Abstract
The nucleoid protein p6 of Bacillus subtilis phage φ29 binds to DNA, recognizing a structural feature rather than a specific sequence. Upon binding to the viral DNA ends, p6 generates an extended nucleoprotein complex that activates the initiation of φ29 DNA replication. Protein p6 also participates in transcription regulation, repressing the early C2 promoter and assisting the viral regulatory protein p4 in controlling the switch from early to late transcription. Proteins p6 and p4 bind cooperatively to an ∼200 bp DNA region located between the late A3 and the early A2c promoters, generating an extended nucleoprotein complex that helps to repress the early A2c promoter and to activate the late A3 promoter. We show that stable assembly of this complex requires interaction between protein p6 and the C-terminus of protein p4. Therefore, at this DNA region, stable polymerization of protein p6 relies on p4-specified signals in addition to the structural features of the DNA. Protein p4 would define the phase and boundaries of the p6–DNA complex.
Keywords: bacterial promoters/nucleoid proteins/phage φ29/protein–protein interactions/transcription regulation
Introduction
Nucleoid proteins (also known as chromatin-associated or histone-like proteins) are a group of DNA-binding proteins that assist in organizing the local as well as the global structure of bacterial chromosomes (reviewed in McLeod and Johnson, 2001). Similar architectural factors are found in eukaryotes, where they perform analogous functions. Besides participating in compacting the chromosome, and in processes such as DNA replication and recombination, they can act as positive or negative modulators of gene expression. Nucleoid proteins bind DNA non-specifically, although some of them show a preference for certain DNA sequences or structures. For example, the Escherichia coli HU protein binds DNA non-specifically, but shows higher affinity for kinked or gapped DNA sequences, and for cruciform structures (Bianchi, 1994; Castaing et al., 1995; Kobryn et al., 1999; Pinson et al., 1999). Other nucleoid proteins, such as FIS (Hengen et al., 1997) or IHF (Goodrich et al., 1990), recognize targets showing a degenerated consensus sequence. Nucleoid proteins can regulate gene expression directly by binding to and affecting the function of a promoter, or can act in combination with sequence-specific regulatory proteins (reviewed in McLeod and Johnson, 2001). In this latter case, the synergy may arise either through the ability of the nucleoid protein to introduce a change in the DNA conformation that hinders or assists the function of the sequence-specific regulatory protein (a passive architectural role), or through direct interactions between the two proteins. In the case of nucleoid proteins recognizing DNA structures, rather than specific sequences, very few examples have been studied. A well characterized example involves the E.coli gal operon. The GalR repressor binds to operators OE and OI, located 113 bp apart. In negatively supercoiled DNA, the nucleoid protein HU binds to a site located between these two operators, bringing the two GalR dimers together to form a higher order complex called the Gal repressosome (Aki and Adhya, 1997; Adhya et al., 1998). Repressosome assembly requires a specific protein–protein interaction between HU and GalR (Kar and Adhya, 2001).
Transcription regulation of the Bacillus subtilis phage φ29 genome provides a different and interesting case of cooperation between a nucleoid and a sequence-specific DNA-binding protein. Expression of phage φ29 genes occurs in two stages, early and late (Salas and Rojo, 1993; Monsalve et al., 1995). The switch from early to late transcription is controlled by the viral proteins p4 and p6. Protein p4 is a dimeric sequence-specific DNA-binding protein that binds to three sites in the φ29 genome (see Figure 1A). Binding of two p4 dimers to site 3 results in repression of the early A2b promoter by preventing RNA polymerase (RNAP) binding to it (Rojo and Salas, 1991), and in activation of the divergently oriented late A3 promoter (Barthelemy and Salas, 1989). Activation of the A3 promoter requires an interaction between the C-end of protein p4 and the C-terminal domain of the RNAP α-subunit (Mencía et al., 1996a,b). This interaction stabilizes the binding of RNAP to the promoter (Nuez et al., 1992). Protein p4 has two low affinity binding sites at the early A2c promoter (Monsalve et al., 1998). In the absence of RNAP, p4 binds to site 1, centred at position –39 relative to the A2c start site (see Figure 1A). Binding of RNAP to the A2c promoter displaces p4 from this site and favours its binding to site 2, which is centred at position –71. When bound at site 2, protein p4 interacts with the RNAP α-subunit and represses transcription by inhibiting promoter clearance (Monsalve et al., 1996a,b, 1997). Protein p6 is a small dimeric nucleoid protein, very abundant in infected cells. It binds to DNA, recognizing a structural feature rather than a specific sequence, forming extended complexes at DNA regions showing a tendency to bend with a defined periodicity (every ∼12 bp). This structural feature exists at the viral DNA ends, where p6 forms a nucleoprotein complex that plays an important role in the initiation of φ29 DNA replication (Serrano et al., 1989). In this complex, DNA forms a right-handed solenoid wrapped around a multimeric p6 core (Serrano et al., 1990). Protein p6 also participates in the regulation of some viral promoters. When bound to the right end of the genome, it represses the early C2 promoter (Whiteley et al., 1986; Barthelemy et al., 1989; Camacho and Salas, 2001a). In addition, protein p6 participates, together with protein p4, in the regulation of the A2c, A2b and A3 promoters. Initial evidence arose from the observation that binding of p4 to site 3 promoted the formation of an extended p6 nucleoprotein complex that covered the A2c promoter, repressing it by inhibiting RNAP binding (Elías-Arnanz and Salas, 1999). The binding of p4 and p6 to DNA was cooperative. It was observed later that a mutation in gene 6, coding for protein p6, affects not only DNA replication but also regulation of the early A2b and A2c promoters in infected cells (Camacho and Salas, 2000). Subsequent analyses suggested that the nucleo protein complex formed by p6 at the A2c promoter was stabilized not only by the p4 molecule bound at the A3 promoter, but also by another p4 molecule bound at the A2c promoter (Camacho and Salas, 2001b). The precise location of this second p4 site was not determined at the time. The cooperative binding of proteins p6 and p4 to DNA suggested that the complex formed might be stabilized by interactions between the two proteins. This issue has now been addressed by investigating the ability of a collection of protein p4 mutants to stimulate formation of the p4–p6 nucleoprotein complex. A number of p4 residues whose modification does not affect specific DNA binding, but impairs formation of the p4–p6 nucleoprotein complex, were found. The location of the second p4 site at this complex has now been mapped. The results reported explain the role of p4 in formation of the extended p4–p6 nucleoprotein complex at the A2c promoter.
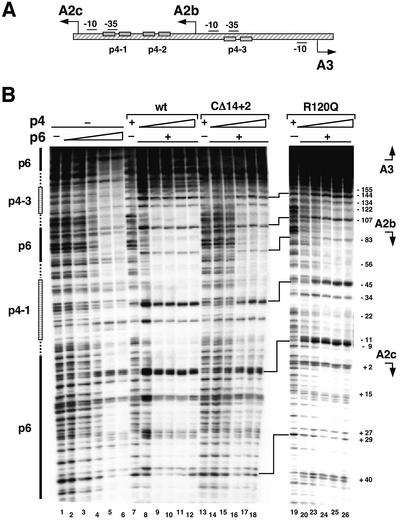
Fig. 1. Effect of mutations at the C-end of protein p4 on repression of the A2c promoter in the presence of protein p6. (A) Scheme illustrating the relative location of promoters A2c, A2b, A3 and C2 in the φ29 genome. The position of the three p4-binding sites (p4-1, p4-2 and p4-3) is indicated, as well as the –10 and –35 boxes for RNAP at each promoter. Arrows indicate the direction and transcription start site of each promoter. (B) In vitro transcription reactions included the complete φ29 genome as template, RNAP (50 nM), protein p6 (10.6 µM) and protein p4 [41, 82, 165, 330 or 660 nM in the case of wild-type p4 and p4(CΔ14 + 2), and 82, 165, 330 or 660 nM for protein p4 mutants p4(R120Q), p4(R118Q) and p4(K122Q)]. The transcripts originated at promoters C2, A2c or A3 are indicated.
Results
Modification of protein p4 C-end impairs p6-mediated repression of the A2c promoter
To study whether the cooperative binding of proteins p4 and p6 to the A2c and A3 promoters requires a direct interaction between the two proteins, the ability of a set of protein p4 mutants to form such a complex was analysed. The p4 derivatives used, which have been described previously (Rojo et al., 1990; Mencía et al., 1993, 1996b), contain substitutions or deletions at the C-end. This region of the protein includes residue R120, an amino acid that is critical for the interaction between protein p4 and the C-terminal domain of the RNAP α-subunit, that leads to activation of the late A3 promoter (Mencía et al., 1993, 1996b) and to repression of the early A2c promoter in the absence of p6 (Monsalve et al., 1996a). All the protein p4 mutant derivatives used conserve their DNA-binding capacity. The ability of these mutant proteins to stimulate protein p6-mediated repression of the A2c promoter was analysed by in vitro transcription assays. The reactions were performed using limiting concentrations of protein p4 that do not achieve full repression of the A2c promoter in the absence of p6, and including protein p6 at concentrations that allow repression of the p4-independent C2 promoter, but which do not affect expression of promoters A2c or A3 in the absence of p4 (see Figure 1B, lanes 1 and 2). As previously described (Elías-Arnanz and Salas, 1999), repression of the early A2c promoter at low protein p4 concentrations was enhanced in the presence of protein p6 (Figure 1B, lanes 3–12). In addition, activation of the late A3 promoter was also more efficient in the presence of protein p6 (Figure 1B, lanes 3–12). As a first approach to analyse the effect of the p4 C-end on this process, mutant protein p4(CΔ14 + 2) was used. This protein lacks the last 14 residues of the p4 C-end (residues 112–125), among them the critical residue R120 required to interact with RNAP. As a consequence, it can neither repress promoter A2c nor activate promoter A3 through interactions with RNAP (Rojo et al., 1990; Mencía et al., 1993, 1996b; see Figure 1B, lanes 13–17). Protein p4(CΔ14 + 2) did not stimulate repression of the A2c promoter by protein p6 (Figure 1B, lanes 18–22). This indicates that the p4 C-end could be important for a correct functional interaction between proteins p4 and p6 when binding to this region of the viral genome. The possible role of residue R120 was analysed with mutant protein p4(R120Q), in which p4 residue R120 has been modified to Q, producing a protein that can bind to DNA but cannot interact with RNAP (Mencía et al., 1993, 1996b). As a consequence, p4(R120Q) is unable to repress the A2c promoter and cannot activate the A3 promoter in the absence of p6 (see Figure 1B, lanes 23–26; Mencía et al., 1996b; Monsalve et al., 1996a). Interestingly, protein p6 restored the ability of p4(R120Q) to repress the A2c promoter (Figure 1B, lanes 27–30), indicating that residue R120 is not essential to generate the p4–p6 nucleoprotein complex that represses this promoter. It should be noted, however, that repression efficiency decreased ∼2-fold with respect to wild-type p4. It is worth noting that protein p6 also partially restored the ability of p4(R120Q) to activate the late A3 promoter (Figure 1B, lanes 27–30). These results prompted us to analyse the effect of other mutations at the protein p4 C-end. Modification of protein p4 residues R118 or K122 into Q, which are not important for contact with RNAP, decreased by 8-fold the ability of the corresponding mutant proteins (R118Q and K122Q) to repress the A2c promoter in the presence of protein p6 (Figure 1B, lanes 31–46). However, activation of promoter A3 by these p4 derivatives was stimulated by protein p6. These results, summarized in Table I, indicate that although the protein p4 C-end is important for p6-assisted repression of the A2c promoter, the determinants involved are different from those used to interact with RNAP.
Table I. Ability of protein p6 to stimulate repression of the early A2c promoter by protein p4 derivatives harbouring mutations at the C-end.
| p4 protein | PA2c repression (x-fold) | p4–p6 complex (x-fold) | PA2c occlusion (x-fold) |
|---|---|---|---|
| Wild-type | 1 | 1 | 1 |
| CΔ14 + 2 | >16 | 4 | 16 |
| R116S | NT | 4 | 4 |
| L117A | NT | 2 | 2 |
| R118Q | 8 | 8 | 8 |
| E119Q | NT | 2 | NT |
| R120Q | 2 | 2–4 | 4 |
| R121S | NT | 16 | NT |
| K122Q | 8 | 8 | 8 |
All the p4 mutants analysed can bind to DNA; their ability to repress the A2c promoter in the absence of protein p6 has been described previously (Monsalve et al., 1996a). The ability of each mutant protein to repress the A2c promoter in the presence of protein p6 is indicated as the increase in protein concentration required for a complete repression in in vitro transcription assays relative to wild-type p4 (left column; values obtained from Figure 1B and assays not shown). The central and right columns show the increase in protein concentration (relative to the wild-type) required to form a p4–p6 nucleoprotein complex, or to impede RNAP binding to the A2c promoter, as determined by DNase I footprinting assays. The values shown were obtained by densitometric scanning of the appearance of the DNase I-hypersensitive band at position –45 (indicative of formation of the p4–p6 complex; see Figure 2), or of the disappearance of the DNase I-hypersensitive band at position –37 (indicative of RNAP binding to promoter A2c; see Figure 4). NT, not tested
Protein p4 C-end has a role in formation of the p4–p6 complex at the A2c promoter
The decrease in repression efficiency of the p4 mutants analysed could be the consequence of an impaired formation of the p4–p6 nucleoprotein complex. This prediction was tested by DNase I footprinting. In the absence of protein p4, protein p6 forms a complex at the DNA region encompassing the A3 and A2c promoters that gives rise to a set of protected regions interspersed by non-protected positions (see Figure 2B, lanes 2–6; Elías-Arnanz and Salas, 1999; Camacho and Salas, 2001b). The simultaneous presence of protein p4, even at low concentrations, increases the affinity of protein p6 for this DNA region >16-fold, allowing the formation of an organized and more stable extended complex, with characteristic protected regions flanked by non-protected or hypersensitive bands (Figure 2B, lanes 8–12). It is worth noting that the presence of protein p4 modified the footprinting pattern of p6 in the region located between the A3 and A2c promoters, but not downstream of the A2c promoter start site. When mutant protein p4(CΔ14 + 2) was used, formation of the p4–p6 nucleoprotein complex was significantly impaired (Figure 2B, lanes 14–18), which explains why p6 and p4(CΔ14 + 2) cannot repress the A2c promoter in the transcription assays. The concentration of protein p4(CΔ14 + 2) required to form a stable p4–p6 complex was 4-fold higher than in the case of wild-type p4. Furthermore, the complex was similar to that formed by the wild-type p4 mainly in the region located between p4-binding site 3 (located at promoter A3) and the A2c promoter, since protections downstream from p4-binding site 1 at the A2c promoter were much weaker when p4(CΔ14 + 2) was used. DNase I footprinting performed in the presence of p6 and p4(R120Q) showed that modification of p4 residue R120 did not have a large effect in the efficiency of complex formation (a 2- to 4-fold decrease; Figure 2B, lanes 20–26), which agrees with its ability to repress the A2c promoter in the presence of p6. However, modification of residues R118 or K122 to Q reduced the efficiency of complex formation by 8-fold (assays not shown; results summarized in Table I), which parallels their decreased ability to repress the A2c promoter in the presence of protein p6. Modification of residues R116 or R121 decreased the efficiency of complex formation by 4- and 16-fold, respectively, while mutation of residues L117 or E119 had a milder effect (assays not shown, see Table I). In summary, the results obtained clearly indicate that the protein p4 C-end has a role in the formation of the p4–p6 nucleoprotein complex that represses the A2c promoter. The DNase I footprints presented, in which the DNA is labelled at the strand coding for the late genes, do not allow the boundaries of the protected regions at the A3 promoter to be defined accurately. Labelling of the complementary strand, however, clearly showed that wild-type p4 modified the footprint generated by p6 at the A3 promoter, organizing it and defining a clear border that ended at the p4-binding site of the A3 promoter (Figure 3). At this promoter, p4 generates a footprint from positions –58 to –104 (relative to the A3 start site), which changed in the presence of p6 upstream from position –85. In the presence of p4, no p6 footprint was observed downstream from position –58 (Figure 3) while, in its absence, p6 could bind to all the DNA region analysed. The presence of this sharp boundary suggests that p4 organizes the p4–p6 complex at specific DNA regions only (from p4 site 3 towards the A2c promoter).
Fig. 2. Analysis of the binding cooperativity of protein p6 and protein p4 derivatives containing mutations at the C-end. (A) Scheme illustrating the relative location of promoters A3, A2b and A2c, as well as the three p4-binding sites in the 362 bp DNA fragment used in (B). (B) DNase I footprinting of the complexes formed by protein p6 and protein p4 (either wild-type or the indicated mutant derivatives) with the DNA fragment mentioned above, labelled at the strand coding for late genes. Protections generated by each protein are indicated on the left. Broken lines indicate regions where the footprint differs depending on whether one or both proteins were present. The DNA was incubated with increasing concentrations of p6 (lanes 2–6: 2.6, 5.3, 10.6, 21.2 and 42.4 µM, respectively), or with 10.6 µM p6 prior to addition of increasing amounts of protein p4 (lanes 8–12: 82, 165, 330, 660 and 1320 nM). When p4 was the only protein added, its concentration was 1320 nM. Numbering on the right indicates positions relative to the transcription start site of the A2c promoter. The transcription initiation sites of promoters A2c, A2b and A3 are indicated with arrows.
Fig. 3. Analysis of the p4–p6 nucleoprotein complex formed at the late A3 promoter. The DNA fragment used in Figure 2, but labelled at the strand coding for early genes, was incubated with protein p6 (5.3, 10.6, 16 and 21.2 µM), or with p6 (10.6 µM) and increasing amounts of protein p4 (82, 165, 330, 660 and 1320 nM). The concentration of p4 in the absence of p6 was 1320 nM. Numbering refers to positions relative to the transcription start site of the A3 promoter; arrows indicate the direction and transcription initiation site of each promoter.
We next investigated the effect of the nucleoprotein complexes formed by p6 and the p4 mutant derivatives on the binding of RNAP to the A2c promoter. RNAP has a strong affinity for the A2c promoter, protecting from DNase I a region spanning from about position –55 to +17 relative to the A2c start site, and generating a characteristic hypersensitive band at position –37 (Monsalve et al., 1996a; see Figure 4, lane 2). The presence of protein p6 at the concentration used does not modify this footprinting pattern (Figure 4, lane 5). When protein p4 is present in addition to RNAP, p4 binds immediately upstream from RNAP to p4-binding site 2, generating a number of protected regions flanked by hypersensitive bands (Monsalve et al., 1996a; Figure 4, lane 6). In the absence of RNAP, p4 binds weakly to site 1 at promoter A2c and more strongly to site 3 at promoter A3 (Barthelemy and Salas, 1989; Monsalve et al., 1998; Figure 4, lane 7). The simultaneous presence of proteins p4 and p6 generates a complex that inhibits RNAP binding to the A2c promoter (note the decrease of the hypersensitive band at position –37 and the appearance of the p4–p6 footprinting along promoter A2c as protein p4 concentration increases; lanes 2, 4 and 8–12; Elías-Arnanz and Salas, 1999; Camacho and Salas, 2001b). When protein p4(CΔ14 + 2) was used instead of the wild-type p4, the footprinting observed corresponded to RNAP bound to the A2c promoter, indicating that the weak p4(CΔ14 + 2)–p6 nucleoprotein complex formed is unable to compete with RNAP for binding to the A2c promoter (Figure 4, lanes 14–18). This agrees with the lack of repression ability of this mutant derivative in the presence of p6 observed in the transcription assays. Note that although p4(CΔ14 + 2) can bind to a high affinity site such as site 3, no footprint is detected at site 2 because p4 binding to this site requires its stabilization by RNAP through interactions between the C-terminal domain of the RNAP α-subunit and the p4 C-end (Monsalve et al., 1996b, 1998), which is missing in p4(CΔ14 + 2). The complex formed by protein p6 and mutant protein p4(R120Q) was able to compete with RNAP for binding to promoter A2c, although with a 4-fold lower efficiency than when the wild-type p4 was used [note the disappearance of the hypersensitive band at position –37 corresponding to RNAP as the concentration of p4(R120Q) increases, and the appearance of the p4–p6 footprint; Figure 4, lanes 20–24]. This again agrees with the results of the in vitro transcription assays. Similar footprinting assays performed with the other p4 mutants analysed also showed that the ability to form the p4–p6 complex, and to displace RNAP from the A2c promoter, parallels in all cases the repressing efficiency observed in the transcription assays (assays not shown; results summarized in Table I).
Fig. 4. Binding of RNAP to the A2c, A2b and A3 promoters in the presence of protein p6 and protein p4 derivatives containing mutations at the C-end. The DNA fragment used in Figure 2 containing promoters A2c, A2b and A3, labelled at the strand coding for late genes, was incubated with protein p6 (10.6 µM), or with p6 (10.6 µM) and increasing amounts of wild-type or mutant protein p4 (82, 165, 330, 660 and 1320 nM). The concentration of p4 in the absence of p6 was 1320 nM. Where indicated, RNAP was also added at 50 nM. The DNase I footprints generated by protein p4 and/or protein p6 are indicated on the left. Numbering on the right refers to positions relative to the transcription start site of the A2c promoter; arrows indicate the direction and transcription initiation site of each promoter. Triangles on the right point to hypersensitivities indicative of RNAP binding to the A2c promoter (position –37) or of p4–p6 complex formation (position –45).
The DNase I footprinting assays described above allow us to determine the effect of the p4–p6 complex on the formation of open RNAP–promoter complexes at the A2c promoter, but, since they were performed at 37°C, they do not show any possible effect on the formation of closed RNAP–promoter complexes. In addition, it is difficult to identify mixed populations of different protein–DNA complexes using this technique. For these reasons, the complexes formed by RNAP, p6 and either p4, p4(CΔ14 + 2) or p4(R120Q) were analysed by gel retardation assays at 4°C, conditions that allow detection of closed RNAP–promoter complexes and identification of individual complexes. The DNA fragment used contained only the A2c promoter, which still allows formation of p4–p6 nucleoprotein complexes (Camacho and Salas, 2001b) and simplifies interpretation of the results. In the presence of protein p6, RNAP and low concentrations of wild-type protein p4, the DNA migrated more slowly than when RNAP and either p4 or p6 were present (see Figure 5, lanes 5 and 6, and 8 and 9). As reported earlier (Camacho and Salas, 2001b), this nucleoprotein complex most probably contains RNAP bound at promoter A2c, protein p4 bound immediately upstream from RNAP, and protein p6 flanking these two proteins (see scheme in Figure 5B, a). Interestingly, this complex disappeared at higher concentrations of protein p4, giving rise to a complex that migrated as the p4–p6–DNA complex (Figure 5B, b and lanes 10–12). Considering that the DNA fragment used does not contain the p4-binding site at the A3 promoter, which helps to organize the p4–p6 complex (Elías-Arnanz and Salas, 1999; Camacho and Salas, 2001b), it seems likely that at low p4 concentrations the p4–p6 complex still allows RNAP binding to the A2c promoter. At higher p4 concentrations, the p4–p6 complex would be able to displace RNAP from the promoter. When mutant protein p4(R120Q) was used instead of wild-type p4, the lowest mobility complex was not observed, and all DNA migrated as a p4–p6–DNA complex (Figure 5B, b and lanes 14–18). This is to be expected given that p4(R120Q) cannot interact with RNAP to bind upstream from it at p4 site 2. In the case of p4(CΔ14 + 2), three bands were observed, indicative of several populations of protein–DNA complexes. The lowest mobility complex migrated as DNA bound to RNAP, in agreement with the transcription and footprinting assays indicating that the p6–p4(CΔ14 + 2) complex is inefficient at repressing the A2c promoter. The band of fastest mobility migrated as DNA bound to p4(CΔ14 + 2). Finally, a band of intermediate mobility was present that migrated as a p4(CΔ14 + 2)–p6–DNA complex (see scheme in Figure 5B, c and lanes 20–24). The presence of these complexes agrees with the DNase I footprinting assays, which showed that the p4(CΔ14 + 2)–p6 nucleoprotein complex is not formed efficiently and could therefore co-exist with other protein–DNA complexes. Altogether, these results confirm the importance of the p4 C-end in formation of a p4–p6 complex, and show that the presence of a stable p4–p6 complex blocks RNAP binding to the A2c promoter.
Fig. 5. Formation of closed complexes by RNAP at the A2c promoter in the presence of protein p6 and either wild-type p4 or protein p4 mutants R120Q and (CΔ14 + 2). (A) Scheme of the DNA fragment used, containing promoter A2c. The transcription start point and –10 and –35 boxes are indicated, as well as the binding regions for RNAP and protein p4, in the latter case in both the absence (site 1) and presence (site 2) of RNAP. (B) Complexes formed by proteins p4, p6 and RNAP with the DNA fragment shown in (A), analysed in a band shift assay performed at 4°C. Protein p6 and RNAP were added at 10.6 µM and 50 nM, respectively. Wild-type or mutant protein p4 was added at 41, 82, 165, 330 or 660 nM, as indicated. When it was the only protein present in the reaction mixture, protein p4 was added to 660 nM and protein p6 to 10.6 µM. FD, free DNA; p4, p4–DNA complex; p6, p6–DNA complex; p4 + p6, p4–p6–DNA complex; RNAP, RNAP–DNA complex; RNAP + p4 + p6, RNAP–p4–p6–DNA complex. The schemes on the bottom show the interpretation of some of the complexes formed. The use of a discontinuous line indicates poor p6 binding, according to Figure 2.
Identification of the protein p4-binding site at promoter A2c in the presence of protein p6
Previous analyses of the p4–p6 DNA complex had indicated that it is formed by a series of p6 dimers organized and stabilized by two protein p4 tetramers, one of them bound at the A3 promoter (Elías-Arnanz and Salas, 1999) and the other at the A2c promoter (Camacho and Salas, 2001b). However, the resolution of the DNase I footprinting assays did not allow us to distinguish whether the p4 molecules at promoter A2c were bound at site 1 or site 2 (centred at positions –39 and –71, respectively, relative to the A2c start site). Protein p4-binding sites consist of an inverted repeat formed by two 6 bp arms separated by a 15 bp spacer (Figure 6). Modification of residues important for p4 recognition at either site 1 or site 2 of the A2c promoter impairs binding of protein p4 (Monsalve et al., 1998). Mutant promoters with modifications at site 1 or site 2 were used to investigate which is the target of protein p4 at promoter A2c in the p4–p6 nucleoprotein complex. Modification of the p4 recognition sequences at site 2 did not impair formation of the p4–p6 nucleoprotein complex, while mutations at site 1 impaired formation of this complex (Figure 6). DNase I footprinting confirmed that p6 could assist protein p4 in repressing the A2c promoter when modifications affected site 2, but not when they affected site 1 (not shown). We conclude that formation of a p4–p6 nucleoprotein complex requires binding of protein p4 to target site 1 of the A2c promoter.
Fig. 6. Role of p4-binding sites 1 and 2 at the A2c promoter in formation of a stable p4–p6 nucleoprotein complex. (A) Sequence of the A2c promoter indicating the residues recognized by protein p4 at sites 1 and 2 (grey boxes). Each site is formed by two 6 bp boxes, which conform to an inverted repeat. Site 1 is recognized in the absence of RNAP, while site 2 is the target when RNAP is present. Positions modified in mutant promoters A2c-X, A2c-dn and A2c-up are indicated in bold and with asterisks. (B) Band shift assay showing the complexes formed by protein p6 (10.6 µM) in the absence or presence of increasing amounts of p4 (82, 165, 330 and 660 nM), with a DNA fragment containing either the wild-type A2c promoter, or each of the mutant promoters indicated in (A). When no other proteins were present, p4 was added at 660 nM. FD, free DNA; p6, p6–DNA complex; p4 + p6, p4–p6–DNA complex.
Discussion
The main aim of this work was to unravel the molecular basis of the cooperative binding of proteins p4 and p6 over the DNA region spanning from the early A2c to the late A3 promoter, a complex that leads to repression of the A2c promoter. Since protein p4 binds efficiently to a specific target, but protein p6 does not, and considering that p6 binds poorly to this DNA region in the absence of p4, it was proposed that protein p4 promotes polymerization of p6 along this DNA region, generating a stable complex (Elías-Arnanz and Salas, 1999; Camacho and Salas, 2001b). The results presented here indicate that formation of the p4–p6 nucleoprotein complex requires not only the binding of protein p4 to DNA, but also the integrity of the p4 C-end. Deletion of the last 14 residues of p4, which does not alter its ability to bind DNA (Rojo et al., 1990), significantly decreased its ability to promote formation of the p6 nucleoprotein complex along the A2c promoter. Increasing the concentration of this p4 deletion derivative by 4-fold allowed formation of the p4–p6 nucleoprotein complex, but was inefficient at repressing the A2c promoter. This suggests that this complex is less stable than the one formed by wild-type p4 and does not compete successfully with RNAP, so that transcription initiation can occur. The behaviour of this p4 deletion mutant, named p4(CΔ14 + 2), is consistent with a model in which the cooperative binding of p4 and p6 requires a contact between the two proteins. To identify individual residues involved in this interaction, the ability of a number of single substitution mutants in this region of protein p4 to bind cooperatively with p6, and to repress the A2c promoter, was analysed. Residues L117 and E119 appeared not to be important for the p4–p6 interaction either. However, modification of residues R116, R118, R120, R121 or K122 impaired formation of the p4–p6 complex to different extents and allowed only a partial repression of the A2c promoter. This suggests that the contact between proteins p4 and p6 relies on a network of interactions involving several positively charged residues of p4.
The ability of p4 to promote polymerization of p6 appears to depend on the DNA region considered. In the absence of p4, protein p6 could bind to a large DNA region including promoters A2c and A3, and downstream from both, forming unstable complexes. However, the presence of p4 bound at site 3 promoted polymerization of a stable p6 complex only in the direction towards promoter A2c, but not downstream from promoter A3. When the DNA fragment used contained p4 site 1, but not p4 site 3, p4 could cover the DNA on both sides of p4 (see scheme in Figure 5B, b). This behaviour can be explained assuming that the stability of the p4–p6 complex depends not only on the p4–p6 interaction, but also on the ability of the DNA to bend in a direction favourable for p6 binding. It is known that the preferential binding of protein p6 to the φ29 DNA ends is due to their characteristic bendability that facilitates wrapping of the DNA over a p6 core (Serrano et al. 1989, 1990). Accordingly, the DNA region between p4 sites 1 and 3 would favour the binding of p6 with the phase imposed by p4, but the region downstream of promoter A3 would not. By using a series of mutants at the A2c promoter, the second p4 target in the p4–p6 complex has now been mapped to p4-binding site 1, which overlaps the –35 region of the A2c promoter. Although important for p6 polymerization, this second p4 site does not impose a border to the complex, since the p6 complex continues at the other side of the p4 tetramer bound to site 1, continuing downstream of promoter A2c. Importantly, however, deletion of the last 14 residues of p4 decreased p6 polymerization downstream of promoter A2c. The p4–p6 complex formed by protein p4(CΔ14 + 2), which was observed only at high p4(CΔ14 + 2) concentrations, spanned mainly from p4 site 1 to site 3. Altogether, the results presented suggest that the role of protein p4 in promoting polymerization of protein p6 is to define the phase and boundaries of the p6–DNA complex. This implies that polymerization of protein p6 over the analysed DNA region relies on p4-specified signals in addition to the structural features of the DNA. It is interesting to note that, in the absence of p4, binding of p6 to this region generated a characteristic DNase I footprint, which is indicative of a complex having a defined periodicity. However, p4 changed this pattern in the region located between p4 sites 1 and 3, although not beyond site 1. It seems, therefore, that p4 forces a change in the phase of p6 binding to DNA between p4 sites 1 and 3. The complex formed in the presence of p4 is more stable than that formed in its absence, indicating that the interaction between p4 and p6 compensates for the repositioning of the p6 dimers. Probably, upon repositioning of the first p6 dimers next to p4, additional p6 dimers can bind efficiently, forming a stable p6 filament with a defined periodicity. Repositioning of a transcriptional regulator by another regulator has been observed for sequence-specific proteins, such as, for example, repositioning of MalT forced by CRP at the malKp promoter (Richet et al., 1991). In this case, repositioning is due in part to the DNA bending induced by CRP on the DNA (Richet and Sogaard-Andersen, 1994). Protein p4 also bends DNA upon binding to site 3 at the A3 promoter (Barthelemy and Salas, 1989; Rojo et al., 1990). However, DNA bending is not sufficient to promote p6 polymerization. Although mutant protein p4(CΔ14 + 2) generates 23% less bending than the wild-type p4, DNA bending by mutant proteins R118Q, R121S or K122Q is essentially as strong as that generated by the wild-type p4 (Rojo et al., 1990; Mencía et al., 1996b), and they still showed a clear decrease in their ability to promote polymerization of p6.
Promoter regulation by nucleoid proteins is not infrequent (reviewed in McLeod and Johnson, 2001). Since the levels of several nucleoid proteins vary at different stages of cell growth (Azam et al., 1999), their involvement in gene regulation can be used to connect the activity of many promoters with the physiological status of the cell. In some cases, it is the interplay of several nucleoid proteins that is used to regulate transcription, a strategy that can provide substantial flexibility (Browning et al., 2002). The repression mechanism of the p4–p6 nucleoprotein complex has some resemblance to that used by the nucleoid protein HN-S at certain promoters, where it oligomerizes starting at a site distant from the promoter, eventually inhibiting promoter function (Jordi and Higgins, 2000). HN-S acts in these cases as a regional silencer, but it is not clear whether repression occurs by a modification of DNA topology, or by a direct interference with RNAP function. The case of the p4–p6 complex has an added element of complexity, since p4 helps to promote p6 oligomerization. This allows for a temporal control of p6 action, favouring its participation at late times of infection, when p6 is more abundant. The repression mechanism of the p4–p6 complex has some resemblance to the silencing mechanism of the P1 plasmid ParB protein, which polymerizes along the DNA from a nucleation site at parS helped by IHF, silencing flanking genes (Rodionov et al., 1999).
It is worth noting that the p4–p6 complex not only repressed the A2c promoter, but also stimulated p4 activation of the late A3 promoter (Elías-Arnanz and Salas, 1999; this work). At low p4 concentration, activation may be explained by the cooperative binding of both proteins to DNA. However, ∼2-fold stimulation of the A3 promoter was also observed at high p4 concentrations, which suggests that the effect is not solely to facilitate the binding of protein p4 to the A3 promoter. It is known that p4 activates promoter A3 by stabilizing the RNAP at the promoter as a closed complex (Nuez et al., 1992). The effect of p6 at saturating concentrations of p4 could be due to the stimulation of further steps of the transcription initiation process.
Since the strong early A2c promoter can be repressed through several mechanisms, the question arises as to which could be the prevalent one in vivo. One of the mechanisms relies solely on protein p4 (Monsalve et al., 1996a), while the other two also require protein p6 (Elías-Arnanz and Salas, 1999; Camacho and Salas, 2001b). In the absence of protein p6, protein p4 binds upstream from RNAP, interacting with it and inhibiting promoter clearance (Figure 7A; Monsalve et al., 1996a). Protein p6 facilitates alternative mechanisms that are probably more effective. If formation of the p4–p6 complex is not very efficient, a situation that can be visualized in vitro at low p4 concentrations (Figure 7B), p6 would still allow RNAP binding to promoter A2c, although the transition from closed to open complex would be impaired (Camacho and Salas, 2001b). However, when a stable p4–p6 complex is formed, a situation that occurs in vitro at higher p4 concentrations, the p4–p6 nucleoprotein complex covers the A2c promoter, preventing RNAP binding (Figure 7C). It is likely that all these mechanisms co-exist in infected cells. The p4–p6 complexes are expected to be rather dynamic in infected cells, because, among other reasons, the DNA replication process probably forces their disassembly. The prevalence of each mechanism will probably depend on the relative amounts of p4, p6 and DNA, which change along the infection cycle. Protein p4 is not abundant in infected cells (Monsalve et al., 1995), but its concentration increases as infection proceeds. Protein p6 is very abundant, reaching concentrations as high as 1 mM at late infection times (Abril et al., 1997). On the other hand, the amount of viral DNA rapidly increases after 10 min of infection (Monsalve et al., 1995), although part of it eventually is encapsidated. It is interesting to note that other phages evolutionarily related to φ29, which also contain promoters equivalent to A2c, A2b and A3, do not have a binding site 2 for protein p4 (Nuez and Salas, 1993; Calles, 2002). Therefore, in these phages, protein p4 cannot repress the A2c promoter on its own, and should rely on p6 to achieve this.
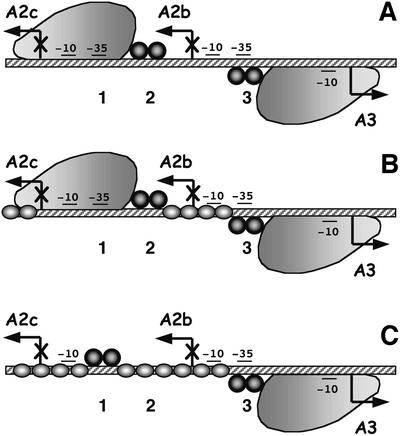
Fig. 7. Strategies to regulate the switch from early to late transcription in phage φ29. (A) In the absence of protein p6, repression of the early A2b and A2c promoters, and activation of the late A3 promoter, is mediated by protein p4. Binding of protein p4 to site 3 activates the late A3 promoter by stabilizing the binding of RNAP as a closed complex, and represses the early A2b promoter by inhibiting binding of RNAP to it. Protein p4 binds to site 2 only in the presence of RNAP, and represses the early A2c promoter by preventing promoter clearance. Activation of the late A3 promoter and repression of the early A2c promoter involve a direct interaction between p4 residue R120 and the C-terminal domain of the RNAP α-subunit (reviewed in Rojo et al., 1998). (B) In the presence of p6 and low concentrations of p4, a p4–p6 complex is formed, but RNAP can still bind to the A2c promoter as a closed complex, although open complexes are not formed (Camacho and Salas, 2001b). (C) In the presence of p6 and higher concentrations of protein p4, a stable nucleoprotein complex is formed that blocks transcription from the A2c promoter. In this complex, protein p4 is bound to sites 1 and 3, and RNAP cannot bind to promoter A2c. Moreover, in the presence of p6, binding of p4 to site 3 is enhanced, as is activation of the A3 promoter. The large protein represents RNAP, the dark spheres depict protein p4 dimers (which bind to DNA as a tetramer), and the grey oblate ellipses represent protein p6. The –10 and –35 boxes for the vegetative RNAP are indicated.
Materials and methods
DNA substrates
The 364 bp DNA fragment containing promoters A2c, A2b and A3 used in the DNase I footprinting was obtained by PCR amplification from φ29 DNA with primers 5′-GATTTCTCTCTGCATCA-3′ and 5′-CAAAATATCTTCGTGTTC-3′. The fragment containing only promoter A2c was obtained by PCR amplification from plasmid pMM13 (Monsalve et al., 1996a). Fragments containing promoters A2c-X, A2c-dn and A2c-up were obtained using the same method, using as tem plates the corresponding plasmids described in Monsalve et al. (1998) and the 24mer universal M13-mp18 sequencing primers. To label these DNA fragments at the 5′ end, one of the primers was treated with polynucleotide kinase and [γ-32P]ATP prior to the PCR amplification. The DNA template for in vitro transcription reactions was full-length φ29 DNA with the terminal protein covalently attached to each 5′ end (TP-DNA).
Proteins
Bacillus subtilis σA-RNA polymerase, protein p6 and protein p4 were purified essentially as described previously by Sogo et al. (1979), Pastrana et al. (1985) and Mencía et al. (1993), respectively.
In vitro transcription reactions
Primer extension assays were performed using as templates full-length φ29 DNA (TP-DNA). Reactions contained, in 25 µl, 2 nM of template DNA, 200 µM each ATP, CTP, GTP and UTP, 25 mM Tris–HCl pH 7.5, 10 mM MgCl2, 100 mM KCl, 2 µg of poly[d(I–C)], 2 mM dithiothreitol, 7.5 U of RNasin ribonuclease inhibitor and 50 nM B.subtilis σA-RNAP. Where indicated, protein p6 and/or protein p4 were also added. In reactions containing p6, the DNA was pre-incubated with the protein at 37°C for 10 min prior to the addition of RNAP and/or p4. Mixtures were incubated in the absence of NTPs for 10 min at 37°C, and transcription was started by addition of the NTPs. After 20 min at 37°C, the reactions were stopped with EDTA (20 mM final concentration), and the RNAs precipitated with 0.3 M potassium acetate and ethanol in the presence of 10 µg of carrier tRNA. RNAs were analysed by primer extension with appropriate primers as described previously (Monsalve et al., 1995). The cDNAs obtained were resolved by denaturing PAGE and quantified with a BAS-IIIs Fuji imaging analyser. The primers were selected to produce cDNAs of 68, 78 and 98 nucleotides from promoters A3, A2c or C2, respectively.
Band shift assays
Binding reactions contained, in 20 µl, 0.1 nM end-labelled DNA, 25 mM Tris–HCl pH 7.5, 10 mM MgCl2, 100 mM KCl, 2 µg of poly[d(I–C)], 2 µg of bovine serum albumin (BSA) and, where indicated, different amounts of protein p4, 10.6 µM protein p6 and/or 50 nM B.subtilis σA-RNAP. Reactions containing protein p6 were pre-incubated for 10 min on ice before the addition of other proteins. Samples were chilled on ice for an additional 10 min and loaded onto a non-denaturing 4% polyacrylamide gel containing 100 mM KCl after addition of 4 µl of 30% (v/v) glycerol. Electrophoresis was performed at 4°C.
DNase I footprinting
Binding reactions contained, in 20 µl, end-labelled DNA (1 nM), 25 mM Tris–HCl pH 7.5, 10 mM MgCl2, 100 mM KCl, 2 µg of poly[d(I–C)], 2 µg of BSA and, where indicated, protein p4, protein p6 and/or RNAP at the concentrations indicated in the figure legends. In reactions containing p6, the DNA fragment was pre-incubated with the protein for 10 min at 37°C prior to the addition of DNase I or any other protein. Mixtures were incubated for an additional 10 min at 37°C. The footprinting reaction was initiated by the addition of DNase I (0.05 U). Digestion was allowed to proceed for 2 min at 37°C, stopped with EDTA (20 mM final concentration), and the DNA was precipitated with 0.3 M potassium acetate, 10 µg of tRNA and ethanol, and analysed in denaturing 6% polyacrylamide gels.
Acknowledgments
Acknowledgements
We are grateful to W.Meijer for helpful discussions, to A.Camacho for critical reading of the manuscript, and to J.M.Lázaro and L.Villar for protein purification and technical assistance. This investigation was supported by grants 2R01 GM27242-23 from the National Institutes of Health and PB98-0645 from the Dirección General de Investigación Científica y Técnica to M.S., by grant BIO2000-0939 from Comisión Interministerial de Ciencia y Tecnología to F.R., and by an institutional grant from Fundación Ramón Areces to the Centro de Biología Molecular ‘Severo Ochoa’. B.C. was a holder of a predoctoral fellowship from the Spanish Ministry of Education and Culture.
References
- Abril A.M., Salas,M., Andreu,J.M., Hermoso,J.M. and Rivas,G. (1997) Phage φ29 protein p6 is in a monomer–dimer equilibrium that shifts to higher association states at the millimolar concentrations found in vivo. Biochemistry, 36, 11901–11908. [DOI] [PubMed] [Google Scholar]
- Adhya S., Geanacopoulos,M., Lewis,D.E., Roy,S. and Aki,T. (1998) Transcription regulation by repressosome and by RNA polymerase contact. Cold Spring Harbor Symp. Quant. Biol., 63, 1–9. [DOI] [PubMed] [Google Scholar]
- Aki T. and Adhya,S. (1997) Repressor induced site-specific binding of HU for transcriptional regulation. EMBO J., 16, 3666–3674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azam T.A., Iwata,A., Nishimura,A., Ueda,S. and Ishihama,A. (1999) Growth phase-dependent variation in protein composition of the Escherichia coli nucleoid. J. Bacteriol., 181, 6361–6370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barthelemy I. and Salas,M. (1989) Characterization of a new prokaryotic transcriptional activator and its DNA recognition site. J. Mol. Biol., 208, 225–232. [DOI] [PubMed] [Google Scholar]
- Barthelemy I., Mellado,R.P. and Salas,M. (1989) In vitro transcription of bacteriophage φ29 DNA: inhibition of early promoters by the viral replication protein p6. J. Virol., 63, 460–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi M.E. (1994) Prokaryotic HU and eukaryotic HMG1: a kinked relationship. Mol. Microbiol., 14, 1–5. [DOI] [PubMed] [Google Scholar]
- Browning D.F., Beatty,C.M., Wolfe,A.J., Cole,J.A. and Busby,S.J. (2002) Independent regulation of the divergent Escherichia coli nrfA and acsP1 promoters by a nucleoprotein assembly at a shared regulatory region. Mol. Microbiol., 43, 687–701. [DOI] [PubMed] [Google Scholar]
- Calles B. (2002) Transcription regulation of the A2c and A3 promoters from the Bacillus subtilis phage φ29: role of the p4 and p6 proteins. PhD thesis, Department of Molecular Biology, Universidad Autónoma de Madrid, Madrid, Spain.
- Camacho A. and Salas,M. (2000) Pleiotropic effect of protein p6 on the viral cycle of bacteriophage φ29. J. Bacteriol., 182, 6927–6932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camacho A. and Salas,M. (2001a) Repression of bacteriophage φ29 early promoter C2 by viral protein p6 is due to impairment of closed complex. J. Biol. Chem., 276, 28927–28932. [DOI] [PubMed] [Google Scholar]
- Camacho A. and Salas,M. (2001b) Mechanism for the switch of φ29 DNA early to late transcription by regulatory protein p4 and histone-like protein p6. EMBO J., 20, 6060–6070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castaing B., Zelwer,C., Laval,J. and Boiteux,S. (1995) HU protein of Escherichia coli binds specifically to DNA that contains single-strand breaks or gaps. J. Biol. Chem., 270, 10291–10296. [DOI] [PubMed] [Google Scholar]
- Elías-Arnanz M. and Salas,M. (1999) Functional interactions between a phage histone-like protein and a transcriptional factor in regulation of φ29 early–late transcriptional switch. Genes Dev., 13, 2502–2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodrich J.A., Schwartz,M.L. and McClure,W.R. (1990) Searching for and predicting the activity of sites for DNA binding proteins: compilation and analysis of the binding sites for Escherichia coli integration host factor (IHF). Nucleic Acids Res., 18, 4993–5000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hengen P.N., Bartram,S.L., Stewart,L.E. and Schneider,T.D. (1997) Information analysis of Fis binding sites. Nucleic Acids Res., 25, 4994–5002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordi B.J. and Higgins,C.F. (2000) The downstream regulatory element of the proU operon of Salmonella typhimurium inhibits open complex formation by RNA polymerase at a distance. J. Biol. Chem., 275, 12123–12128. [DOI] [PubMed] [Google Scholar]
- Kar S. and Adhya,S. (2001) Recruitment of HU by piggyback: a special role of GalR in repressosome assembly. Genes Dev., 15, 2273–2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobryn K., Lavoie,B.D. and Chaconas,G. (1999) Supercoiling-dependent site-specific binding of HU to naked Mu DNA. J. Mol. Biol., 289, 777–784. [DOI] [PubMed] [Google Scholar]
- McLeod S.M. and Johnson,R.C. (2001) Control of transcription by nucleoid proteins. Curr. Opin. Microbiol., 4, 152–159. [DOI] [PubMed] [Google Scholar]
- Mencía M., Salas,M. and Rojo,F. (1993) Residues of the Bacillus subtilis phage φ29 transcriptional activator required both to interact with RNA polymerase and to activate transcription. J. Mol. Biol., 233, 695–704. [DOI] [PubMed] [Google Scholar]
- Mencía M., Monsalve,M., Rojo,F. and Salas,M. (1996a) Transcription activation by phage φ29 protein p4 is mediated by interaction with the α subunit of Bacillus subtilis RNA polymerase. Proc. Natl Acad. Sci. USA, 93, 6616–6620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mencía M., Monsalve,M., Salas,M. and Rojo,F. (1996b) Transcriptional activator of phage φ29 late promoter: mapping of residues involved in interaction with RNA polymerase and in DNA bending. Mol. Microbiol., 20, 273–282. [DOI] [PubMed] [Google Scholar]
- Monsalve M., Mencía,M., Rojo,F. and Salas,M. (1995) Transcription regulation in Bacillus subtilis phage φ29: expression of the viral promoters throughout the infection cycle. Virology, 207, 23–31. [DOI] [PubMed] [Google Scholar]
- Monsalve M., Mencía,M., Rojo,F. and Salas,M. (1996a) Activation and repression of transcription at two different phage φ29 promoters are mediated by interaction of the same residues of regulatory protein p4 with RNA polymerase. EMBO J., 15, 383–391. [PMC free article] [PubMed] [Google Scholar]
- Monsalve M., Mencía,M., Salas,M. and Rojo,F. (1996b) Protein p4 represses phage φ29 A2c promoter by interacting with the α subunit of Bacillus subtilis RNA polymerase. Proc. Natl Acad. Sci. USA, 93, 8913–8918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monsalve M., Calles,B., Mencía,M., Salas,M. and Rojo,F. (1997) Transcription activation or repression by phage φ29 protein p4 depends on the strength of the RNA polymerase–promoter interactions. Mol. Cell, 1, 99–107. [DOI] [PubMed] [Google Scholar]
- Monsalve M., Calles,B., Mencía,M., Rojo,F. and Salas,M. (1998) Binding of phage φ29 protein p4 to the early A2c promoter: recruitment of a repressor by the RNA polymerase. J. Mol. Biol., 283, 559–569. [DOI] [PubMed] [Google Scholar]
- Nuez B. and Salas,M. (1993) Bacteriophage Nf DNA region controlling late transcription: structural and functional homology with bacteriophage φ29. Nucleic Acids Res., 21, 2861–2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuez B., Rojo,F. and Salas,M. (1992) Phage φ29 regulatory protein p4 stabilizes the binding of the RNA polymerase to the late promoter in a process involving direct protein–protein contacts. Proc. Natl Acad. Sci. USA, 89, 11401–11405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastrana R., Lázaro,J.M., Blanco,L., García,J.A., Méndez,E. and Salas,M. (1985) Overproduction and purification of protein p6 of Bacillus subtilis phage φ29: role in the initiation of DNA replication. Nucleic Acids Res., 13, 3083–3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinson V., Takahashi,M. and Rouviere-Yaniv,J. (1999) Differential binding of the Escherichia coli HU, homodimeric forms and heterodimeric form to linear, gapped and cruciform DNA. J. Mol. Biol., 287, 485–497. [DOI] [PubMed] [Google Scholar]
- Richet E. and Sogaard-Andersen,L. (1994) CRP induces the repositioning of MalT at the Escherichia coli malKp promoter primarily through DNA bending. EMBO J., 13, 4558–4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richet E., Vidal-Ingigliardi,D. and Raibaud,O. (1991) A new mechanism for coactivation of transcription initiation: repositioning of an activator triggered by the binding of a second activator. Cell, 66, 1185–1195. [DOI] [PubMed] [Google Scholar]
- Rodionov O., Lobocka,M. and Yarmolinsky,M. (1999) Silencing of genes flanking the P1 plasmid centromere. Science, 238, 546–549. [DOI] [PubMed] [Google Scholar]
- Rojo F. and Salas,M. (1991) A DNA curvature can substitute phage φ29 regulatory protein p4 when acting as a transcriptional repressor. EMBO J., 10, 3429–3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojo F., Zaballos,A. and Salas,M. (1990) Bend induced by the phage φ29 transcriptional activator in the viral late promoter is required for activation. J. Mol. Biol., 211, 713–25. [DOI] [PubMed] [Google Scholar]
- Rojo F., Mencía,M., Monsalve,M. and Salas,M. (1998) Transcription activation and repression by interaction of a regulator with the α subunit of RNA polymerase: the model of phage φ29 protein p4. Prog. Nucleic Acid Res. Mol. Biol., 60, 29–46. [DOI] [PubMed] [Google Scholar]
- Salas M. and Rojo,F. (1993) Replication and transcription of bacteriophage φ29 DNA. In Hoch,J.A. and Losick,R. (eds), Bacillus subtilis and Other Gram-positive Bacteria: Biochemistry, Physiology and Molecular Genetics. American Society for Microbiology, Washington, DC, pp. 843–857.
- Serrano M., Gutiérrez,J., Prieto,I., Hermoso,J.M. and Salas,M. (1989) Signals at the bacteriophage φ29 DNA replication origins required for protein p6 binding and activity. EMBO J., 8, 1879–1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano M., Salas,M. and Hermoso,J.M. (1990) A novel nucleoprotein complex at a replication origin. Science, 248, 1012–1016. [DOI] [PubMed] [Google Scholar]
- Sogo J.M., Inciarte,M.R., Corral,J., Viñuela,E. and Salas,M. (1979) RNA polymerase binding sites and transcription of the DNA of Bacillus subtilis phage φ29. J. Mol. Biol., 127, 411–436. [DOI] [PubMed] [Google Scholar]
- Whiteley H.R., Ramey,W.D., Spiegelman,G.B. and Holder,R.D. (1986) Modulation of in vivo and in vitro transcription of bacteriophage φ29 early genes. Virology, 155, 392–401. [DOI] [PubMed] [Google Scholar]