Abstract
Pre-protein translocation into chloroplasts is accomplished by two distinct translocation machineries in the outer and inner envelope, respectively. We have isolated the translocon at the inner envelope membrane (Tic complex) by blue-native PAGE and describe a new Tic subunit, Tic62. Tic62, together with Tic110 and Tic55, forms a core translocation unit. The N-terminus of Tic62 shows strong homologies to NAD(H) dehydrogenases in eukaryotes and to Ycf39-like proteins present in cyanobacteria and non-green algae. The stromal-facing C-terminus of Tic62 contains a novel, repetitive module that interacts with a ferredoxin-NAD(P)+ oxidoreductase. Ferredoxin-NAD(P)+ oxidoreductase catalyses the final electron transfer of oxygenic photosynthesis from ferredoxin to NAD(P). Substrates that interfere with either NAD binding, such as deamino-NAD, or influence the ratio of NAD(P)/NAD(P)H, such as ruthenium hexamine trichloride, modulate the import characteristics of leaf-specific ferredoxin-NAD(P)+ oxidoreductase isologues differently. We conclude that the Tic complex can regulate protein import into chloroplasts by sensing and reacting to the redox state of the organelle.
Keywords: chloroplast/redox state/Tic complex/translocation
Introduction
Photosynthesis localized at chloroplasts mainly provides green plants with energy in the form of ATP and reduced NADs. During their development, these organelles undergo significant structural and metabolic changes dependent on environmental factors. Each stage of plastid development requires a specific set of proteins, which are encoded mainly by the nucleus. Therefore, it seems sensible to assume that not only transcription and translation but also import of nuclear-encoded proteins into chloroplasts is modulated by environmental conditions.
The outer envelope membrane contains a general pre-protein translocon, the Toc complex (Küchler and Soll, 2001). The hydrolysis and binding of GTP seem to play a prominent role in regulation of pre-protein translocation at the Toc complex. A GTP/GDP binding cycle modulates the association of the pre-protein with a receptor protein of the Toc complex, Toc34 (Sveshnikova et al., 2000). Like Toc34, another receptor protein, Toc159, contains a GTP-binding site (Hirsch et al., 1994; Kessler et al., 1994), and the putative GTPase activity of Toc159 is thought to regulate the interaction of pre-proteins with the Toc complex (Chen et al., 2000). While the first interaction with the receptor proteins seems reversible and independent of ATP hydrolysis (Perry and Keegstra, 1994), the hydrolysis of low concentrations of ATP results in an irreversible association of the pre-protein with the Toc complex (Olsen and Keegstra, 1992). At this stage, pre-proteins have partially passed through the outer envelope membrane and are in contact with components of the Tic complex, which is the translocon at the inner envelope membrane of chloroplasts (Caliebe et al., 1997; Kouranov and Schnell, 1997; Nielsen et al., 1997). At the Tic complex, the participation of nucleoside triphosphates other than ATP in pre-protein translocation is excluded (Theg et al., 1989).
The Tic complex comprises at least five components: Tic55, Tic40, Tic22, Tic20 and Tic110 (Kessler and Blobel, 1996; Lübeck et al., 1996; Caliebe et al., 1997; Kouranov et al., 1998; Stahl et al., 1999). Recently, the function of Tic110 as the pre-protein translocation pore at the inner envelope membrane was established (Heins et al., 2002). Tic20 and Tic22 were identified by label-transfer cross-linking experiments using a chimeric pre-protein (Kouranov et al., 1998). They were suggested to function as a part of the pre-protein translocation pore and in directing the pre-protein in the intermembrane space on its way from the Toc to the Tic complex, respectively. Tic40 is an essential protein in Arabidopsis (Budziszweski et al., 2001) and might recruite molecular chaperones to the Tic translocon (Stahl et al., 1999). Using blue-native polyacrylamide gel electrophoresis (BN-PAGE), Tic55 and Tic110 co-purified with a complex comprising several unknown proteins (Caliebe et al., 1997). Tic55 belongs to the class of Rieske-type iron–sulfur proteins, and import of pre-proteins was inhibited specifically at the inner envelope membrane using diethylpyrocarbonate, a Rieske- type protein-modifying reagent (Caliebe et al., 1997). Therefore, Tic55 could play a role as a redox sensor during pre-protein translocation in chloroplasts.
Here we describe a refined BN-PAGE, which was used to isolate a Tic core complex. This complex consists of Tic110, Tic55 and a 60 kDa protein. The 60 kDa protein, which is referred to here as Tic62, binds pyridine nucleotides at its N-terminus. The C-terminal domain containing a repetitive module associates with a ferredoxin-NAD(P)+ oxidoreductase (FNR). Protein import into isolated chloroplasts is affected in the presence of nicotinamide hypoxanthine dinucleotide (deamino-NAD), which functions as electron acceptor of reductases and hydrogenases. We propose a model that involves NAD(P)-binding proteins regulating the translocation of pre-proteins at the chloroplast inner envelope.
Results
Purification of the Tic core complex
Different detergents such as decyl maltoside, Triton X-100 and SDS as control were used to solubilize inner envelope membranes from pea chloroplasts prior to BN-PAGE. Both non-ionic detergents had similar solubilization efficiencies, complex distribution and polypeptide pattern in BN-PAGE and SDS–PAGE, respectively (Figure 1A). SDS completely solubilized the inner envelope membrane (Figure 1A). We therefore developed a refined BN-PAGE to isolate a Tic core complex from purified inner envelope vesicles using decyl maltoside. The Tic complex migrated at ∼230 kDa (Figure 1B and upper panel of D), it was electro-eluted and subjected to a second BN-PAGE in which the Tic complex again migrated at ∼230 kDa (Figures 1B and 2D). Protein complexes with a higher or lower apparent molecular weight were not observed in the second dimension, indicating that other protein complexes did not co-migrate with the Tic complex after the first BN dimension (Figure 1B). Furthermore, the Tic complex obtained after the second BN-PAGE confirmed that the 230 kDa complex represents a stable core complex. The composition of the 230 kDa Tic complex was analysed by denaturating SDS–PAGE. Prominent proteins in this core complex were Tic110, Tic55 and an unknown 60 kDa protein (Figure 1B, lower panel). The identity of Tic110 and Tic55 was verified by immunodecoration (data not shown). The 36 and 45 kDa protein observed after the first BN-PAGE (Caliebe et al., 1997) did not co-purify with this core complex (Figure 1A, lower panel). Single proteins with a mol. wt ≤36 kDa are still observed. However, they migrated separately from the Tic core complex (Figure 1B, lower panel). Sucrose density centrifugation of the Tic complex electro-eluted after the first BN-PAGE revealed a composition similar to that observed after the second BN-PAGE: it mainly included Tic110, Tic55 and the unknown 60 kDa protein (Figure 1B, fraction 11). However, in several fractions of the sucrose gradient, proteins with an apparent mol. wt of ≤36 kDa were found to co-migrate with the Tic core complex (Figure 1B, fractions 5–9), demonstrating that the resolution of BN-PAGE was higher than that of sucrose density centrifugation. These proteins might be subunits of a larger Tic complex, which are less tightly associated. The affiliation of the 60 kDa protein with the Tic complex was supported by the finding that antisera against Tic110 or Tic55 co-immunoprecipitated the 60 kDa protein from the Tic complex purified by electro-elution (Figure 1C). Conversely, after immunoprecipitation with an antiserum against the 60 kDa protein, Tic110 was detected (Figure 1C). Inner envelope vesicles, which were solubilized with decyl maltoside, also contained a Tic complex comprising the 60 kDa protein (see below). Antisera against the 60 kDa protein co-precipitated the 60 kDa protein and further Tic components such as Tic110 and Tic40 (see below). Together, these results provide convincing evidence that the 60 kDa protein is a component of the Tic complex, it will be referred to here as Tic62.
Fig. 1. Analysis of the Tic core complex by BN-PAGE and immunoprecipitation. (A) Purified inner envelope vesicles (200–400 µg of protein) were solubilized with either 3% decyl maltoside (DeMa), 0.5% Triton X-100 (TX-100) or decyl maltoside followed by treatment with 2% SDS (SDS). Protein complexes were separated on a blue native gel of 10–12% acrylamide (left panel). A Coomassie Blue-stained BN gel is shown. RUBISCO (∼540 kDa), catalase (∼240 kDa) and bovine serum albumin (BSA; ∼66 kDa) are indicated as size markers. Gel slices (indicated by asterisks) corresponding to the protein complex of ∼230 kDa were cut from each BN lane and subjected to a second dimension under denaturing conditions. A silver-stained gel is shown in the right panel. The molecular weight of standard proteins in kDa is given on the right. (B) Purified inner envelope vesicles (200 µg of protein) were solubilized with 3% decyl maltoside. In the first dimension, protein complexes were separated on a BN gradient gel of 6.5–12% acryl amide (1D). Next, a protein complex of ∼230 kDa was electro-eluted and subjected to a second dimension of BN-PAGE (2D). Size markers were as in (A). The entire lane of the second dimension was analysed further by SDS–PAGE. Tic110 and Tic55 are labelled, and the 60 kDa protein is indicated by an asterisk. A silver-stained gel is shown. In a second approach, the electro-eluted 230 kDa complex was subjected to a continuous sucrose density gradient (5–25% sucrose). The fractions were analysed by SDS–PAGE. A silver-stained gel is shown. At the top, the number of fractions is indicated and the molecular weight of standard proteins in kDa is given. (C) After electro-elution of the 230 kDa complex, its constituents were co- immunoprecipitated with antisera (α) against Tic110, Tic55 and Tic62. As a control, pre-immune serum of Tic62 (pre) was used. The precipitated constituents were detected with antisera against Tic110 and Tic62.
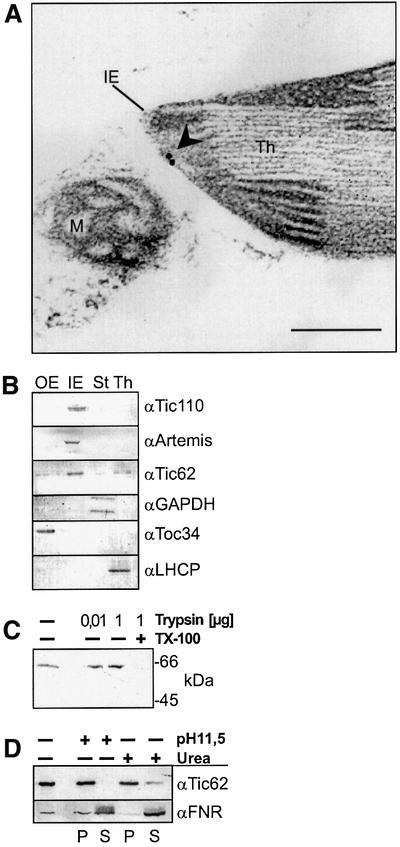
Fig. 2. psTic62 is localized at the inner envelope membrane of chloroplasts. (A) Ultrathin sections of pea leaves were incubated with antiserum against Tic62. The panel shows immunogold labelling of the inner envelope membrane of an intact chloroplast. Thylakoids, Th; inner envelope, IE; mitochondrion, M. The scale bar represents 0.2 µm. (B) Intact chloroplasts were fractionated. Outer (OE) and inner envelope (IE) membranes, thylakoids (Th) and stroma (St) (10 µg of protein) were subjected to SDS–PAGE. Then, proteins were transferred to nitrocellulose and immunodecorated with antisera against Toc34 (outer envelope), Tic110, Artemis (Fulgosi et al., 2002), Tic62 (inner envelope), glyceraldehyde phosphate dehydrogenase (GAPDH, stroma) and LHCP (thylakoids). (C) Tic62 is resistant to protease treatment. Inner envelope vesicles (10 µg of protein) were incubated with 10 ng or 1 µg of protease with or without 1% Triton X-100 (TX-100). The proteolytic treatment was stopped finally by the addition of 2 mM PMSF. The samples were subjected to SDS–PAGE. An immunodecoration with antiserum against Tic62 is shown. (D) Inner envelope vesicles (20 µg of protein) were extracted with 0.1 M Na2CO3 pH 11.5 or 6 M urea for 15 min at 4°C. The insoluble protein (P) and the soluble fraction (S) were subjected to SDS–PAGE. An immunodecoration with antiserum against Tic62 and FNR is shown.
Ultrathin sections of pea leaves were used to obtain evidence for the localization of Tic62. Immunogold staining resulted in the labelling of chloroplasts only (Figure 2A). Within the chloroplast, 93% of gold grains were confined to the inner envelope (evaluation of ≥50 fields). Gold grains confined to the inner envelope were localized mostly to the stromal face of the membrane (Figure 2A). The antiserum was raised against the C-terminal portion of Tic62, indicating that this module might be exposed to the stroma. The location of Tic62 at the inner envelope was supported by the immunodecoration of fractions of pea chloroplasts purified by sucrose density centrifugation (Figure 2B). Tic62 resided almost exclusively in the enriched inner envelope membrane fraction. Treatment of isolated inner envelope vesicles with the protease trypsin did not result in low molecular weight degradation products of Tic62, even at a higher concentration of trypsin (Figure 2C). This result corroborates our notion that the C-terminus of Tic62 is deeply embedded in the membrane or that it faces the stromal side of the inner envelope membrane. After alkaline treatment of isolated inner envelope vesicles or treatment with 6 M urea, Tic62 remained within the membrane (Figure 2D), indicating that it is an integral membrane protein. FNR which is also detected at the inner envelope fraction (see below) could be extracted by this treatment.
Molecular characterization of Tic62
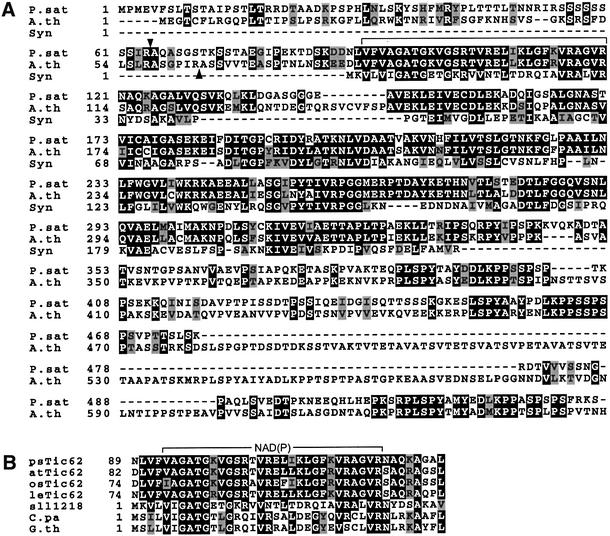
Since the N-terminus of Tic62 was inaccessible to sequencing, endoproteinase lys-C was used to generate internal peptides of Tic62. An oligonucleotide mixture derived from two different amino acid sequences was then used to isolate a cDNA clone of 1149 bp encoding a protein of 382 amino acids. The deduced amino acid sequence contains all the peptide sequences obtained from the proteolytic fragments (EQPLSPYTAY and EEQHLEP), demonstrating that the isolated cDNA encodes Tic62; however, the 5′ end was truncated. To obtain the full-length sequence of psTic62, we performed a 5′-RACE cDNA amplification and were able to isolate a full-length clone of 1944 bp which encodes a protein of 534 amino acids with a calculated mol. wt of 56.9 kDa. Using the programs ChloroP 1.1 or TargetP (Emanuelsson and von Heijne, 2001), a chloroplast targeting signal and a processing site for the pre-protein at amino acids R64–A65 was predicted for psTic62, giving a mature protein of 470 amino acids with a calculated mol. wt of 49.8 kDa. Homologues of psTic62 were found in Arabidopsis thaliana (DDBJ/EMBL/GenBank accession No. AAC26697) and Oryza sativa (DDBJ/EMBL/GenBank accession No. AC079632). The A.thaliana gene product showed ∼60% identity for the deduced mature sequence and had a calculated mol. wt of 62.1 kDa (Figure 3A). Based on A.thaliana as the generally accepted model, we name the 60 kDa protein Tic62 in both A.thaliana (at) and Pisum sativum (ps) (Figure 3A) (Schnell et al., 1997). The N-terminal half of both psTic62 and atTic62 resembles a putative protein of unknown function, Ycf39, which is present in Synechocystis PCC6803 (sll1218), Cyanophora paradoxa (DDBJ/EMBL/GenBank accession No. AAA81188) and non-green algae such as Gulliardia theta (DDBJ/EMBL/GenBank accession No. AAC35663) (Ermakova-Gerdes and Vermaas, 1999). These Ycf39-like proteins are probably soluble proteins, which have a pyridine nucleotide-binding site at the N-terminus comprising similarity to dehydrogenases. The amino acid sequence of at/psTic62 includes a pyridine nucleotide-binding site at amino acids V84/91–R113/120 (Figure 3B). Furthermore, both proteins have a hydrophobic region of ∼75 amino acids at their N-termini, which probably anchors the plant proteins in the envelope membrane. At the C-terminus, Tic62 contains repetitive, highly conserved sequence modules consisting of 20 amino acids, which are separated by a region of ∼40 amino acids with multiple charged residues (Figure 3A). Computer analysis revealed a mainly random coiled structure for the C-terminal domain. Several deduced amino acid sequences, i.e. Zea mays (DDBJ/EMBL/GenBank accession No. BG320430), Solanum tuberosum (DDBJ/EMBL/GenBank accession No. BE340947), Medicago trunculata (DDBJ/EMBL/GenBank accession Nos AW696271 and BE322329), Lycopersicum esculentum (DDBJ/EMBL/GenBank accession No. AW442902) and Glycine max (DDBJ/EMBL/GenBank accession No. AW202191), show similarity to Tic62 including this repetitive sequence module. However, none of them encodes a full-length protein.
Fig. 3. Comparison of psTic62 with putative homologues. (A) The amino acid sequence of Tic62 from P.sativum (ps) is compared with the deduced amino acid sequence of an A.thaliana (atTic62) cDNA clone. The sequence data of psTic62 have been deposited in the EMBL database (EMBL accession No. AJ344551). The predicted processing sites of psTic62 and atTic62 are indicated by arrowheads. The pyridine nucleotide-binding-module is framed. Identical amino acids are boxed in black, and similar residues in grey. The numbers at the left indicate the position of the amino acids. (B) The pyridine nucleotide-binding module of Ycf39-like proteins and Tic62. The amino acid sequence of Cyanophora paradoxa (C.pa), Gulliardia theta (G.th), Synechocystis sp. PCC6803 (sll1218), P.sativum (psTic62), A.thaliana (atTic62), Oryza sativa (osTic62) and Lycopersicum esculentum (leTic62) were compared. The numbers indicate the position of the amino acids.
Tic62 binds pyridine nucleotides
Since the known Tic62 homologues carry a highly conserved pyridine nucleotide-binding site at their N-termini, we investigated the capacity of psTic62 to bind NAD. Inner envelope vesicles were solubilized with either Triton X-100 or SDS and kept on ice in the presence of 32P-labelled NAD+. Cross-linking was induced by UV light. In non-solubilized vesicles or membranes solubilized with Triton X-100, a cross-linking product of 32P-labelled NAD+ with an apparent mol. wt of 60 kDa occurred (Figure 4A, left panel). Inner envelope vesicles solubilized with SDS were not capable of binding 32P-labelled NAD+ (Figure 4A, left panel). The polypeptide, which was recognized by the Tic62 antiserum, migrated identically to the cross-linking product (Figure 4A, right panel). Further evidence that Tic62 was indeed labelled by [32P]NAD+ was obtained by immunoprecipitation. Only with Tic62 antibodies was a cross-linking product precipitated, which had an apparent mol. wt of 60 kDa (Figure 4B). The binding of 32P-labelled NAD+ was suppressed when solubilized inner envelope vesicles were incubated with unlabelled pyridine nucleotides or with analogous nucleotides prior to the addition of 32P-labelled NAD+ (Figure 4C). In the presence of 10 µM glucose-6-phosphate, UDP or ATP, the binding is not affected, while with 10 µM NAD+ or deamino-NAD the binding of 32P-labelled NAD+ is significantly decreased (Figure 4C). Deamino-NAD interacts with pyridine nucleotide-binding proteins (Zickermann et al., 2000). In summary, these findings clearly demonstrate that psTic62 indeed has a functional pyridine nucleotide-binding site most probably localized at the extreme N-terminus of the protein.
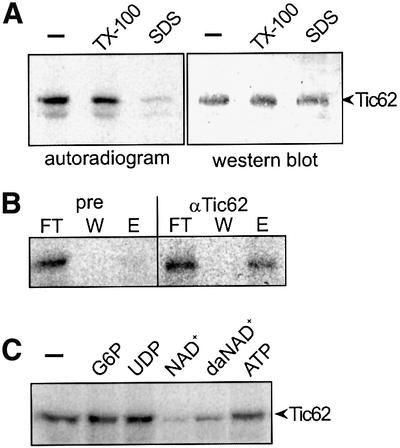
Fig. 4. The N-terminus of psTic62 binds pyridine nucleotides. (A) Inner envelope vesicles (50 µg of protein) were incubated with 32P-labelled NAD+. After UV illumination, the samples were subjected to SDS– PAGE, transferred to nitrocellulose and analysed by autoradiography (left panel) and simultaneously by immunodecoration with antiserum against Tic62 (right panel). The addition of Triton X-100 (TX-100) did not affect the NAD+-binding capacity of Tic62, while in the presence of SDS, binding is abolished (left panel). (B) NAD+-bound Tic62 was immunoprecipitated from inner envelope vesicles in the presence of SDS. As a control, pre-immune serum (pre) was used. An autoradiograph of aliquots of the flow through (FT), last washing step (W) and the eluted protein (E) is shown. (C) After solubilization of inner envelope vesicles with Triton X-100, pyridine nucleotides compete for the binding of 32P-labelled NAD+ to psTic62. Nicotinamide hypoxanthine dinucleotide, da-NAD; glucose-6-phosphate, G-6-P.
A novel repetitive module of psTic62 interacts with FNR
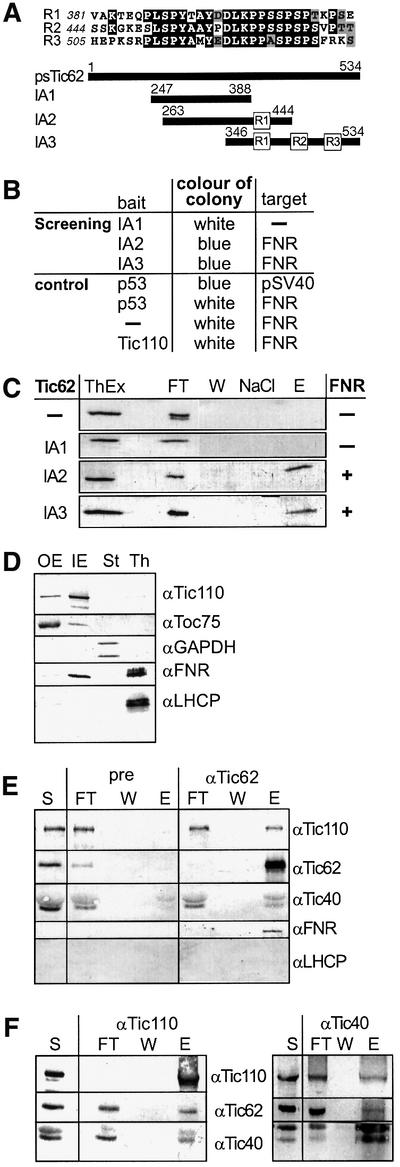
The function of the repetitive sequence module (Figure 5A) at the C-terminus of psTic62 was investigated first using a yeast two-hybrid system. Three cDNA fragments, IA1, IA2 and IA3, were subcloned into the bait vector of the HybriZAP Two-hybrid™ cDNA library. The protein product IA1 covered a region of psTic62 without a repetitive module, while IA2 and IA3 included one or three repetitive modules, respectively (Figure 5A). After the transformation into yeast, the expression of both protein products, IA2 and IA3 resulted in several colonies showing β-galactosidase activity. Surprisingly, all coloured colonies contained FNR (DDBJ/EMBL/GenBank accession No. X12446) as an interacting partner of the C-terminus of psTic62 (Figure 5B). After transformation of IA1, which did not encode a repetitive module, an interacting protein was not detected. Control transformations were performed according to the manufacturer’s instructions. (i) The expression of FNR only did not cause β-galactosidase activity. (ii) FNR did not interact with the murine phosphoprotein p53 and the Tic110 polypeptide T233–F959. (iii) Co-expression of the murine phosphoprotein p53 and the SV40 large T antigen allowed β-galactosidase activity (Figure 5B).
Fig. 5. psTic62 interacts with FNR. (A) Comparison of the repetitive modules at the C-terminus of psTic62. The numbers indicate the position of the amino acids. A scheme of the polypeptides used to investigate the interaction of the C-terminus of psTic62 with partner proteins is presented. (B) Overview of the results of the yeast two-hybrid screening. The bait and the target proteins obtained after screening or used for control are shown. (C) Interaction of polypeptides containing the repetitive module with FNR. Proteins extracted from thylakoids (ThEx) were mixed with recombinant C-terminal stretches of psTic62 (see A). After metal affinity chromatography, FNR co-purifies with IA2 and IA3. As a control, only the column material (–) was incubated with the thylakoid extract. The samples were subjected to SDS–PAGE and transferred to nitrocellulose. An immunodecoration with antiserum against FNR is shown. Flow through, FT; washing step, W; washing with NaCl, NaCl; eluted proteins, E. (D) Localization of FNR in chloroplasts. Intact chloroplasts were fractionated, and outer (OE) and inner envelope (IE) membranes, thylakoids (Th) and stroma (St) (10 µg of protein) were subjected to SDS–PAGE. Proteins were transferred to nitrocellulose and immunodecorated with antisera against Toc75 (outer envelope), Tic110 (inner envelope), GAPDH (stroma), FNR and LHCP (thylakoids). (E) Inner envelope vesicles (400 µg of protein) were solubilized with 3% decyl maltoside. After centrifugation, the supernatant was incubated with Tic62 antibodies coupled to AF-Tresyl 650M. Aliquots of the supernatant (S), the flow through (FT) of the Tic62 column, the last washing step (W) and the eluted (E) protein (1/5) were subjected to SDS–PAGE and transferred to nitrocellulose. Antisera raised against Tic110, Tic62, Tic40 and FNR were used to analyse the co-immunoprecipitated proteins. (F) Co-immunoprecipitation of the Tic complex with antibodies against different Tic components. Inner envelope vesicles were solubilized with 3% decyl maltoside. After centrifugation, the supernatant was incubated with Tic110 antibodies coupled to AF-Tresyl 650M (left panel) or Tic40 antibodies coupled to protein A–Sepharose (right panel). Aliquots of the supernatant (S), the flow through (FT), the last washing step (W) and the eluted protein (E) were subjected to SDS–PAGE and transferred to nitrocellulose. Antisera raised against Tic110, Tic62 and Tic40 were used to analyse the co-immunoprecipitated proteins.
Next, the association of FNR with the C-terminus of psTic62 was studied in vitro using FNR extracted from thylakoids with the recombinant polypeptides IA1, IA2 and IA3 (Figure 5C). The identity of the polypeptides was verified by immunodetection with antiserum against Tic62. The purified protein products IA1, IA2 and IA3 were mixed with protein extracted from thylakoids. Then, IA1, IA2, IA3 and interacting proteins were isolated by metal affinity chromatography. With IA2 and IA3, which include the repetitive modules, FNR co-purified as verified by immunodetection with antiserum against FNR (Figure 5C). Using IA1 as bait, none of the proteins extracted from thylakoids were detected. No interaction was observed after incubation of the extracted proteins with only the column material (Figure 5C). We conclude that the C-terminus of Tic62 functions in recruiting FNR. However, FNR is not a constituent of the Tic core complex (Figure 1A) and is generally assumed to be bound to the thylakoid membrane. To elucidate these somewhat controversial findings, we examined the location of FNR by immunodecoration of purified fractions of pea chloroplasts. The results show that FNR is present at the inner envelope membrane in addition to its expected localization at the thylakoids (Figure 5D). The highly abundant light-harvesting chlorophyll a/b-binding protein (LHCP) was not detected in inner envelopes, demonstrating that the FNR signal is not due to a thylakoidal contamination of this membrane fraction. In a second approach, inner envelope vesicles were solubilized with 3% decyl maltoside, and Tic62 antibodies were used for immunoprecipitation (Figure 5E). Tic110 and Tic40 co-precipitate with Tic62. Tic22 was also detected, but the signal was very faint (data not shown). Since the IgG heavy chains migrate at an apparent molecular weight identical to that of Tic55, detection of Tic55 was not possible. Surprisingly, FNR was significantly enriched in the immunoprecipitated fraction (Figure 5E, αTic62, lane E) in comparison with the starting material (Figure 5E, lane S). An LHCP antiserum was used as a control, which did not react with any of the precipitated proteins (Figure 5E). Using the pre-immune serum of Tic62, neither FNR nor Tic components were co-precipitated (Figure 5E, pre, lane E). Immuno precipitations with antisera against Tic110 and Tic40 were also done to confirm the affiliation of Tic62 with the complex. Antisera against both Tic110 (Figure 5E, left panel) and Tic40 (Figure 5E, right panel) immunoprecipitated Tic110, Tic62 and Tic40 (Figure 5E). The stoichiometry of the complex cannot be deduced from this experiment because each Tic subunit seems to be present also as a monomer under the experimental conditions, resulting in an enrichment in the respective immunoprecipitation. In conclusion, we suggest that FNR is involved in the action of the Tic complex and that the C-terminus of Tic62, which faces the stroma, recruits FNR.
The import of FNR isologues is differentially affected by deamino-NAD and HAR
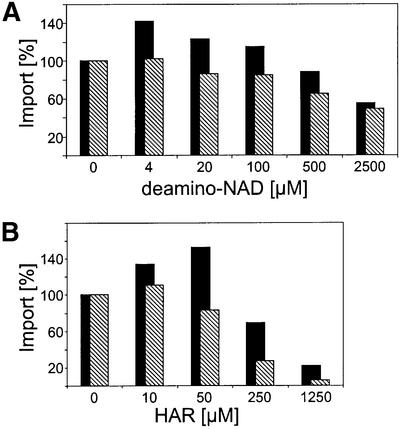
The identification of Tic55 and Tic62 as a potential electron carrier or redox partner led us to assume that import into chloroplasts could be redox regulated. Indeed, a recent report (Hirohashi et al., 2001) indicated that ferredoxin I, which is expressed predominantly in the light, imported equally well in the light and in the dark, while ferredoxin III, which is constitutively expressed, was imported correctly only in the dark but was mis-sorted to the intermembrane space in the light. Similar results were obtained for the import of FNR isoforms. Therefore, we investigated the effect of deamino-NAD as well as the influence of ruthenium hexamine trichloride (HAR) on the import of different leaf-specific Arabidopsis isoforms of FNR (see Materials and methods). In a first series of experiments, the competitor deamino-NAD was added to isolated chloroplasts 15 min prior to the import reaction. Deamino-NAD in concentrations up to 2.5 mM did not alter the binding capacity of intact chloroplasts for either pFNR-L1 or pFNR-L2 (data not shown). However, the import of pFNR-L1 was stimulated by a low concentration (4–20 µM) of deamino-NAD by ∼40% (Figure 6A). Only a concentration ≥500 µM deamino-NAD caused some inhibition of pFNR-L1 import. On the other hand, pFNR-L2 import was not stimulated but decreased in the presence of ≥20 µM deamino-NAD. The maximum import inhibition was ∼50% (Figure 6A). When chloroplasts were pre-incubated with HAR, import of pFNR-L2 was strongly inhibited and completely abolished at 1.25 mM HAR (Figure 6B). The import of pFNR-L1 was, however, again stimulated at low concentrations of effector (≤50 µM) and increased ∼50–60% (Figure 6B). Only at a higher concentration did an inhibitory effect of HAR become predominant, and import of pFNR-L1 was also inhibited. However, HAR never completely inhibited pFNR-L1 import, in contrast to pFNR-L2 (Figure 6B). The differential effect of HAR on pFNR-L1 import might be caused by a biphasic inhibition. At low concentrations, HAR non-enzymatically oxidizes the NAD(P)H pool in the stroma. This initial and still selective effect causes an increase of pFNR-L1 import at a high NAD(P)/NAD(P)H ratio. Higher concentrations of HAR will probably oxidize non-specifically other redox components, such as glutathione, thioredoxine and others, which causes a non-specific inhibition of import, as seen for both pFNR-L1 and pFNR-L2. The stimulatory effect on import caused by both deamino-NAD and HAR indicates that pFNR-L1 is translocated preferentially at a high NAD(P)/NAD(P)H ratio, i.e. in the dark, while pFNR-L2 is not influenced specifically.
Fig. 6. Import of different FNR isologues shows distinct characteristics. Import of precursor forms of FNR-L1 (dark bars) and FNR-L2 (hatched bars) into isolated chloroplasts of pea pre-incubated with different concentrations of either deamino-NAD (A) or HAR (B). Prior to the import reaction, chloroplasts (15 µg of chlorophyll) were incubated with deamino-NAD and HAR for 15 min in the dark. Import was performed for 4 min at 25°C. The chloroplasts were recollected and analysed by SDS–PAGE and autoradiography. The mature peptides were quantified using a phosphoimager. Means of at least five independent experiments are given.
Discussion
In this report, we show that in isolated inner envelope vesicles, Tic110, Tic55 and Tic62 form a stable Tic core complex in the absence of a pre-protein. In BN-PAGE, these three components constitute a complex, while after sucrose density centrifugation additional proteins were observed, which co-purify in the fraction of the Tic core complex (Figure 1B). In the fractions obtained after sucrose density centrifugation, Tic110, Tic55 and Tic62 were abundant in a different ratio (Figure 1B, sucrose gradient), indicating a dynamic composition of the Tic complex. It may be argued that the co-migration of Tic110, Tic55 and Tic62 happened coincidentally in BN-PAGE as well as after sucrose density centrifugation. This objection seems highly unlikely, because antibodies against Tic110, Tic62 and Tic55 efficiently co-immunoprecipitate the other respective Tic components (Figures 1C, and 5E and F), though the results do not reflect the stoichiometry in the complex as each antibody also precipitates its respective antigen in its monomeric state. Furthermore, when envelope membranes were solubilized by Triton X-100 prior to BN-PAGE, a Tic core complex of identical composition could be isolated, while SDS completely destroyed the complex (Figure 1A).
Since the conditions and methods used to prepare and characterize the Tic complex vary, the composition of the Tic complex has been discussed controversially (Caliebe et al., 1997; Kouranov et al., 1998; Stahl et al., 1999). In mitochondria, subcomplexes of the pre-protein translocase of the outer mitochondrial membrane were examined successfully using BN-PAGE together with co-immunoprecipitation (Dekker et al., 1998). Furthermore, the existence of different translocases of the inner mitochondrial membrane corresponding to different subsets of pre-proteins was shown (Koehler, 2000). Therefore, it could be that in chloroplasts, different subcomplexes of the Tic translocon exist, which use Tic110 as a central translocation pore, but have distinct additional subunits to form translocons of different specificity or regulation.
The amino acid sequence of Tic62 can be subdivided in two functional regions (Figure 3). The C-terminal region of Tic62 contains a novel repetitive sequence module, which associated with FNR at the inner envelope membrane of chloroplasts. In isolated inner envelope vesicles, the C-terminus of Tic62 was protected against treatment with protease, as shown by immunodetection with an antibody raised against a C-terminal 14 kDa portion of Tic62 (Figure 2D). The association of FNR with the C-terminus of Tic62 was investigated by use of genetic and biochemical methods (Figure 5). A minor distinction between Tic62 from pea and Arabidopsis is the number of three or four beaded repetitive modules at their C-termini, respectively. However, the genetic as well as the biochemical approaches verified that even one module is enough to bind FNR. The interaction of FNR with its usual redox partner ferredoxin involves electrostatic forces: positively charged residues of FNR interact with negatively charged residues of ferredoxin (Dorowski et al., 2001) but, in addition, FNR forms a hydrophobic cavity of unknown function (Bruns and Karplus, 1995; Serre et al., 1996). The repetitive sequence modules at the C-terminus of Tic62, which interacted with FNR in vitro (Figure 5), are actually separated by spacers with a negative net charge. It is not clear whether the repetitive module of hydrophobic amino acids or the spacer region containing negatively charged amino acids is actually responsible for the interaction of Tic62 with FNR. Usually, FNR catalyses the electron transfer from ferredoxin to the electron acceptor NAD(P)+, raising the question of the function of the FNR associated with Tic62. Further questions are whether an unknown type of signal induces binding of FNR to Tic62 or if it is bound permanently. Palatnik et al. (1997), for example, demonstrated the release of FNR from thylakoids under oxidative stress, which might provide an FNR pool to bind to the inner envelope. The transcription of FNR itself was suggested to be dependent on the redox state of the plastoquinone pool (Pfannschmidt et al., 2001). However, the expression of an FNR construct comprising the transit peptide was shown to be independent of the plastoquinone pool but dependent on an unknown redox signal (Pfannschmidt et al., 2001). Since the transcription and expression of FNR are sensitive to the redox state of the chloroplast, its association with Tic62 might reflect a signal cascade involved in sensing the redox state of the chloroplast. FNR could be involved in an electron transfer to the pyridine nucleotide, which is a co-factor of Tic62.
The database searches show that Tic62 is only present in plants. The N-proximal region of Tic62 resembles a Ycf39-like family of isoflavone reductases. These proteins utilize pyridine nucleotides as a co-factor (Babiychuk et al., 1995). The capacity of psTic62 to bind pyridine nucleotides was shown in vitro (Figure 4B). The NAD binding of psTic62 was only competed for by the ribosyl nicotinamide, but not by the adenosyl-diphosphate moiety (Figure 4C). Ycf39- and isoflavone reductase-like proteins are supposed to have broader functions, including a chaperone-like function involved in insertion of plastoquinone A into PSII centres in Synechocystis sp. PCC6803 (Ermakova-Gerdes and Vermaas, 1999). Another isoflavone reductase-like protein responded to oxidative stress in A.thaliana (Babiychuk et al., 1995). We demonstrated that Tic62 is an integral protein of the inner envelope membrane (Figure 2B) supported by computer analysis predicting one or two transmembrane helices in the N-terminal region of Tic62. This result is inconsistent with the localization suggested for Ycf39- and isoflavone reductase-like proteins. Therefore, the conversion to a membrane-bound protein and the C-terminal extension of Tic62 indicate that in chloroplasts the function is different from that of Ycf39- and isoflavone reductase-like proteins.
Two regulatory levels are presented commonly for chloroplast protein import, i.e. the phosphorylation of precursor proteins in the cytosol and the nucleotide-dependent control of precursor binding and translocation across the outer chloroplast envelope. In addition, for several nuclear-encoded chloroplast proteins, regulation of gene expression by redox mechanisms has been argued (Escoubas et al., 1995; Oswald et al., 2001; Pfannschmidt et al., 2001). Therefore, the adaptation of chloroplast import to the reductant homeostasis of the chloroplast could represent another checkpoint of import regulation of a subset of proteins. The influence of light and, consequently, the redox state of the chloroplast was suggested recently to influence chloroplast import in vitro (Hirohashi et al., 2001). In the presence of light, the non-photosynthetic ferredoxin III, which is usually localized in the stroma, was mis-sorted to the intermembrane space between the envelope membranes (Hirohashi et al., 2001). To investigate the influence of a pyridine nucleotide-binding protein such as Tic62 on the import of pre-proteins into chloroplasts, deamino-NAD or HAR were added to the import reaction. HAR is a water-soluble electron acceptor that supports a high rate of pyridine nucleotide oxidation (Zickermann et al., 2000). The import of pFNR-L1 was stimulated by a low concentration of HAR, while a general overriding inhibition was observed at higher concentrations. This opposite biphasic effect of HAR is most likely to be due to an initial selective oxidation of the NAD(P)H pool with little or no indirect influence on the import capacity of the chloroplasts. At higher concentrations, the general oxidative effect of HAR is prevalent and causes a general decrease of import capacity. However, in contrast to pFNR-L2, HAR does not cause a complete block of pFNR-L1 import, an indication that translocation of both proteins is differentially regulated. Similarly, deamino-NAD, an NAD analogue containing a hypoxanthine instead of the adenosyl phosphate moiety, also stimulated the import of pFNR-L1 at concentrations ≤100 µM. Again pFNR-L2 was not influenced by these deamino-NAD concentrations. We conclude that a high NAD(P)/NAD(P)H ratio stimulates pFNR-L1 import, while pFNR-L2 does not react to a change in the NAD(P)/NAD(P)H ratio. We could not show that the effect of HAR and deamino-NAD resulted directly from a change of the redox state of Tic62 or a concomitant conformational change. However, Tic62 is tightly associated with Tic110 (Figure 1), which is at least a major part of the translocation pore of the Tic complex. It is therefore likely that HAR and deamino-NAD acted directly and indirectly, respectively, on Tic62 when affecting pre-protein import. Several potassium channels comprise pore-forming α-subunits and auxiliary β-subunits (Bähring et al., 2001). The β-subunits, which utilize pyridine nucleo tides as a cofactor, recently were shown to regulate the activity of a potassium channel by a redox mechanism (Bähring et al., 2001; Zhou et al., 2001). Therefore, a function of Tic62 in regulating the translocation pore Tic110 dependent on its redox state is conceivable. Further experiments are needed to show whether an entire Tic complex is regulated possibly by a NAD(H)/NAD(P)H turnover correlating with FNR binding/release. On the other hand, different Tic subcomplexes with distinct regulatory import features may account for divergent import characteristics of alternating protein sets.
In summary, strong evidence was provided that at the inner envelope of chloroplasts, Tic62 associated with FNR might be a checkpoint of pre-protein translocation regulated by a novel redox mechanism. Together with Tic55, which has a Rieske-type iron–sulfur centre (Caliebe et al., 1997), Tic62 and FNR could sense the redox state of the chloroplast to affect the transport of a subset of chloroplast pre-proteins.
Materials and methods
Preparation of chloroplasts and chloroplast subfractions
Intact chloroplasts were isolated from 12- to 14-day-old pea plants (Pisum sativum L., var. Golf) as previously described (Keegstra and Youssif, 1986; Waegemann and Soll, 1991). Outer and inner envelope membrane vesicles were recovered from the supernatant by sucrose density centrifugation (Waegemann and Soll, 1991).
BN-PAGE
The first dimension of BN-PAGE was performed basically as described by Caliebe et al. (1997) except that protein complexes of interest were subjected to a second round of BN-PAGE. To do this, the band containing the Tic complex was cut and electro-eluted (Electroeluter/concentrator, CBS Scientific, Canada) in 0.1% phenylmethylsulfonyl fluoride (PMSF), 25 mM Tricine, 7.5 mM Bis-Tris pH 7.0 at 4°C overnight. The eluate was subjected to a second BN-PAGE. A complete lane or cut bands containing the Tic complex of the second dimension were soaked in 1% β-mercaptoethanol, 1% SDS for 20 min. Denaturating SDS–PAGE was used to identify the constituents of the Tic core complex.
Isolation of a cDNA clone encoding the C-terminal portion of Tic62
Protein complexes of the inner envelope membranes were isolated using BN-PAGE and SDS–PAGE as described above. Proteins blotted onto PVDF membranes were sequenced either from the N-terminus of the protein or after digestion with endoproteinase lys-C (Boehringer, Mannheim, Germany). A degenerate oligonucleotide was derived and used for screening a cDNA expression library (Uni Zap XR, Stratagene, CA) (Caliebe et al., 1997).
Generation of a full-length cDNA clone encoding psTic62 by 5′-RACE technology
The isolation of poly(A)+ RNA from 125 mg of leaf material of 12- to 14-day-old light-grown pea seedlings (P.sativum L., var. Golf) was performed using the PolyATtract® System 1000 (Promega, USA) according to the manufacturer’s instructions. The isolated poly(A)+ RNA was used for a 5′-RACE cDNA amplification using the SMART™ RACE cDNA Amplification Kit (Clontech, USA) and a gene specific primer for the 5′-RACE PCR. Database searches were performed with TBLASTN and BLAST & BEAUTY (Worley et al., 1995), and sequence alignments with CLUSTALW 1.6 (Thompson et al., 1994) at the BCM Search Launcher (Houston, TA).
Raising of antibodies
A nucleotide fragment encoding K412–S534 of psTic62 was subcloned into the pET21d vector (Novagen, USA), and expressed in Escherichia coli BL21 (DE3) (Novagen, USA). The expressed 12 kDa peptide was purified from the soluble compartment by its C-terminal polyhistidine tag using Ni-NTA–agarose (Qiagen, Germany).
Binding of NADs and analogous nucleotides
In a final volume of 40 µl, inner envelope vesicles equivalent to 50 µg of protein were resuspended in 20 mM Tris–HCl pH 7.4 in the presence of 35 pmol of 32P-labelled NAD+ (NEN Life Science Products, USA). Optionally, 0.1% Triton X-100 or 0.2% SDS were included. Analogous nucleotides (10 µM) used for the competition were added prior to the binding of 32P-labelled NAD+. After 10 min on ice, the samples were illuminated with UV light (Soll and Fischer, 1988). Finally, the samples were subjected to SDS–PAGE, transferred to nitrocellulose and analysed by autoradiography or immunodecoration.
Immunoprecipitation of Tic components
The Tic complex purified by BN-PAGE was electro-eluted (see above). The eluate was diluted with 0.13% decyl maltoside and IP buffer (0.01% egg albumin, 150 mM NaCl, 25 mM Tricine, 7.5 mM Bis-Tris pH 7.0). A 5 µl aliquot of antisera raised against Tic110, Tic55, Tic62 and pre-immune serum of Tic62 were mixed with the eluate (100 µg protein). After cross-linking with 32P-labelled NAD+, inner envelope vesicles were solubilized with 0.4% SDS prior to immunoprecipitation. The antibody complexes were isolated using protein A–Sepharose (Amersham Pharmacia Biotech, Germany). Isolated inner envelope vesicles equivalent to 400 µg of protein were first solubilized with 3% decyl maltoside, 50 mM aminocaproic acid, 20 mM Tris pH 7.4. After centrifugation, the supernatant was incubated with either pre-immune serum, or Tic62 or Tic110 antiserum coupled to AF-Tresyl 650M (TosoHas, Germany) in the presence of 250 mM NaCl, 0.3% decyl maltoside, 0.01% egg albumin, 20 mM Tris pH 7.4. Immunoprecipitation with Tic40 antiserum was performed using a modified IP buffer (150 mM NaCl, 25 mM HEPES–NaOH pH 7.6) and protein A–Sepharose.
Screening of a yeast two-hybrid cDNA library
Three cDNA fragments encoding A247–L388 (IA1), M263–S444 (IA2) or V346–S534 (IA3) of psTic62 were subcloned into the pBD vector (Stratagene, USA) of a HybriZAP two-hybrid™ cDNA library made from 8-day-old light-grown pea seedlings (P.sativum L. var. Golf). The screening procedure, the identification of interacting proteins and control transformations were performed according to the manufacturer’s instructions (Stratagene, USA).
Binding assay of FNR and Tic62
FNR was enriched by extraction of thylakoids equivalent to 300 µg of chlorophyll. Thylakoids were resuspended in 0.5 M NaCl, 10 mM Tricine pH 7.8 for 30 min at 4°C. After centrifugation at 12 000 g for 10 min at 4°C, the supernatant was used as thylakoid extract. After subcloning of the cDNA fragments into the pET21d vector, proteins with a C-terminal polyhistidine tag were expressed in E.coli BL21 (DE3) and purified using Ni-NTA–agarose. Imidazole was removed by dialysis against 100 mM NaCl, 10 mM Tricine pH 7.6. A 10 µg aliquot of IA1, IA2 and IA3, respectively, was mixed with a thylakoid extract in 50 mM NaCl, 10 mM Tricine pH 7.6 at 4°C overnight. Then, the Tic62 polypeptides and interacting proteins were isolated by binding to Ni-NTA–agarose. One-four-hundredth of the supernatant, the flow through, the last washing step and the eluted protein were subjected to SDS–PAGE, transferred to nitrocellulose and analysed by immunodecoration.
Import into chloroplasts
Isolation of chloroplasts and import reactions were carried out as described previously (Waegemann and Soll, 1991). We used expressed sequence tag (EST) clones for pFNR-L1 (accession Nos AV527056 and At5g66190) and pFNR-L2 (accession Nos AV526098 and At1g20020) from Kazusa DNA Research Institute (Chiba, Japan). After translation in a reticulocyte lysate, 35S-labelled pFNR-L1 and pFNR-L2 subcloned to pSP65 were mixed with chloroplasts equivalent to 15 µg of chlorophyll for 4 min at 25°C. Chloroplasts were recollected and analysed by SDS–PAGE and autoradiography. The mature peptides were quantified using a phosphoimager (Bas-1500; Fuji, USA). Tina 2.0 software was used.
Acknowledgments
Acknowledgements
We thank Ralf Bernd Klösgen for the generous gift of antiserum against FNR, and Christel Glockmann for performing the electron microscopy. This work was supported in part by a grant of the Deutsche Forschungsgemeinschaft and Fonds der Chemischen Industrie.
References
- Babiychuk E., Kushnir,S., Belles-Boix,E., Van Montagu,M. and Inze,D. (1995) Arabidopsis thaliana NADPH oxidoreductase homologs confer tolerance of yeast toward the thiol-oxidizing drug diamide. J. Biol. Chem., 270, 26224–26231. [DOI] [PubMed] [Google Scholar]
- Bähring R. et al. (2001) Coupling of voltage-dependent potassium channel inactivatition and oxidoreductase active site of Kvβ subunits. J. Biol. Chem., 276, 22923–22929. [DOI] [PubMed] [Google Scholar]
- Bruns C.M. and Karplus,P.A. (1995) Refined crystal structure of spinach ferredoxin reductase at 1.7 Å resolution: oxidised, reduced and 2′-phospho-5′-AMP bound states. J. Mol. Biol., 247, 125–145. [DOI] [PubMed] [Google Scholar]
- Budziszweski G.J. et al. (2001) Arabidopsis genes essential for seedling viability: isolation of insertional mutants and molecular cloning. Genetics, 159, 1765–1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caliebe A., Grimm,R., Kaiser,G., Lübeck,J., Soll,J. and Heins,L. (1997) The chloroplastic protein import machinery contains a Rieske-type iron–sulfur cluster and a mononuclear iron-binding protein. EMBO J., 16, 7342–7350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K., Chen,X. and Schnell,D.J. (2000) Initial binding of preproteins involving the Toc159 receptor can be bypassed during protein import into chloroplasts. Plant Physiol., 122, 813–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekker P.J.T., Ryan,M.T., Brix,J., Müller,H., Hönlinger,A. and Pfanner,N. (1998) Preprotein translocase of the outer mitochondrial membrane: molecular dissection and assembly of the general import pore complex. Mol. Cell. Biol., 18, 6515–6524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorowski A., Hofmann,A., Steegborn,C., Boicu,M. and Huber,R. (2001) Crystal structure of paprika ferredoxin-NADP+ reductase. J. Biol. Chem., 276, 9253–9263. [DOI] [PubMed] [Google Scholar]
- Emanuelsson O. and von Heijne,G. (2001) Prediction of organellar targeting signals. Biochim. Biophys. Acta, 1541, 114–119. [DOI] [PubMed] [Google Scholar]
- Ermakova-Gerdes S. and Vermaas,W. (1999) Inactivation of the open reading frame slr0399 in Synechocystis sp. PCC6803 functionally complements mutations near the QA niche of photosystem II. J. Biol. Chem., 274, 30540–30549. [DOI] [PubMed] [Google Scholar]
- Escoubas J.M., Lomas,M., LaRoche,J. and Falkowski,P.G. (1995) Light intensity regulation of cab gene transcription is signaled by the redox state of the plastoquinione pool. Proc. Natl Acad. Sci. USA, 92, 10237–10241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulgosi H., Gerdes,L., Westpfahl,S., Glockmann,C. and Soll,J. (2002) Cell and chloroplast division requires ARTEMIS. Proc. Natl Acad. Sci. USA, 99, 11501–11506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heins L., Mehrle,A., Hemmler,R., Wagner,R., Küchler,M., Hörmann,F., Sveshnikov,D. and Soll,J. (2002) The preprotein conducting channel at the inner envelope membrane of plastids. EMBO J., 21, 2616–2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirohashi T., Hase,T. and Nakai,M. (2001) Maize non-photosynthetic ferredoxin precursor is mis-sorted to the intermembrane space of chloroplasts in the presence of light. Plant Physiol., 125, 2154–2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch S., Muckel,E., Heemeyer,F., von Heijne,G. and Soll,J. (1994) A receptor component of the chloroplast protein translocation machinery. Science, 266, 1989–1992. [DOI] [PubMed] [Google Scholar]
- Keegstra K. and Yousif,A.E. (1986) Isolation and characterization of chloroplast envelope membranes. Methods Enzymol., 118, 316–325. [Google Scholar]
- Kessler F. and Blobel,G. (1996) Interaction of the protein import and folding machineries in the chloroplast. Proc. Natl Acad. Sci. USA, 93, 7684–7689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler F., Blobel,G., Patel,H.A. and Schnell,D.J. (1994) Identification of two GTP-binding proteins in the chloroplast protein import machinery. Science, 266, 1035–1039. [DOI] [PubMed] [Google Scholar]
- Koehler C.M. (2000) Protein translocation pathways of the mitochondrion. FEBS Lett., 476, 27–31. [DOI] [PubMed] [Google Scholar]
- Kouranov A. and Schnell,D.J. (1997) Analysis of the interactions of preproteins with the import machinery over the course of protein import into chloroplasts. J. Cell Biol., 139, 1677–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouranov A., Chen,X., Fuks,B. and Schnell,D.J. (1998) Tic20 and Tic22 are new components of the protein import apparatus at the chloroplast inner envelope membrane. J. Cell Biol., 143, 991–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Küchler M. and Soll,J. (2001) From nuclear genes to chloroplast localized proteins. Plant Sci., 161, 379–389. [Google Scholar]
- Lübeck J., Soll,J., Akita,M., Nielsen,E. and Keegstra,K. (1996) Topology of IEP110, a component of the chloroplastic protein import machinery present in the inner envelope membrane. EMBO J., 15, 4230–4238. [PMC free article] [PubMed] [Google Scholar]
- Nielsen E., Akita,M., Davila-Aponte,J. and Keegstra,K. (1997) Stable association of chloroplastic precursors with protein-translocation complexes that contain proteins from both envelope membranes and a stromal Hsp100 molecular chaperone. EMBO J., 16, 935–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen L.J. and Keegstra,K. (1992) The binding of precursor proteins to chloroplasts requires nucleotide triphosphates in the intermembrane space. J. Biol. Chem., 267, 433–439. [PubMed] [Google Scholar]
- Oswald O., Martin,T., Dominy,P.J. and Graham,I.A. (2001) Plastid redox state and sugars: interactive regulators of nuclear-encoded photosynthetic gene expression. Proc. Natl Acad. Sci. USA, 98, 2047–2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palatnik J.F., Valle,E.M. and Carillo,N. (1997) Oxidative stress causes ferredoxin-NADP+ reductase solubilization from the thylakoid membranes in methyl viologen-treated plants. Plant Physiol., 115, 1721–1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry S.E. and Keegstra,K. (1994) Envelope membrane proteins that interact with chloroplastic precursor proteins. Plant Cell, 6, 93–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfannschmidt T., Schütze,K., Brost,M. and Oelmüller,R. (2001). A novel mechanism of nuclear photosynthesis gene regulation by redox signals from the chloroplast during photosystem stoichiometry adjustment. J. Biol. Chem., 276, 36125–36130. [DOI] [PubMed] [Google Scholar]
- Schnell D., Blobel,G., Keegstra,K., Kessler,F., Ko,K. and Soll,J. (1997) A consensus nomenclature for the protein-import components of the chloroplast envelope. Trends Cell Biol., 7, 303–304. [DOI] [PubMed] [Google Scholar]
- Serre L., Vellieux,F.M.D., Medina,M., Gomez-Moreno,C., Fontecilla-Camps,J.C. and Frey,M. (1996) X-ray structure of the ferredoxin:NADP+ reductase from the cyanobacterium Anaebaena PCC 7119 at 1.8 Å resolution and crystallographic studies of NADP+ binding at 2.25 Å resolution. J. Mol. Biol., 262, 20–39. [DOI] [PubMed] [Google Scholar]
- Soll J. and Fischer,I. (1988) Analysis of chloroplast envelope membranes using photoaffinity label. J. Plant Physiol., 132, 631–635. [Google Scholar]
- Stahl T., Glockmann,C., Soll,J. and Heins,L. (1999) Tic40, a new ‘old’ subunit of the chloroplast protein import. J. Biol. Chem., 274, 37467–37472. [DOI] [PubMed] [Google Scholar]
- Sveshnikova N., Soll,J. and Schleiff,E. (2000) Toc34 is a preprotein receptor regulated by GTP and phosphorylation. Proc. Natl Acad. Sci. USA, 97, 4973–4978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theg S.M., Bauerle,C., Olsen,L.J., Selman,B.R. and Keegstra,K. (1989) Internal ATP is the only energy requirement for the translocation of precursor proteins across chloroplastic membranes. J. Biol. Chem., 264, 6730–6736. [PubMed] [Google Scholar]
- Thompson J.D., Higgins,D.G. and Gibson,T.J. (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignments through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res., 22, 4673–4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waegemann K. and Soll,J. (1991) Characterization of the protein import apparatus in isolated outer envelopes of chloroplasts. Plant J., 1, 149–158. [Google Scholar]
- Worley K.C., Wiese,B.A. and Smith,R.F. (1995) BEAUTY: an enhanced BLAST-based search tool that integrates multiple biological information resources into sequence similarity results. Genome Res., 5, 173–184. [DOI] [PubMed] [Google Scholar]
- Zhou M., Morals-Cabral,J.H., Mann,S. and MacKinnon,R. (2001) Potassium channel receptor site for the inactivation gate and quaternary amine inhibitors. Nature, 411, 657–661. [DOI] [PubMed] [Google Scholar]
- Zickermann V., Kurki,S., Kervinen,M., Hassinen,I. and Finel,M. (2000) The NADH oxidation domain of complex I: do bacterial and mitochondrial enzymes catalyze ferricyanide reduction similarly? Biochim. Biophys. Acta, 1459, 61–68. [DOI] [PubMed] [Google Scholar]