, there remains the possibility currently under investigation that SGT and/or other unknown proteins are part of the αGDI–Hsp90 chaperone complex that facilitates Rab3A recycling.
Regulation of Rab3A recycling and neurotransmitter release in response to GA
While the identification of a chaperone complex containing αGDI represents an important step forward in defining novel binding partners participating in membrane association and Rab recycling, we have provided evidence that this complex may be physiologically important during neurotransmitter release. For this purpose, we made use of both GA and radicicol, specific inhibitors of Hsp90 function. GA/radicicol have been demonstrated to tightly bind to the ATP site of Hsp90. Binding of GA/radicicol is currently thought to prevent assembly of the Hsp90-containing complexes from intermediate Hsc70/Hsp40-containing complexes and thereby block the activity of Hsp90-regulated signaling pathways (Whitesell et al., 1994; Prodromou et al., 1997; Stebbins et al., 1997). We provided evidence that both drugs inhibited αGDI activity in Rab3A extraction. Thus, the effect of GA/adicicol on Rab3A recycling may be to inhibit the Hsp90 chaperone-mediated function of the client molecule αGDI.
Of particular interest is the fact that GA is also known to be an inhibitor of a number of kinase-regulated cascades through its effect on Hsp90 co-chaperone activity (Richter and Buchner, 2001; Young et al., 2001). Recently, Gruenberg and colleagues (Cavalli et al., 2001) have demonstrated that the MAP kinase p38, a serine protein kinase sensitive to the inhibitor SB203580, is involved in the regulation of GDI function in recycling of the Rab5 GTPase directing endosomal trafficking (Cavalli et al., 2001). p38 can phosphorylate Ser121 of αGDI, a residue adjacent to the MEL region that directs interaction with RRF (Luan et al., 2000). In contrast to the potential role of p38 in Rab5 recycling, we found no effect of SB203580 on the ability of αGDI to recycle Rab3A in synaptosomes, suggesting that the pathways regulating GDI function may differ for different Rab GTPases.
In addition to the effects of GA/adicicol on αGDI-mediated Rab3A recycling, a correlation was observed between the effects of these drugs on inhibition of Rab3A retrieval from membranes and their ability to inhibit Ca2+-dependent glutamate release. Disruption of MEL reduced the ability of wild-type αGDI to promote Rab3A recycling, interact with Hsp90 and interfere with neurotransmitter release in vitro. Consistent with these effects on intact and DPS, we have also observed a partial effect of GA on neurotransmitter release from culture hippocampal neurons (C.Stevens and W.E.Balch, unpublished observations). While further work is necessary to elucidate the basis for these results, one possibility is that Hsp90 facilitates an unknown step in addition to the αGDI-mediated Rab3A recycling, triggering vesicle targeting/fusion. Alternatively, and consistent with our evidence, a second possibility is that by interfering with the cycling of αGDI through the Hsp90-containing chaperone complex, a Rab3A-containing priming complex (Castillo et al., 2002; Schoch et al., 2002) is unable to efficiently mature in response to Rab3A hydrolysis. Thus, the presence of Rab-GDP may have an unsuspected effect on protein interactions dictating downstream targeting/fusion events related to synaptic plasticity (long-term potentiation; LTP). Our results are consistent with the current view that Rab3A regulates the availability of synaptic vesicles for membrane fusion (Geppert and Sudhof, 1998).
A model for the αGDI–Hsp90 chaperone cycle in Rab3A recycling
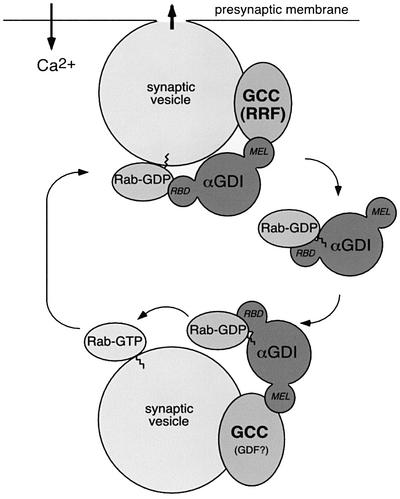
αGDI plays a crucial role in the recycling Rab GTPases from membranes through its ability to interact with hydrophobic geranylgeranyl lipid groups attached to their C-terminus. Structural analyses of αGDI (Schalk et al., 1996; Luan et al., 2000; W.E.Balch, unpublished observations) suggest that a hydrophobic pocket normally reserved for interaction with Rab prenyl groups may need to be protected from the hydrophilic solvent. Therefore, in the first step of the GDI cycle (Figure 7), delivery of Rab3A to synaptic vesicles through MEL by the Hsp90-containing chaperone complex may facilitate both transfer of lipid to the bilayer and stabilization of αGDI (by analogy to steroid hormone Hsp90 chaperone cycles). At this step, the Hsp90-containing complex on synaptic vesicles could potentially function as the proposed GDI displacement factor (GDF) (Dirac-Svejstrup et al., 1997). In subsequent steps, our evidence is consistent with the hypothesis that the chaperone complex is used to coordinate the function of αGDI with extraction of Rab following vesicle fusion in a multi-step pathway involving both the RBD found in domain I and the lower domain II containing MEL (Figure 7). Thus, in the case of the synapse, we suggest that RRF, the proposed membrane-associated component that facilitates Rab recycling (Luan et al., 1999, 2000), is the αGDI–Hsp90-containing chaperone complex. The coupling of synaptic vesicle targeting/fusion to the Rab3A recycling pathway through an αGDI–Hsp90 chaperone complex is consistent with changes in synaptic plasticity observed in the αGDI knock-out mouse strain (Ishizaki et al., 2000). Moreover, it emphasizes the importance of αGDI in human disease whereby mutations triggering decreased ability of αGDI to extract Rab3A lead to non-syndromic mental retardation (D’Adamo et al., 1998).
Fig. 7. A working model for αGDI–Hsp90 chaperone interactions in Rab3A recycling. The αGDI–Rab3A cytosolic complex delivers Rab3A to the synaptic vesicle. On the synaptic vesicle, the αGDI–Hsp90 chaperone complex (GCC) may function as GDF to promote Rab3A dissociation. Following Rab3A release, αGDI is retained on the membrane by GCC through the MEL. During vesicle fusion associated with neurotransmitter release, GCC functions as RRF (Luan et al., 2000). At this step, Rab3A–GDP is extracted from the membrane by sequential steps involving both the RBD of αGDI and the chaperone activity of GCC through MEL. Hsp90 may facilitate the transfer of prenyl groups from the lipid bilayer to αGDI by analogy to steroid hormone receptor pathways.
Materials and methods
Antibodies
Anti-αGDI mouse monoclonal antibody (Cl 81.2) was a kind gift from Dr R.Jahn (The Max-Plank Institute for Biophysical Chemistry, Göttingen, Germany). Recombinant Bot B and E toxin light chains were kindly provided by Dr R.Stevens (The Scripps Research Institute, La Jolla, CA). Other materials are described in Supplementary figure 1.
Preparation of permeabilized synaptosomes
Intact synaptosomes were suspended at a protein concentration of 1 mg/ml in buffer A [50 mM HEPES–KOH pH 7.2, 100 mM KCl, 5 mM MgCl2, 2 mM EGTA, 1 mM DTT and complete protease inhibitor cocktail (Sigma)] and incubated with 50 µg/ml digitonin for 10 min on ice. Digitonin-treated synaptosomes were washed with buffer A and resuspended in buffer B (50 mM HEPES–KOH pH 7.2, 40 mM KCl, 5 mM MgCl2, 2 mM EGTA, 1 mM DTT and complete protease inhibitor cocktail) for 10 min at 4°C to allow for washout of soluble proteins.
Cross-linking of synaptosome proteins
Intact synaptosomes were resuspended at a protein concentration of 1 mg/ml in buffer C (20 mM HEPES–NaOH pH 7.4, 140 mM NaCl, 5 mM KCl, 5 mM NaHCO3, 1.2 mM Na2HPO4, 1 mM MgCl2 and 10 mM glucose) and pre-warmed for 5 min at 30°C. Cross-linker (dissolved in DMSO) was then added to yield a final concentration of 1 mM (1% v/v of the solvent). After incubation with stirring for 15 min at 30°C, 50 mM Tris–HCl at pH 7.4 was added as a quencher, followed by osmotic shock. Synaptosomes were subsequently fractionated by differential centrifugation as described previously (Stahl et al., 1996) and αGDI-interacting proteins recovered by immunoprecipitation.
Solubilization and isolation of αGDI complexes from synaptosome membranes
DPS were prepared as described in Supplementary figure 2, resuspended in buffer A and solubilized with 1% Triton X-100 for 1 h. Insoluble material was removed by centrifugation, the supernatant diluted with two volumes of buffer A and the diluted sample (1 ml containing 1 mg of protein) immunoprecipitated by the addition of 15 µg of the anti-αGDI covalently coupled to 50 µl of protein A–Sepharose 4B beads (Pharmacia). A mouse immunoglobulin G was used as a non-specific control. For CSP immunoprecipitation, the detergent extract was incubated with 20 µg of an anti-CSP rabbit polyclonal antibody covalently coupled to 50 µl of protein A–Sepharose 4B beads.
Glutamate release assay
The glutamate release assay was performed using enzyme-linked fluorescent detection of released glutamate (Nicholls and Sihra, 1986; Supplementary figure 2).
Release of Rab3A from DPS
DPS were resuspended with buffer B (1 mg of protein per 1.5 ml of buffer). After incubating with stirring for 5 min at 30°C, DPS were stimulated with 10 µM CaCl2, incubated for an additional 15 min, and membranes were collected by centrifugation. The supernatant was incubated with the anti-αGDI mouse monoclonal antibody. Fifty percent of the immunoprecipitate and 50 µl of the membrane pellet were resuspended in a total of 500 µl of SDS sample buffer, separated using SDS–PAGE and immunoblotted with anti-αGDI and anti-Rab3A rabbit polyclonal antibodies.
Retrieval of Rab3A by GST–αGDI and His6-αGDI
Twenty-five micrograms of SM and the indicated concentration of GST–αGDI (see Results) were supplemented with 250 µM GDP in a 200 µl final volume of buffer A. After incubation for 20 min at 37°C, the samples were chilled on ice and membranes pelleted by centrifugation for 20 min at 250 000 g. The membrane pellet was resuspended in a total of 80 µl of SDS sample buffer. The supernatant was incubated with 50 µl of glutathione–Sepharose (GS) beads for 1 h at 4°C. Fifty percent of GS–bead recovered αGDI and the 40 µl of the solubilized membrane pellet were analyzed using SDS–PAGE and immunoblotting with anti-αGDI and Rab3A rabbit polyclonal antibodies. Where indicated in the Results, the SM were preincubated with either GA, SB 203580 and DMSO (control) for 10 min at 30°C prior to incubation with GST–αGDI. His6-αGDI was incubated with cobalt beads (TALON metal affinity beads; Clontech) to recover the His6-αGDI–Rab3A complex.
Binding of wild-type and mutant His6-αGDI to Rab3A
Twenty-five micrograms of 0.6% CHAPS-extracted SM (diluted below the critical micelle concentration at 0.18%) and the indicated concentration of His6-αGDI (see Results) were supplemented with 250 µM GDP in a 200 µl final volume of buffer A supplemented with CHAPS (final concentration 0.18%). After incubation for 20 min at 37°C, the samples were chilled on ice, centrifuged for 20 min at 250 000 g, and the supernatant incubated with 50 µl of cobalt beads for 1 h at 4°C. Cobalt bead-recovered His6-αGDI (50% of total) was analyzed by SDS–PAGE and immunoblotting with the Rab3A rabbit polyclonal antibody.
αGDI–chaperone complex reconstitution
Purified His6-αGDI was incubated with protein A–Sepharose 4B beads covalently coupled with the αGDI monoclonal antibody to generate αGDI–beads (0.5 µg of His6-αGDI per 50 µl of beads). αGDI–beads were washed with 0.5 M KCl and 5 mM ATP for 30 min at 4°C to remove unbound protein. Subsequently, αGDI–beads were incubated with 20 µg of purified Hsp90 (Stressgen), 20 µg of purified Hsc70 from bovine brain prepared as described previously (Newmyer and Schmid, 2001) and 2 µg of recombinant Escherichia coli-expressed His6-CSP (pQE30-CSP vector; a kind gift from Dr R.Burgoyne, University of Liverpool, Liverpool, UK) in buffer A containing 20 mM sodium molybdate and the ATP-regenerating system. In the presence of AMP-PNP, an ATP-regenerating system with AMP-PNP instead of ATP was used. The assay mixtures were incubated for 30 min at 30°C with shaking. At the end of the incubation, the pellets were washed three times with buffer A containing 20 mM sodium molybdate. Complex formation was examined by SDS–PAGE followed by Coomassie Blue staining. Where indicated, GA was added at the indicated concentration and incubated for 10 min at 30°C prior to the addition of αGDI–beads.
Mass spectrometry
Protein bands were excised from the colloidal Coomassie Blue (Sigma)-stained SDS–PAGE gel and digested in-gel with trypsin and analyzed by the TSRI Mass Spectroscopy Core Facility.
Supplementary data
Supplementary data are available at The EMBO Journal Online.
Acknowledgments
Acknowledgements
This work was supported by a grant to W.E.B. from National Institutes of Health (NIH) (GN33301) and Core B of the National Cancer Institute (CA58689). T.S. was supported by a fellowship from the Human Frontier Science Program Organization. This is manuscript 15048-CB from TSRI.
References
- Alory C. and Balch,W.E. (2001) Organization of the Rab-GD/HM superfamily: the functional basis for choroideremia disease. Traffic, 2, 532–543. [DOI] [PubMed] [Google Scholar]
- Bittner M.A. and Holz,R.W. (1992) Kinetic analysis of secretion from permeabilized adrenal chromaffin cells reveals distinct components. J. Biol. Chem., 267, 16219–16225. [PubMed] [Google Scholar]
- Blatch G.L. and Lassle,M. (1999) The tetratricopeptide repeat: a structural motif mediating protein–protein interactions. BioEssays, 21, 932–939. [DOI] [PubMed] [Google Scholar]
- Bronk P., Wenniger,J.J., Dawson-Scully,K., Guo,X., Hong,S., Atwood,H.L. and Zinsmaier,K.E. (2001) Drosophila Hsc70-4 is critical for neurotransmitter exocytosis in vivo. Neuron, 30, 475–488. [DOI] [PubMed] [Google Scholar]
- Buchner J. (1999) Hsp90 & Co.—a holding for folding. Trends Biochem. Sci., 24, 136–141. [DOI] [PubMed] [Google Scholar]
- Calakos N. and Scheller,R.H. (1996) Synaptic vesicle biogenesis, docking and fusion: a molecular description. Physiol. Rev., 76, 1–29. [DOI] [PubMed] [Google Scholar]
- Castillo P.E., Schoch,S., Schmitz,F., Sudhof,T.C. and Malenka,R.C. (2002) RIM1a is required for presynaptic long-term potentiation. Nature, 415, 327–330. [DOI] [PubMed] [Google Scholar]
- Cavalli V., Vilbois,F., Corti,M., Marcote,M.J., Tamura,K., Karin,M., Arkinstall,S. and Gruenberg,J. (2001) The stress-induced MAP kinase p38 regulates endocytic trafficking via the GDI:Rab5 complex. Mol. Cell, 7, 421–432. [DOI] [PubMed] [Google Scholar]
- Chamberlain L.H. and Burgoyne,R.D. (2000) Cysteine-string protein: the chaperone at the synapse. J. Neurochem., 74, 1781–1789. [DOI] [PubMed] [Google Scholar]
- D’Adamo P. et al. (1998) Mutations in GDI1 are responsible for X-linked non-specific mental retardation. Nat. Genet., 19, 134–139. [DOI] [PubMed] [Google Scholar]
- Desnoyers L., Anant,J.S. and Seabra,M.C. (1996) Geranylgeranylation of Rab proteins. Biochem. Soc. Trans., 24, 699–703. [DOI] [PubMed] [Google Scholar]
- Dirac-Svejstrup A.B., Sumizawa,T. and Pfeffer,S.R. (1997) Identification of a GDI displacement factor that releases endosomal Rab GTPases from Rab-GDI. EMBO J., 16, 465–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geppert M. and Sudhof,T.C. (1998) RAB3 and synaptotagmin: the yin and yang of synaptic membrane fusion. Annu. Rev. Neurosci., 21, 75–95. [DOI] [PubMed] [Google Scholar]
- Hohfeld J., Minami,Y. and Hartl,F.U. (1995) Hip, a novel cochaperone involved in the eukaryotic Hsc70/Hsp40 reaction cycle. Cell, 83, 589–598. [DOI] [PubMed] [Google Scholar]
- Ishizaki H. et al. (2000) Role of rab GDP dissociation inhibitor α in regulating plasticity of hippocampal neurotransmission. Proc. Natl Acad. Sci. USA, 97, 11587–11592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luan P., Balch,W.E., Emr,S.D. and Burd,C.G. (1999) Molecular dissection of guanine nucleotide dissociation inhibitor function in vivo. Rab-independent binding to membranes and role of Rab recycling factors. J. Biol. Chem., 274, 14806–14817. [DOI] [PubMed] [Google Scholar]
- Luan P., Heine,A., Moyer,B.D., Greasely,S.E., Kuhn,P., Balch,W.E. and Wilson,I.A. (2000) A new functional domain of guanine nucleotide dissociation inhibitor (α-GDI) involved in Rab recycling. Traffic, 1, 270–281. [DOI] [PubMed] [Google Scholar]
- Matveeva E. and Whiteheart,S.W. (1998) The effects of SNAP/SNARE complexes on the ATPase of NSF. FEBS Lett., 435, 211–214. [DOI] [PubMed] [Google Scholar]
- Morgan J.R., Prasad,K., Jin,S., Augustine,G.J. and Lafer,E.M. (2001) Uncoating of clathrin-coated vesicles in presynaptic terminals. Roles for hsc70 and auxilin. Neuron, 32, 289–300. [DOI] [PubMed] [Google Scholar]
- Newmyer S.L. and Schmid,S.L. (2001) Dominant-interfering Hsc70 mutants disrupt multiple stages of the clathrin-coated vesicle cycle in vivo. J. Cell Biol., 152, 607–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls D.G. and Sihra,T.S. (1986) Synaptosomes possess an exocytotic pool of glutamate. Nature, 321, 772–773. [DOI] [PubMed] [Google Scholar]
- Pereira-Leal J.B. and Seabra,M.C. (2001) Evolution of the Rab family of small GTP-binding proteins. J. Mol. Biol., 313, 889–901. [DOI] [PubMed] [Google Scholar]
- Prodromou C., Roe,S.M., O’Brien,R., Ladbury,J.E., Piper,P.W. and Pearl,L.H. (1997) Identification and structural characterization of the ATP/ADP-binding site in the Hsp90 molecular chaperone. Cell, 90, 65–75. [DOI] [PubMed] [Google Scholar]
- Ranjan R., Bronk,P. and Zinsmaier,K.E. (1998) Cysteine string protein is required for calcium secretion coupling of evoked neurotransmission in Drosophila but not for vesicle recycling. J. Neurosci., 18, 956–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter K. and Buchner,J. (2001) Hsp90: chaperoning signal transduction. J. Cell Physiol., 188, 281–290. [DOI] [PubMed] [Google Scholar]
- Roe S.M., Prodromou,C., O’Brien,R., Ladbury,J.E., Piper,P.W. and Pearl,L.H. (1999) Structural basis for inhibition of the Hsp90 molecular chaperone by the antitumor antibiotics radicicol and geldanamycin. J. Med. Chem., 42, 260–266. [DOI] [PubMed] [Google Scholar]
- Schalk I., Zeng,K., Wu,S.-K., Stura,E., Matteson,J., Huang,M., Tandon,A., Wilson,I. and Balch,W.E. (1996) Structure and mutational analysis of Rab GDP-dissociation inhibitor. Nature, 381, 42–48. [DOI] [PubMed] [Google Scholar]
- Schoch S., Castillo,P.E., Jo,T., Mukherjee,K., Geppert,M., Wang,Y., Schmitz,F., Malenka,R.C. and Sudhof,T.C. (2002) RIM1a forms a protein scaffold for regulating neurotransmitter release at the active zone. Nature, 415, 321–326. [DOI] [PubMed] [Google Scholar]
- Sollner T., Whiteheart,S.W., Brunner,M., Erdjument-Bromage,H., Geromanos,S., Tempst,P. and Rothman,J.E. (1993) SNAP receptors implicated in vesicle targeting and fusion. Nature, 362, 318–324. [DOI] [PubMed] [Google Scholar]
- Stahl B., Chou,J.H., Li,C., Sudhof,T.C. and Jahn,R. (1996) Rab3 reversibly recruits rabphilin to synaptic vesicles by a mechanism analogous to raf recruitment by Ras. EMBO J., 15, 1799–1809. [PMC free article] [PubMed] [Google Scholar]
- Stebbins C.E., Russo,A.A., Schneider,C., Rosen,N., Hartl,F.U. and Pavletich,N.P. (1997) Crystal structure of an Hsp90–geldanamycin complex: targeting of a protein chaperone by an antitumor agent. Cell, 89, 239–250. [DOI] [PubMed] [Google Scholar]
- Takai Y., Sasaki,T. and Matozaki,T. (2001) Small GTP-binding proteins. Physiol. Rev., 81, 153–208. [DOI] [PubMed] [Google Scholar]
- Tandon A., Bannykh,S., Kowalchyk,J.A., Banerjee,A., Martin,T.F. and Balch,W.E. (1998) Differential regulation of exocytosis by calcium and CAPS in semi-intact synaptosomes. Neuron, 21, 147–154. [DOI] [PubMed] [Google Scholar]
- Tobaben S., Thakur,P., Fernandez-Chacon,R., Sudhof,T.C., Rettig,J. and Stahl,B. (2001) A trimeric protein complex functions as a synaptic chaperone machine. Neuron, 31, 987–999. [DOI] [PubMed] [Google Scholar]
- Ungewickell E., Ungewickell,H., Holstein,S.E., Lindner,R., Prasad,K., Barouch,W., Martin,B., Greene,L.E. and Eisenberg,E. (1995) Role of auxilin in uncoating clathrin-coated vesicles. Nature, 378, 632–635. [DOI] [PubMed] [Google Scholar]
- Whitesell L., Mimnaugh,E.G., De Costa,B., Myers,C.E. and Neckers,L.M. (1994) Inhibition of heat shock protein HSP90–pp60v–src heteroprotein complex formation by benzoquinone ansamycins: essential role for stress proteins in oncogenic transformation. Proc. Natl Acad. Sci. USA, 91, 8324–8328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S.K., Zeng,K., Wilson,I.A. and Balch,W.E. (1996) Structural insights into the function of the Rab GDI superfamily. Trends Biochem. Sci., 21, 472–476. [DOI] [PubMed] [Google Scholar]
- Wu S.K., Luan,P., Matteson,J., Zeng,K., Nishimura,N. and Balch,W.E. (1998) Molecular role for the Rab binding platform of guanine nucleotide dissociation inhibitor in endoplasmic reticulum to Golgi transport. J. Biol. Chem., 273, 26931–26938. [DOI] [PubMed] [Google Scholar]
- Young J.C., Moarefi,I. and Hartl,F.U. (2001) Hsp90: a specialized but essential protein-folding tool. J. Cell Biol., 154, 267–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerial M. and McBride,H. (2001) Rab proteins as membrane organizers. Nat. Rev. Mol. Cell Biol., 2, 107–119. [DOI] [PubMed] [Google Scholar]
- Zinsmaier K.E. and Bronk,P. (2001) Molecular chaperones and the regulation of neurotransmitter exocytosis. Biochem. Pharmacol., 62, 1–11. [DOI] [PubMed] [Google Scholar]