Abstract
Transport of secretory proteins out of the endoplasmic reticulum (ER) is mediated by vesicles generated by the COPII coat complex. In order to understand how cargo molecules are selected by this cytoplasmic coat, we investigated the functional role of the Sec24p homolog, Lst1p. We show that Lst1p can function as a COPII subunit independently of Sec24p on native ER membranes and on synthetic liposomes. However, vesicles generated with Lst1p in the absence of Sec24p are deficient in a distinct subset of cargo molecules, including the SNAREs, Bet1p, Bos1p and Sec22p. Consistent with the absence of any SNAREs, these vesicles are unable to fuse with Golgi membranes. Furthermore, unlike Sec24p, Lst1p fails to bind to Bet1p in vitro, indicating a direct correlation between cargo binding and recruitment into vesicles. Our data suggest that the principle role of Sec24p is to discriminate cargo molecules for incorporation into COPII vesicles.
Keywords: cargo packaging/COPII/ER/transport vesicle/yeast
Introduction
In eukaryotic cells, transport between the compartments of the secretory pathway is mediated by vesicles that ensure vectorial transfer of cargo molecules. Vesicles are generated on a donor membrane by recruitment of cytoplasmic coat proteins that deform the membrane to drive vesicle formation, while also recruiting cargo destined for forward transport. This specific recruitment is thought to occur as a result of direct interactions between sorting signals present on cytosolic domains of cargo molecules and subunits of the coat machinery (Schekman and Orci, 1996). The interplay between sorting signal and coat protein has been best characterized for the endocytic signals of various plasma membrane proteins that interact with the clathrin adaptor complex, AP2 (Kirchhausen, 1999). Recent structural characterization of the AP2 complex suggests that phosphorylation of the µ2 subunit causes a conformational change that allows binding to a canonical tyrosine-based endocytic signal (Collins et al., 2002). In contrast to this relatively well-defined sorting system, considerably less is known about the sorting signals and their recognition at the level of forward transport out of the endoplasmic reticulum (ER).
Export of proteins out of the ER is driven by the COPII coat, composed of the small G-protein, Sar1p, the Sec23/24p complex and the Sec13/31p complex (Barlowe et al., 1994). These three coat components form the basic machinery required for budding and cargo sorting on ER membranes as revealed by studies using synthetic liposomes in place of native membranes (Matsuoka et al., 1998a,b). Sar1p appears to be the master regulator of COPII coat formation, and Sec23p is the GTPase-activating protein (GAP) for Sar1p (Yoshihisa et al., 1993); however, the mechanism of cargo recruitment by the COPII coat remains to be fully understood.
Several lines of evidence implicate the Sec24p subunit in the process of cargo recruitment. Cargo-containing ‘pre-budding complexes’ can be isolated from ER membranes after addition of Sar1p and Sec23/24p, suggesting that this partial coat can act to recruit proteins destined for incorporation into vesicles (Aridor et al., 1998; Kuehn et al., 1998). Furthermore, the Sec23/24p complex, and in some cases Sec24p alone, has been shown to bind to cytoplasmic domains of a variety of cargo molecules. For example, the v-SNARE, Bet1p, binds to Sec23/24p in a Sar1p-dependent manner (Springer and Schekman, 1998), whereas the Golgi t-SNARE, Sed5p, binds to Sec24p independently of both Sec23p and Sar1p (Peng et al., 1999). Importantly, two recent reports describe the interaction between Sec23/24p and cargo proteins containing di-acidic sorting motifs (Aridor et al., 2001; Votsmeier and Gallwitz, 2001). Mutation of the di-acidic motif abrogates Sec23/24p binding and reduces incorporation into ER-derived vesicles, suggesting a direct relationship between the ability of a cargo molecule to bind Sec23/24p and its incorporation into a budding vesicle. One limitation of many studies that examine the interaction between COPII proteins and putative sorting signals lies in the use of cytosol to demonstrate binding efficiency. Thus, direct interaction between a sorting signal and a distinct subunit of the COPII coat has only been demonstrated in a minority of cases (Springer and Schekman, 1998; Peng et al., 1999).
Additional evidence for the role of Sec24p in cargo selection comes from the observation that the Sec24p homolog, Lst1p, is required for efficient transport of the plasma membrane H+-ATPase, Pma1p (Roberg et al., 1999; Shimoni et al., 2000). Mutant cells lacking Lst1p accumulate Pma1p in the ER, resulting in a pH-sensitive growth phenotype (Roberg et al., 1999). Furthermore, using in vitro vesicle budding assays, Shimoni et al. (2000) demonstrated that Lst1p is necessary for efficient packaging of Pma1p into COPII vesicles. Together, these data suggest that Lst1p functions as a cargo-specific adaptor protein that facilitates uptake of Pma1p into COPII vesicles. Although Lst1p is found in complex with Sec23p (Roberg et al., 1999; Shimoni et al., 2000) and appears to be able to form COPII vesicles, albeit inefficiently (Shimoni et al., 2000), overexpression of Lst1p cannot compensate for loss of Sec24p function (Peng et al., 2000). We now report conditions that allow the Lst1p subunit to substitute for Sec24p but that result in a dramatically altered capture of cargo proteins.
Results
Lst1p forms a COPII coat on synthetic liposomes
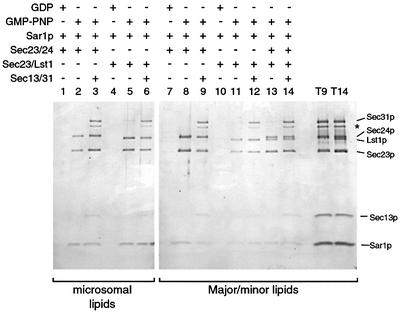
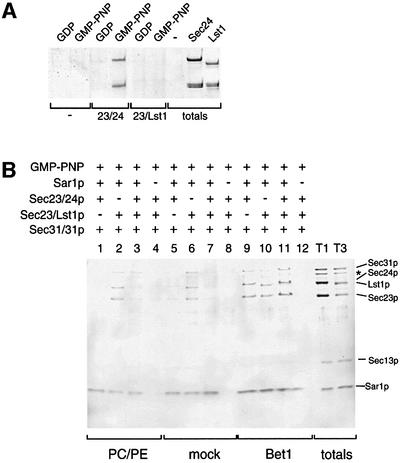
Assembly of the COPII coat has been reconstituted using synthetic liposomes (Matsuoka et al., 1998b). This process occurs in a stepwise manner by the recruitment of Sar1p which binds to the lipid surface during GDP to GTP exchange, followed by association of Sec23/24p and Sec13/31p (Matsuoka et al., 1998b; Antonny et al., 2001). We tested the ability of the Sec23/Lst1p complex to replace Sec23/24p in this reconstituted system. Liposomes derived from ER-enriched microsomal membranes were incubated with various combinations of the COPII coat proteins as indicated in Figure 1. Like Sec23/24p, Sec23/Lst1p bound to these liposomes in a Sar1p- and GMP-PNP-dependent manner, and each was active in the recruitment of Sec13/31p (lanes 1–6). Similarly, Sec23/Lst1p formed a Sar1p- and GMP-PNP-dependent complex on synthetic liposomes composed of ‘major–minor mix’ lipids (lanes 7–12), previously defined as the optimal composition for coat recruitment (Matsuoka et al., 1998b). Although binding of Sec23/Lst1p to major–minor liposomes was marginally reduced compared with Sec23/24p, subsequent recruitment of Sec13/31p was supported. When both Sec23/24p and Sec23/Lst1p were included in the reaction, both complexes were recruited to similar levels (lanes 13 and14).
Fig. 1. Sec23/Lst1p can form a COPII coat on synthetic liposomes. Liposomes (200 µM lipids) composed of either microsomal lipids or synthetic lipids were incubated with COPII components (20 µg/ml Sar1p, 30 µg/ml Sec23/24p or Sec23/Lst1p, 45 µg/ml Sec13/31p) and nucleotide (100 µM) as indicated. Liposomes were collected by flotation and bound proteins were analyzed by SDS–PAGE and SYPRO red staining. A portion of the total reactions was loaded in lanes T9 and T14. The protein marked by an asterisk is a proteolytic fragment of Sec31p.
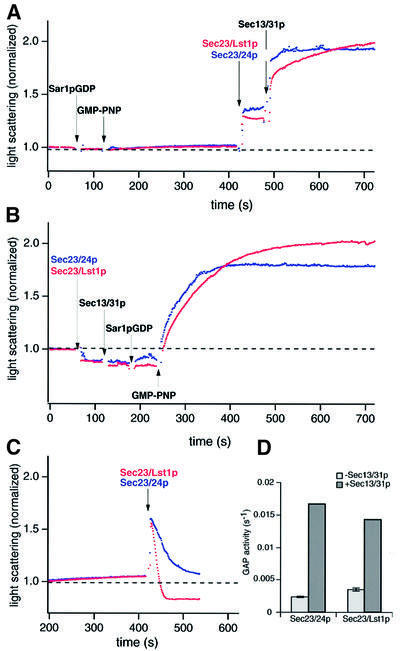
Because Sec23/Lst1p behaved essentially identically to Sec23/24p in liposome-binding assays, we assessed the kinetics of formation of the Sec23/Lst1p-containing coat using a light-scattering assay. Binding of coat proteins to liposomes causes an increase in mass that can be detected by monitoring changes in light scattering (Antonny et al., 2001). Using the standard COPII components, characteristic binding events were observed (Antonny et al., 2001). A small and slow increase in light scattering was observed upon addition of GMP-PNP to a mixture of liposomes and Sar1p, corresponding to the slow exchange of GDP for GMP-PNP to form membrane-bound Sar1p-GMP-PNP (Figure 2A). Addition of Sec23/Lst1p yielded a rapid increase in light scattering that was almost identical to that obtained with Sec23/24. A second rapid increase in light scattering was observed on addition of Sec13/31p. The rate of Sec13/31p recruitment to Sec23/Lst1p-loaded liposomes was slightly reduced, but the yield of signal was similar for coats containing Sec23/24p and Sec23/Lst1p. Order of addition experiments demonstrated that, like Sec23/24p, binding of Sec23/Lst1p required the presence of Sar1p and GMP-PNP on the liposomes and that Sec13/31p was not recruited in the absence of Sec23/Lst1p (Figure 2B).
Fig. 2. Real-time recruitment of the COPII coat. (A–C) The light scattering of a suspension of major–minor liposomes (100 µM lipids) was monitored continuously upon the addition of 930 nM Sar1p, 160 nM Sec23/24p or 160 nM Sec23/Lst1p, 260 nM Sec13/31p and 100 µM GMP-PNP (A and B) or GTP (C) at specific time points as indicated. (D) GAP activity of the Sec23/24p or Sec23/Lst1p complex (55 nM) on Sar1p (2 µM) pre-incubated with 100 µM GTP and major–minor liposomes (300 µM lipids) was determined by tryptophan fluorescence in the absence and presence of Sec13/31p (60 nM).
The development of a real-time assay allowed the visualization of a single round of coat assembly and disassembly in the presence of GTP (Antonny et al., 2001). On liposomes pre-loaded with Sar1p and GTP, addition of Sec23/24p yielded an instant increase in light scattering that persisted for ∼2 min (Figure 2C). Addition of Sec23/Lst1p also yielded an instant binding signal, but this signal was more transient, persisting for <1 min. We considered the possibility that the accelerated disassembly of the Sec23/Lst1p coat may be due to an increased GAP activity on Sar1p. We directly assessed the GAP activity of Sec23/Lst1p on Sar1p using a tryptophan fluorescence assay that distinguishes Sar1p-GDP and Sar1p-GTP (Antonny et al., 2001). Sec23/Lst1p was ∼1.5-fold more efficient in promoting GTP hydrolysis on Sar1p than Sec23/24p, in agreement with the more transient binding signal observed by light scattering (Figure 2D). Moreover, as has already been observed for Sec23/24p (Antonny et al., 2001), the GAP activity of Sec23/Lst1p was stimulated dramatically by Sec13/31p (Figure 2D).
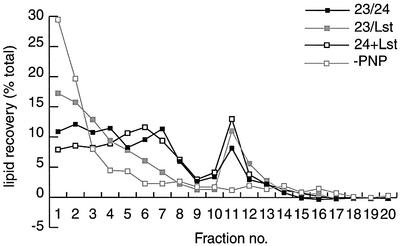
Having demonstrated that Sec23/Lst1p can bind to synthetic liposomes loaded with Sar1p and can in turn recruit Sec13/31p, we next asked whether this alternative coat was sufficient to drive budding from large synthetic liposomes. Extruded liposomes of 400 nm diameter incubated with COPII coat proteins release a population of small (50–90 nm) coated vesicles that can be resolved on linear sucrose gradients (Matsuoka et al., 1998b). Incubation of large liposomes with Sar1p, Sec23/24p and Sec13/31p yielded the characteristic lipid recovery profile composed of large, coated liposomes near the top of the gradient (fractions 3–6) and small, coated vesicles in fractions 10–12 (Figure 3). Incubation with Sec23/Lst1p yielded a very similar profile, with a smaller proportion of large, coated liposomes and a slightly larger fraction of coated vesicles. As expected, no coated vesicles were formed in an incubation lacking GMP-PNP. Incubation with both Sec23/24p and Sec23/Lst1p yielded a slightly larger vesicle peak than when either component was used alone. Morphological examination of each of these fractions by electron microscopy showed coated membrane profiles that were indistinguishable from those generated with Sec23/24p (data not shown). We conclude from these biochemical, kinetic and morphological studies that the intrinsic assembly and disassembly features of the COPII coat on liposomes remain almost identical when Lst1p is substituted for Sec24p.
Fig. 3. Sec23/Lst1p supports budding from synthetic liposomes. Liposomes (100 µg) extruded through 400 nm filters were used in budding reactions in the presence of Sar1p (85 µg/ml), Sec23/24p (85 µg/ml) or Sec23/Lst1p (85 µg/ml), Sec13/31p (100 µg/ml) and GMP-PNP (100 µM). Budded vesicles (fractions 11 and 12) were separated from large, coated liposomes (fractions 3–7) on linear sucrose gradients, and the distribution of lipid quantified by NBD-phospholipid fluorescence.
Vesicles generated with Lst1p lack specific cargo and are fusion deficient
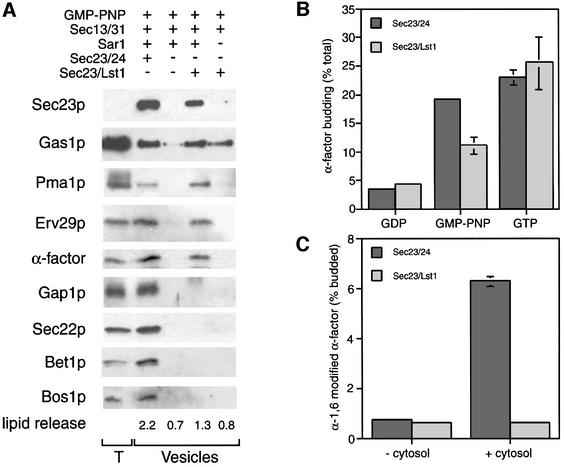
Having established that Sec23/Lst1p was capable of generating a COPII coat in place of Sec23/24p, we wanted to address how this alternative coat might function in generating vesicles from ER membranes. Previous experiments had suggested that Sec23/Lst1p supported vesicle budding to a small extent, but these vesicles seemed to lack any detectable cargo molecules (Shimoni et al., 2000). Because these experiments were performed using relatively crude semi-intact cells, we used enriched ER membranes (microsomes) that allowed the quantitation of phospholipid release to assess overall efficiency of vesicle formation (F.Jiang and R.Schekman, unpublished data). Radiolabeled pre-pro-α-factor was translocated into wild-type microsomes which were then washed extensively with urea and guanine nucleotide to remove any endogenous COPII components. Washed membranes were incubated with either the standard COPII coat proteins or the ‘alternative’ coat containing Sec23/Lst1p in place of Sec23/24p. Following incubation, the donor membranes were removed in a medium speed centrifugation step, and the vesicles remaining in the supernatant were collected by high speed centrifugation. Proteins in the vesicle pellet were analysed by either autoradiography ([35S]α-factor) or immunoblot, and lipid release into the vesicle fraction was assessed by thin-layer chromatography (TLC; Figure 4A). The urea washing step was sufficient to remove any endogenous COPII coat proteins, as Sec23p was undetectable on the donor membranes after washing, and addition of Sar1p and Sec13/31p in the absence of any Sec24 homologs released only background levels of lipid and protein.
Fig. 4. Sec23/Lst1p packages a subset of cargo proteins. (A) Total membranes (T) and budded vesicles were collected from budding reactions containing COPII subunits as indicated. Proteins were analyzed by SDS–PAGE and autoradiography (for 35S-labeled α-factor) or immunoblotting with antibodies against a variety of cargo molecules. Lipid release was quantified by TLC and staining of lipids; numbers given are the percentage of total lipid released into the vesicle fraction. (B) Release of 35S-labeled α-factor into a vesicle fraction after incubation of membranes with Sar1p, Sec23/24p or Sec23/Lst1p, Sec13/31p and guanine nucleotide was determined by precipitation of glycosylated α-factor from the medium speed supernatant. (C) Vesicles generated as in (B) in the presence of either Sec23/24p or Sec23/Lst1p and GTP were incubated with acceptor Golgi membranes in the absence or presence of cytosol. Fusion of vesicles with the Golgi was determined by precipitation with antibodies against α-1,6 mannose residues and scintillation counting.
Bona fide budding of a range of cargo molecules was detected when Sec23/24p was included in the reaction. Quantitation of release of some of these cargo proteins was determined by incubation of immunoblots with radiolabeled secondary antibodies. The SNARE, Sec22p, was packaged with 11.6% efficiency (with 0.2% background budding), while Gas1p was packaged to 1.7% (back ground budding was 0.3%). Quantification of radiolabeled α-factor revealed a budding efficiency of 17% (0.2% background). When Sec23/24p was replaced with Sec23/Lst1p, the amount of lipid released into the vesicle fraction was somewhat reduced (1.3%, compared with 2.2%). This reduction in budding efficiency was mirrored in the reduced recovery of Sec23p in the vesicle fraction. Strikingly, vesicles generated with Sec23/Lst1p contained only a subset of the cargo molecules that we tested. Both α-factor and its packaging receptor, Erv29p (Belden and Barlowe, 2001), were present, again at reduced levels (9.5% packaging of α-factor). Gas1p (1.3% packaging, compared with 0.7% packaging in the absence of Sar1p) and Pma1p (unquantitated) were also present. However, neither the amino acid permease Gap1p nor any of the SNAREs, Sec22p (0.6%), Bet1p or Bos1p, were present in vesicles generated with Lst1p.
The observation that the alternative COPII coat containing Sec23/Lst1p was capable of generating vesicles that contained the model substrate α-factor precursor but lacked the SNAREs required for forward transport led us to investigate whether these vesicles were capable of fusing with acceptor Golgi membranes. Again using urea-washed microsomes, vesicles were generated with either Sec23/24p or Sec23/Lst1p in the presence of GDP, GMP-PNP or GTP (Figure 4B). Release of glycosylated radiolabeled α-factor into the medium speed supernatant was determined by concanavalin A precipitation (Barlowe et al., 1994). Incubation with GMP-PNP facilitated significant packaging of α-factor and, again, replacement of Sec23/24p with Sec23/Lst1p reduced this packaging by about half. However, in the presence of GTP, equivalent levels of α-factor packaging were observed in the presence of either Sec23/24p or Sec23/Lst1p. This apparent disparity in Lst1p-supported budding efficiency may indicate that functional Sec23/Lst1p is limiting in these reactions such that only in the presence of GTP, which allows multiple rounds of budding and uncoating, is optimal vesicle budding observed. Vesicles generated with GTP were used in a second stage fusion reaction that involved incubation with acceptor Golgi membranes, cytosol and nucleotide, followed by immunoprecipitation to isolate α-1,6-modified α-factor precursor (Barlowe et al., 1994). Although vesicles generated with Sec23/24p supported cytosol-dependent fusion, those generated with Sec23/Lst1p were unable to fuse, consistent with the apparent absence of any SNAREs (Figure 4C).
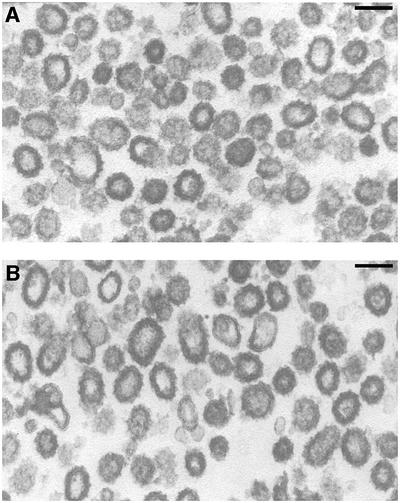
We examined the vesicles generated with either Sec23/24p or Sec23/Lst1p by electron microscopy (Figure 5). Like Sec23/24p vesicles (Figure 5A), Sec23/Lst1p vesicles were largely uniform small profiles, coated with electron-dense material, characteristic of the COPII coat. Vesicles generated with a mixed COPII coat containing both Sec23/24p and Sec23/Lst1p have been shown to differ in size by ∼10 nm (Shimoni et al., 2000; Lee et al., 2002). We performed a similar quantitation of vesicle size and also observed that vesicles generated with a combination of Sec23/24 and Sec23/Lst1p were significantly larger (mean diameter 80 ± 18.1 nm; n = 658) than those generated with Sec23/24p alone (mean diameter 72.1 ± 14.7 nm; n = 607; P < 0.001), whereas vesicles generated with Sec23/Lst1p alone were of intermediate size (mean diameter 75.4 ± 16 nm; n = 548; P < 0.002).
Fig. 5. Sec23/Lst1p vesicles are morphologically identical to Sec23/24p vesicles. Thin-section microscopy of the vesicle-enriched fractions from budding reactions performed with either Sec23/24p (A) or Sec23/Lst1p (B). The overall vesicle morphology and coat appearance are comparable in both samples. The mean size of vesicles generated with Sec23/24p (72.1 ± 14 nm) was moderately smaller than that of those generated with Sec23/Lst1p (75.4 ± 16 nm; P < 0.002). Scale bar = 100 nm.
Pre-budding complexes generated with Lst1p contain a subset of cargo
The surprising observation that vesicles generated with Sec23/Lst1p lacked a specific subset of cargo molecules led us to consider the role that Sec24p and Lst1p may play in cargo selection at early stages in the formation of a vesicle. One of the initial steps in cargo recruitment by the COPII coat can be recapitulated by addition of GST–Sar1p, Sec23/24p and GMP-PNP to ER membranes (Kuehn et al., 1998). Gentle detergent solubilization followed by precipitation of the Sar1p moiety yields cargo-enriched ‘pre-budding complexes’ that are considered to be the precursor to a vesicle proper and are probably required for efficient recruitment of cargo proteins. We isolated pre-budding complexes from detergent-solublized membranes after incubation of urea-washed microsomes with GST–Sar1p, Sec23/24 or monomeric forms of Sec23p and Sec24p and guanine nucleotide (Figure 6A). GST–Sar1p alone was not able to precipitate any cargo proteins; however, addition of Sec23/24p yielded complexes that contained cargo molecules, but not the ER resident Sec61p (Figure 6A). Sec24p is required for the co-precipitation of cargo molecules with Sar1p and Sec23p, because addition of Sec23p alone resulted in the formation of a Sar1p–Sec23p complex that did not contain any cargo molecules. Sec24p alone was also not sufficient to produce any pre-budding complexes, although this is probably due to a lack of interaction between Sar1p and Sec24p in the absence of Sec23p (E.Miller and R.Schekman, unpublished data). Inclusion of both monomeric Sec23p and Sec24p restored the specific recruitment of cargo proteins. Replacement of Sec23/24p with Sec23/Lst1p yielded a complex that contained Sec23/Lst1p as well as the cargo molecules Gas1p and Erv29p (Figure 6B). Interestingly, the Sec23/Lst1p pre-budding complex was enriched for Gas1p compared with the Sec23/24p complex. However, the SNARE proteins Sed5p, Sec22p and Bet1p were absent from Sec23/Lst1p complexes, consistent with the observation that Lst1p fails to package any of these cargo molecules into COPII vesicles. The ER resident protein Sec61p was not precipitated with Sec23/Lst1p, demonstrating the specificity of the cargo selection process.
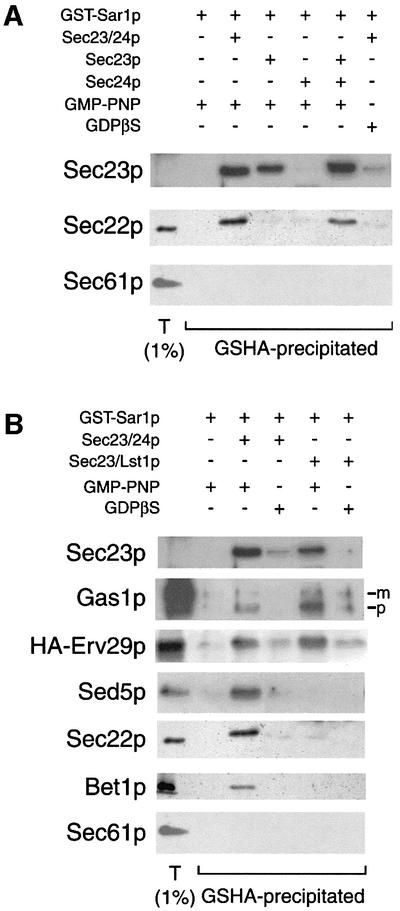
Fig. 6. Recruitment of proteins into pre-budding complexes mirrors vesicle packaging. (A and B) Cargo-enriched pre-budding complexes were isolated from urea-washed membranes after incubation with GST–Sar1p and COPII subunits as indicated. A proportion (1%) of total membranes is included for comparison. The precursor (p) and Golgi-modified mature (m) forms of Gas1p are indicated.
Lst1p does not bind the v-SNARE, Bet1p
The absence of a subset of cargo molecules in both pre-budding complexes and vesicles generated with Sec23/Lst1p led us to address the question of how Sec24p and Lst1p might mediate differential cargo selection. In a soluble binding assay, Sec23/24p was shown previously to bind to GST–Bet1p in the presence of Sar1p and GMP-PNP (Springer and Schekman, 1998). This direct interaction was proposed to mediate recruitment of Bet1p into budding vesicles, and possibly to nucleate COPII coat formation. We tested the ability of Sec23/Lst1p to bind to the cytoplasmic domain of the v-SNARE, Bet1p. Unlike Sec23/24p, Sec23/Lst1p was not recruited to a Sar1p– Bet1p complex (Figure 7A). We next addressed whether Sec23/Lst1p might bind to Bet1p in the context of a lipid bilayer. Full-length Bet1p was incorporated into neutral liposomes that do not normally support the recruitment of Sec23/24p (Matsuoka et al., 1998b; Antonny et al., 2001). The presence of Bet1p in these neutral liposomes facilitated the recruitment of Sec23/24p and the subsequent recruitment of Sec13/31p (Figure 7B, lanes 1, 5 and 9). Neutral liposomes that had been treated with detergent in the absence of Bet1p did not support Sec23/24 binding (Figure 7B, lane 5), demonstrating the specificity imparted by the presence of Bet1p. Although neutral liposomes supported the recruitment of Sec23/Lst1p to a small degree, this binding was not enhanced by the presence of Bet1p (Figure 7B, lanes 2, 6 and 10).
Fig. 7. Sec23/Lst1p does not bind Bet1p. (A) The cytoplasmic domain of the v-SNARE, Bet1p, fused to GST was incubated with Sar1p and either Sec23/24p or Sec23/Lst1p and guanine nucleotide as indicated. Bound proteins were analyzed by SDS–PAGE and SYPRO red staining. (B) Full-length Bet1p was incorporated into neutral liposomes and binding of Sec23/24p or Sec23/Lst1p was assessed by flotation. For comparison, neutral liposomes were either hydrated in buffer and extruded immediately (PC/PE) or hydrated in detergent solution in the absence of Bet1p, dialyzed and extruded as for those containing Bet1p (mock). COPII components were incubated with liposomes as indicated, and proteins bound to liposomes were detected by SDS–PAGE and SYPRO red staining. A portion of the total reactions was loaded in lanes T1 and T3. The protein marked by an asterisk is a proteolytic fragment of Sec31p.
Discussion
The Sec24p homolog, Lst1p, was thought to act as a cargo-specific adaptor protein, required for efficient transport of Pma1p but not sufficient to drive vesicle formation in the absence of Sec24p (Shimoni et al., 2000). This role as a poor cousin of the COPII coat was supported by the observation that overexpression of Lst1p did not complement a Sec24p mutation (Peng et al., 2000). However, we have shown here that Lst1p, in complex with Sec23p, forms a COPII coat that is biochemically and morphologically nearly identical to that formed with Sec23/24p. This coat can form on synthetic liposomes and on ER membranes, and supports vesicle formation.
Morphological characterization of the vesicles generated with Sec23/Lst1p revealed a population of vesicles that was very similar to the standard COPII vesicles that have been characterized previously (Barlowe et al., 1994; Shimoni et al., 2000). Vesicles generated with a mixture of Sec23/24p and Sec23/Lst1p are significantly larger than those generated with Sec23/24p alone (Shimoni et al., 2000; Lee et al., 2002). Because the increased size of mixed coat vesicles does not depend on the membrane lipid composition or the oligomeric state of a major cargo protein, Pma1p, the intrinsic properties of a coat containing Lst1p were postulated to cause the observed increase in vesicle size (Lee et al., 2002). Our observation that vesicles generated with Sec23/Lst1p were intermediate in size between vesicles generated with Sec23/24p and those generated with a mixed coat suggests that the distinct coat architecture that gives rise to a larger vesicle is achieved in part by the distinct properties of Lst1p alone and in part by an altered arrangement of coat subunits in the presence of Lst1p.
The finding that COPII vesicles generated with Sec23/Lst1p contained only a subset of cargo molecules supports the hypothesis that the Sec24p subunit controls cargo selection. The presence of Pma1p in these vesicles is consistent with the observation that Lst1p is required for the efficient ER export of Pma1p (Roberg et al., 1999) and reinforces the suggestion that Lst1p is a cargo-specific adaptor protein (Shimoni et al., 2000; Lee et al., 2002). In addition to Pma1p, Lst1p-generated vesicles also contained the yeast mating pheromone, α-factor, and its receptor, Erv29p, as well as the glycosylphosphatidylinositol (GPI)-anchored protein, Gas1p. Gas1p and other GPI-anchored proteins have been proposed to be segregated into a distinct population of vesicles upon exit from the ER (Muniz et al., 2001). One could envisage a model whereby Sec23/Lst1p may act as a specific adaptor protein to generate this unique population of vesicles containing GPI-anchored proteins. However, several observations are inconsistent with this simple model. Vesicle immuno isolation and density gradient experiments suggested that Gas1p was packaged into vesicles that specifically lacked both the amino acid permease Gap1p and α-factor (Muniz et al., 2001). Whereas we found that Gap1p and Emp24p, the Gas1p receptor, were absent from vesicles generated with Sec23/Lst1p, α-factor was packaged very efficiently into such vesicles. Furthermore, given that vesicles generated with Sec23/Lst1p alone are incapable of fusion (see discussion below), it seems unlikely that these ‘dead-end’ vesicles are generated in vivo. Indeed, the generation of such vesicles may explain both the inability of Lst1p to replace Sec24p function and the toxic effect of overexpression of Lst1p (Roberg et al., 1999; Peng et al., 2000).
If the population of vesicles containing Gas1p and other GPI-anchored proteins is indeed generated by Sec23/Lst1p in vivo, segregation of the different coat subunits would be necessary to allow formation of distinct populations of vesicles. Interestingly, Pma1p and Gas1p, both packaged into Sec23/Lst1p vesicles, are both associated with lipid raft domains (Bagnat et al., 2001). Perhaps Sec23/Lst1p has a propensity to bind more avidly to raft domains than Sec23/24p, increasing the local concentration of the ‘alternative’ coat subunit and enriching in raft-associated proteins while still allowing low-level incorporation of essential cargo such as Bet1p. Clearly, the potential role of Sec23/Lst1p in generating the unique population of vesicles containing GPI-anchored proteins warrants further investigation.
Of particular interest is the observation that vesicles generated with Lst1p specifically lack any SNAREs. Indeed, these vesicles were unable to fuse with acceptor Golgi membranes, consistent with the critical role for vesicle-borne SNARE proteins as part of the fusion machinery (Cao and Barlowe, 2000). Since a number of cytoplasmic factors that are required for vesicle fusion are recruited to vesicles prior to docking, it is also possible that vesicles generated with Lst1p are deficient not only for fusion, but also for tethering and docking (Cao et al., 1998). Further characterization of the complement of proteins that are present in Lst1p-generated vesicles and their ability to recruit tethering and docking factors may provide additional insight into vesicle tethering and docking events.
The specific inability of Lst1p to package SNAREs was correlated with a lack of binding to Bet1p, either as a soluble fusion protein or in the context of a lipid bilayer. This supports the hypothesis that direct interaction between subunits of the COPII coat and the cytoplasmic domains of cargo proteins results in recruitment into pre-budding complexes and then into a completed vesicle (Kuehn et al., 1998; Springer and Schekman, 1998). That we were able to generate COPII vesicles using a subunit that is incapable of binding Bet1p suggests that there is not an absolute requirement for the presence of SNAREs in the nucleation of COPII budding (Springer and Schekman, 1998; Springer et al., 1999). It is possible that other cargo molecules fulfill a similar function for Lst1p, or perhaps the interaction of the COPII coat with SNAREs increases the efficiency but is not essential for coat formation.
Our data provide a direct correlation between the ability of a cargo molecule to bind to a COPII subunit and to be included in a newly formed vesicle. We suggest that cargo specificity is imparted predominantly by the Sec24p subunit and that distinct cargo-binding domains must be present on Sec24p and Lst1p. Some cargo molecules, such as Erv29p, are packaged by both Sec24p and Lst1p, whereas others are packaged solely by Sec24p. Thus, it seems likely that Sec24p has multiple cargo recognition domains. Proteins that are packaged into both Sec24p and Lst1p vesicles would bind to a domain common to each subunit; proteins that are packaged solely by Sec24p would be recognized by a unique region on Sec24p. One interesting consideration is the packaging of Pma1p, which requires Lst1p for efficient transport, but can be packaged independently of Lst1p in the presence of excess Sec24p (Shimoni et al., 2000). This adaptor-independent packaging of Pma1p could be explained by a signal-independent, or bulk flow process. However, no such bulk flow packaging of Bet1p was observed in incubations containing Lst1p in place of Sec24p. Perhaps Pma1p contains a low affinity Sec24p-binding site that is only capable of conferring packaging at high concentrations of Sec23/24p.
Sequence comparison of Sec24p and Lst1p yields few clues to the identity of potential cargo-binding regions, because the overall level of homology is very low (23% identity). The generation of chimeric molecules containing regions of both Sec24p and Lst1p should prove fruitful in deciphering the identity of domains that impart the specificity of cargo recruitment into nascent COPII vesicles.
Materials and methods
Strains, proteins and reagents
Sar1p, Sec23/24p, Sec23/Lst1p and Sec13/31p were prepared as described (Barlowe et al., 1994; Salama et al., 1997; Shimoni et al., 2000). Monomeric Sec23p was produced as a GST fusion protein in Escherichia coli as described (Gimeno et al., 1996). Monomeric Sec24p is a by-product of the Sec23/24p preparation and consititutes the unbound fraction from DEAE chromatography (Barlowe et al., 1994). Lipids were extracted from microsomal membranes as described (Matsuoka and Schekman, 2000). Synthetic lipids were obtained from Avanti Polar Lipids, and the compositions of ‘major–minor mix’ and neutral liposomes have been described previously (Matsuoka et al., 1998b). Microsomal membranes were prepared from either wild-type yeast cells or a strain bearing a hemagglutinin (HA)-tagged version of Erv29p (Belden and Barlowe, 2001) (strains RSY620 and CBY1160, respectively) as described (Wuestehube and Schekman, 1992).
Liposome binding and budding assays
Recruitment of COPII proteins to synthetic liposomes was performed essentially as described previously (Matsuoka et al., 1998b), with the following modifications. Small-scale reactions (75 µl) containing liposomes, nucleotide and COPII proteins were incubated at room temperature to allow COPII recruitment. Binding reactions were mixed with 50 µl of 2.5 M sucrose in HKM (20 mM HEPES pH 6.8, 160 mM KOAc, 1 mM MgCl2), transferred to tubes suitable for a TLA100 rotor (Beckman), overlaid with 100 µl of 0.75 M sucrose in HKM and 20 µl of HKM before centrifugation at 400 000 g for 10 min at room temperature. Floated liposomes were collected, fluorescent lipid quantified and bound proteins were analyzed by SDS–PAGE and SYPRO red staining (Molecular Probes) after adjusting the sample volume to normalize for lipid recovery.
Budding of COPII vesicles from synthetic liposomes (Matsuoka et al., 1998b) and light scattering and tryptophan fluorescence assays (Antonny et al., 2001) were performed as described previously.
Vesicle budding and fusion
Vesicle budding from ER membranes was performed as described (Barlowe et al., 1994), with several modifications. After translocation of 35S-labeled pre-pro-α-factor, microsomal membranes were washed three times with 2.5 M urea in B88 [20 mM HEPES pH 6.8, 250 mM sorbitol, 150 mM KOAc, 5 mM Mg(OAc)2], twice with 0.1 mM GTP in B88 and three times with B88. Microsomes (1.5 mg) were incubated with Sar1p (20 µg/ml), Sec23/24p or Sec23/Lst1p (20 µg/ml) and Sec13/31p (40 µg/ml) in the presence of 0.1 mM guanine nucleotide. A fraction of this reaction was removed for analysis as the total membrane fraction (T). The remaining material was subjected to centrifugation at 13 000 g in a swinging bucket rotor. The supernatant from this medium speed spin was collected and subjected to centrifugation at 100 000 g to yield a vesicle pellet that was resuspended in 40 µl of B88. A portion of this vesicle fraction (10 µl) was removed for analysis by SDS–PAGE and immunoblot, while the remaining vesicles were extracted with CHCl3/MeOH by the Bligh–Dyer method (New, 1997). Extracted lipids were subjected to TLC, stained with primuline (0.002% in 65:25:5 CHCl3:MeOH:HOAc) and quantified using a Typhoon 9400 Imager (Molecular Dynamics) as described previously (Matsuoka and Schekman, 2000).
Forward transport assays were performed as described previously (Barlowe et al., 1994) except that microsomal membranes were urea washed as described above.
Electron microscopy
Vesicles were generated from microsomal membranes as described above, and glutaraldehyde was added to the medium speed supernatant to a final concentration of 2%. Vesicles were collected by high speed centrifugation at 100 000 g and the vesicle pellet was processed further as described (Antonny et al., 2001). Quantitation of vesicle size was performed as described (Lee et al., 2002).
Bet1p binding assays
GST–Bet1p was expressed in E.coli, purified and used in binding assays essentially as described previously (Springer and Schekman, 1998). Briefly, purified GST–Bet1p was immobilized on glutathione–agarose beads and incubated with Sar1p (30 µg/ml) and Sec23/24p or Sec23/Lst1p (30 µg/ml) in the presence of either GDP or GMP-PNP (0.1 mM) in binding buffer (20 mM HEPES pH 6.8, 5 mM MgCl2, 1 mM EDTA, 2% glycerol, 300 mM KOAc, 1 mM dithiothreitol, 0.8% N-octylglucoside). Proteins were incubated for 30 min at 25°C to allow complex formation before immobilized complexes were washed extensively in binding buffer and eluted with SDS sample buffer for analysis by SDS–PAGE and SYPRO red staining.
Full-length Bet1p was incorporated into liposomes essentially as described (Wagner and Tamm, 2001). Briefly, the Bet1p open reading frame was amplified by PCR and inserted downstream of the GST gene in the expression vector, pETGEXCT (Sharrocks, 1994). Protein was expressed in E.coli, purified by glutathione affinity chromatography and cleaved from the GST moiety by treatment with thrombin (2 U) in TCB (25 mM Tris–HCl pH 8.0, 5 mM CaCl2, 250 mM KOAc) containing 0.8% N-octylglucoside. Neutral lipids (Matsuoka et al., 1998b) were dried to a lipid film and hydrated in either Bet1p solution (100 µg/ml) or TCB containing 0.8% N-octylglucoside. The lipid emulsion was diluted 2-fold with TCB without detergent, dialyzed extensively against HK buffer (20 mM HEPES pH 7.0, 160 mM KOAc) and extruded through 400 nm filters to generate liposomes for use in binding assays as described above.
Isolation of pre-budding complexes
Pre-budding complexes were isolated from membranes that had been urea washed as described above. Complex formation and isolation were performed as described previously (Kuehn et al., 1998).
Acknowledgments
Acknowledgements
We thank M.C.S.Lee, C.F.Chan and Y.Shimoni for the preparation of COPII proteins, M.Welsh for the use of the fluorimeter, and C.Barlowe for providing anti-Erv29p serum and the HA-Erv29p strain. We acknowledge the support and critical comments of members of the Schekman laboratory, especially M.C.S.Lee, D.Madden, P.Malkus, S.Springer and R.Valdivia. This work was supported by HHMI (R.S.) and CNRS (B.A.) E.M. is a Fellow of the Jane Coffin Childs Memorial Fund for Medical Research.
References
- Antonny B., Madden,D., Hamamoto,S., Orci,L. and Schekman,R. (2001) Dynamics of the COPII coat with GTP and stable analogues. Nat. Cell Biol., 3, 531–537. [DOI] [PubMed] [Google Scholar]
- Aridor M., Weissman,J., Bannykh,S., Nuoffer,C. and Balch,W.E. (1998) Cargo selection by the COPII budding machinery during export from the ER. J. Cell Biol., 141, 61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aridor M., Fish,K.N., Bannykh,S., Weissman,J., Roberts,T.H., Lippincott-Schwartz,J. and Balch,W.E. (2001) The Sar1 GTPase coordinates biosynthetic cargo selection with endoplasmic reticulum export site assembly. J. Cell Biol., 152, 213–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagnat M., Chang,A. and Simons,K. (2001) Plasma membrane proton ATPase Pma1p requires raft association for surface delivery in yeast. Mol. Biol. Cell, 12, 4129–4138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlowe C. et al. (1994) COPII: a membrane coat formed by Sec proteins that drive vesicle budding from the endoplasmic reticulum. Cell, 77, 895–907. [DOI] [PubMed] [Google Scholar]
- Belden W.J. and Barlowe,C. (2001) Role of Erv29p in collecting soluble secretory proteins into ER-derived transport vesicles. Science, 294, 1528–1531. [DOI] [PubMed] [Google Scholar]
- Cao X. and Barlowe,C. (2000) Asymmetric requirements for a Rab GTPase and SNARE proteins in fusion of COPII vesicles with acceptor membranes. J. Cell Biol., 149, 55–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao X., Ballew,N. and Barlowe,C. (1998) Initial docking of ER-derived vesicles requires Uso1p and Ypt1p but is independent of SNARE proteins. EMBO J., 17, 2156–2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins B.M., McCoy,A.J., Kent,H.M., Evans,P.R. and Owen,D.J. (2002) Molecular architecture and functional model of the endocytic AP2 complex. Cell, 109, 523–535. [DOI] [PubMed] [Google Scholar]
- Gimeno R.E., Espenshade,P. and Kaiser,C.A. (1996) COPII coat subunit interactions: Sec24p and Sec23p bind to adjacent regions of Sec16p. Mol. Biol. Cell, 7, 1815–1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchhausen T. (1999) Adaptors for clathrin-mediated traffic. Annu. Rev. Cell Dev. Biol., 15, 705–732. [DOI] [PubMed] [Google Scholar]
- Kuehn M.J., Herrmann,J.M. and Schekman,R. (1998) COPII–cargo interactions direct protein sorting into ER-derived transport vesicles. Nature, 391, 187–190. [DOI] [PubMed] [Google Scholar]
- Lee M.C., Hamamoto,S. and Schekman,R. (2002) Ceramide biosynthesis is required for the formation of the oligomeric H+-ATPase Pma1p in the yeast endoplasmic reticulum. J. Biol. Chem., 277, 22395–22401. [DOI] [PubMed] [Google Scholar]
- Matsuoka K. and Schekman,R. (2000) The use of liposomes to study COPII- and COPI-coated vesicle formation and membrane protein sorting. Methods, 20, 417–428. [DOI] [PubMed] [Google Scholar]
- Matsuoka K., Morimitsu,Y., Uchida,K. and Schekman,R. (1998a) Coat assembly directs v-SNARE concentration into synthetic COPII vesicles. Mol. Cell, 2, 703–708. [DOI] [PubMed] [Google Scholar]
- Matsuoka K., Orci,L., Amherdt,M., Bednarek,S.Y., Hamamoto,S., Schekman,R. and Yeung,T. (1998b) COPII-coated vesicle formation reconstituted with purified coat proteins and chemically defined liposomes. Cell, 93, 263–275. [DOI] [PubMed] [Google Scholar]
- Muniz M., Morsomme,P. and Riezman,H. (2001) Protein sorting upon exit from the endoplasmic reticulum. Cell, 104, 313–320. [DOI] [PubMed] [Google Scholar]
- New R.R.C. (1997) Liposomes. A Practical Approach. IRL Press, Oxford, UK.
- Peng R., Grabowski,R., De Antoni,A. and Gallwitz,D. (1999) Specific interaction of the yeast cis-Golgi syntaxin Sed5p and the coat protein complex II component Sec24p of endoplasmic reticulum-derived transport vesicles. Proc. Natl Acad. Sci. USA, 96, 3751–3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng R., De Antoni,A. and Gallwitz,D. (2000) Evidence for overlapping and distinct functions in protein transport of coat protein Sec24p family members. J. Biol. Chem., 275, 11521–11528. [DOI] [PubMed] [Google Scholar]
- Roberg K.J., Crotwell,M., Espenshade,P., Gimeno,R. and Kaiser,C.A. (1999) LST1 is a SEC24 homologue used for selective export of the plasma membrane ATPase from the endoplasmic reticulum. J. Cell Biol., 145, 659–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salama N.R., Chuang,J.S. and Schekman,R.W. (1997) Sec31 encodes an essential component of the COPII coat required for transport vesicle budding from the endoplasmic reticulum. Mol. Biol. Cell, 8, 205–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schekman R. and Orci,L. (1996) Coat proteins and vesicle budding. Science, 271, 1526–1533. [DOI] [PubMed] [Google Scholar]
- Sharrocks A.D. (1994) A T7 expression vector for producing N- and C-terminal fusion proteins with glutathione S-transferase. Gene, 138, 105–108. [DOI] [PubMed] [Google Scholar]
- Shimoni Y., Kurihara,T., Ravazzola,M., Amherdt,M., Orci,L. and Schekman,R. (2000) Lst1p and Sec24p cooperate in sorting of the plasma membrane ATPase into COPII vesicles in Saccharomyces cerevisiae. J. Cell Biol., 151, 973–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer S. and Schekman,R. (1998) Nucleation of COPII vesicular coat complex by endoplasmic reticulum to Golgi vesicle SNAREs. Science, 281, 698–700. [DOI] [PubMed] [Google Scholar]
- Springer S., Spang,A. and Schekman,R. (1999) A primer on vesicle budding. Cell, 97, 145–148. [DOI] [PubMed] [Google Scholar]
- Votsmeier C. and Gallwitz,D. (2001) An acidic sequence of a putative yeast Golgi membrane protein binds COPII and facilitates ER export. EMBO J., 20, 6742–6750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner M.L. and Tamm,L.K. (2001) Reconstituted syntaxin1a/SNAP25 interacts with negatively charged lipids as measured by lateral diffusion in planar supported bilayers. Biophys. J., 81, 266–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wuestehube L.J. and Schekman,R.W. (1992) Reconstitution of transport from endoplasmic reticulum to Golgi complex using endoplasmic reticulum-enriched membrane fraction from yeast. Methods Enzymol., 219, 124–136. [DOI] [PubMed] [Google Scholar]
- Yoshihisa T., Barlowe,C. and Schekman,R. (1993) Requirement for a GTPase-activating protein in vesicle budding from the endoplasmic reticulum. Science, 259, 1466–1468. [DOI] [PubMed] [Google Scholar]