Introduction
Community-acquired meningitis is associated with high morbidity and mortality. The epidemiology of community-acquired meningitis has changed over the past 15 years with the use of new vaccines and with the development of resistance to antibiotics. Bacterial meningitis would appear to be the most frequent by far, but most viral aetiologies are very often poorly recognized. The annual incidence of purulent community-acquired meningitis in France was estimated at 22 cases per million inhabitants in 1993. Age is a major risk factor in bacterial meningitis. The most affected group is children under 2 years of age, in whom the infection rate ranges from 10 to 110 cases per 100000 infants per year. Such cases must be considered as an absolute medical emergency. This review is limited to meningitis in immunocompetent patients.
Bacterial epidemiology
From birth until the first month of life, Streptococcus agalactiae, Listeria monocytogenes and Enterobacteriaceae (especially Escherichia coli) are the main pathogens. From 2 months until 4 years of age, meningitis is principally due to Haemophilus influenzae type B (the incidence of which is dramatically decreasing because of widespread vaccination), Neisseria meningitidis and Streptococcus pneumoniae. In children, teenagers and young adults, N. meningitidis and S. pneumoniae represent more than 80% of the cases. Above the age of 60 years, S. pneumoniae and H. influenzae are the most frequently isolated bacteria [1,2].
When all of these bacteria are taken together, the elements that indicate a poor prognosis are as follows: age less than 1 month or greater than 45 years; worsening level of consciousness on admission; and forms with purpura fulminans [3,4,5,6]. In the absence of a specific treatment, purulent meningitis is ultimately fatal.
Pneumococcal meningitis
Pneumococcal meningitis is the most frequent cause of bacterial meningitis in those older than 30 years, and was responsible for 20% of cases of purulent community meningitis in children and 60% of adult cases in France in 1993. It is also more severe in terms of mortality (15–30%) and morbidity (deafness).
The increasing resistance of S. pneumoniae to penicillin (in more than 10% of isolates) has created a problem for therapy, which at present cannot be completely optimized and requires a knowledge of the sensitivity phenotype of the bacteria in question. Penicillin-resistant pneumococci (PRP) include pneumococci with an intermediate sensitivity [minimum inhibitory concentration (MIC) of between 0.12 and 1 mg/l] and highly resistant pneumococci (MIC = 1 mg/l).
Resistance to β-lactams is related to modifications of the genes coding for penicillin-linked proteins (PLPs), which are the active sites for all β-lactams, especially PLP2b and PLP2x [7]. Such genomic change is due either to a genetic transfer or to on-off mutations. The main serotypes involved in the resistance to β-lactams are 6A, 6B, 14, 19A, 19F and 23F, and 99% of the serotypes are present in antipneumococcal vaccine of 23 valences. This evolution in resistance should lead to a renewed interest in antipneumococcal vaccination.
Resistance to β-lactam antibiotics in pneumococci has been increasing in a disturbing manner, especially in Europe. The first strains resistant to penicillin were described in Boston in 1964 and in Chicago in 1974, and the first case of pneumococcal meningitis resistant to ceftriaxone was reported in 1991. The progression of resistance has been constant in the USA; from 1979 to 1987 the rate of resistance was below 10%, but since 1987 there has been an increase of up to 30% in PRP. In Europe, Spain has a rate of almost 60% of PRP, followed by Bavaria with 50% resistance, but the rate is lower for the rest of Germany. France is next with 36% in children and 25% in adults in 1994. The magnitude of resistance was high in half of the cases.
Among the cephalosporins, cefaclor, cefuroxime and cefixime are no longer active on strains resistant to penicillin, and a direct relationship has even been noted between cefaclor and penicillin resistance. Of PRP strains 10% are cross-resistant to macrolides: erythromycin, as well as clarithromycin and azithromycin.
In children, the risk factors for acquiring infection with resistant S. pneumoniae are hospitalization, living in closed populations, β-lactam treatment in the preceding months and recurring otitis media. In adults the risk factors are immunodeficiency, nosocomial infections and β-lactam treatment in the preceding months. Limiting the spread of S. pneumoniae resistance to antibiotics therefore depends on the following: more rational utilization of antibiotics and perhaps reduction of the systematic prophylaxis of acute otitis media in children with recurrent otitis; evaluation of the optimal duration of antibiotic therapy; evaluation of posologies and the intake required in order to avoid long periods with serum levels nearly at subinhibition concentrations and favouring the selection of resistant mutants; alternating different classes of antibiotics; and development of the antipneumococcal vaccine.
Meningococcal meningitis
Meningococcal meningitis occurs in endemics, with cases that are usually sporadic causing minor endemia that are limited in time and space, particularly in closed populations of children and young adults. There is a distinct preponderance of group B (60%) over group C (30%) organisms. The mean mortality rate of 10% is principally linked to the occurrence of purpura fulminans [8].
Haemophilus influenzae meningitis
Widespread vaccination is currently disrupting the epidemiology of H. influenzae meningitis, which is caused by type B strains. Approximately 50% produce β-lactamases, making them resistant to amino-penicillins. Despite this increase in resistance, the mortality rate has gone from 10 to 3%.
Physiopathology
The bacteria responsible for purulent meningitis are of the extracellular multiplication type, and have properties that make them resistant to nonspecific defence factors and allow them to develop in the host tissues. They also have specific properties that enable them to invade the meninges and cause inflammation [9,10].
Bacterial penetration in the cerebrospinal fluid
There are various arguments that support the hypothesis of penetration of bacteria into the cerebrospinal fluid (CSF) by the haematogenous route, with secondary penetration through the haematomeningeal barrier. In the case of experimental infection with H. influenzae, rhinopharyngeal administration of bacteria is followed by bacteremia and then invasion of the CSF. Similarly, intraperitoneal injection of N. meningitidis in newborn rats or intravenously in macaques causes secondary colonization of the CSF [11].
The haematomeningeal barrier is only one of the elements of the haematoencephalic barrier, and pathogenic bacteria are hypothesized to invade the CSF via the blood by two different routes: direct penetration of the meningeal capillary endothelium; or by direct penetration at the level of the choroid plexus, which produces CSF by secretion. The latter of these would appear to be the most plausible hypothesis following postmortem and experimental observations of major and selective concentrations of bacteria in the choroid plexus capillaries.
Inflammatory mechanisms of the subarachnoid space
Once the bacterium has invaded the CSF, little can prevent it from multiplying because there is almost no complement fractions and the immunoglobulin concentration is very low. On the other hand, poor nutrition slows bacterial multiplication.
In order to understand the cascade of events that occurs as soon as the bacterium has invaded the CSF, experimental meningitis models have been designed by direct injection of bacteria into rabbit and rat cisterna magna, making it possible to short-circuit the haematomeningeal barrier.
In-situ production of cytokines
The most important event that follows bacterial penetration into the CSF is the production of cytokines [12]. In animal experiments, intracisternal injection of lipopolysaccharide triggers production of tumour necrosis factor (TNF)-α, interleukin (IL)-1 and IL-6, preceding the appearance of inflammatory exudate, which can be partially prevented by the injection of anti-TNF-α and/or anti-IL-1 antibodies. Injection of TNF-α and IL-1 into the cisterna magna is followed by an elevation in the protein concentration in the CSF, an afflux of polynuclear cells and an increase in the weight of the brain, reflecting oedema. It would therefore follow that the production of cytokines in the CSF is a prerequisite to episodes of meningitis. This production occurs in situ, is independent of all systemic cytokine production because the molecules cannot penetrate the haematomeningeal barrier and therefore comes from the macrophage cells within the meninges itself [13,14].
Polynuclear afflux
Cytokine release allows for recruitment of polynuclear cells in the CSF by an adhesion phenomenon between the neutrophils and the endothelial cells. In fact, TNF-α and IL-1 induce the expression of adhesion molecules at the surface of the endothelium that favours loose adhesion of polynuclear cells and their rolling on the endothelial surface. Under the influence of IL-1, the endothelial cell produces IL-8, which leads to the detachment of the polynuclear L-selectin and interrupts polynuclear rolling because of the increase in integrin activity. These phenomena provide for even tighter adhesion between the polynuclear cells and the endothelium, leading to polynuclear extravasation in the infected tissue [15,16].
Alteration of the haematomeningeal barrier
The other consequence of cytokine production is a reduction in the tightness of the hematomeningeal barrier, which is essentially related to local production of IL-1 and synergistically assisted by TNF-α, creating a loosening of the tight cerebral capillary junctions.
Consequences of bacterial meningitis
All of the events that occur during an episode of bacterial meningitis are the result of polynuclear afflux and alteration of the haematomeningeal barrier. The result is a mixed cerebral oedema (ie both interstitial, because it is linked to the resorption of CSF at the level of the arachnoid villi, and vasogenic, because it is linked to an increase in the permeability of the haematomeningeal barrier). In addition, this major meningeal inflammation can lead to major vascular alterations of the meningeal vessels, as occurs in vasculitis, facilitating microthromboses and participating in profound alterations in cerebral blood flow and anoxia [17].
Meningococcus
N. meningitidis is a Gram-negative, oxidase-positive aerobic diplococcus that has a polysaccharide capsule. It is a saprophyte, and colonizes most humans at some point in their lives without producing any particular symptoms. The development of disease after exposure to meningococci is the result of a poorly known mechanism of host factors that have an influence on bacterial invasion of mucosal surfaces. Among the elements that influence host response, genetic control of the response to the acute stage and cytokine secretion (TNF-α, IL-1, IL-6) is of importance in making the prognosis. Moreover, a deficiency in complement predisposes the patient to a meningococcus infection with unusual serogroups (X, Y, Z, W135, 29E) [18,19].
Listeria monocytogenes
L. monocytogenes is an occasional intracellular Gram-positive bacteria that is capable of multiplying in the macrophages and in most of the cells of the infected host tissue. From the sites of intracellular multiplication, L. monocytogenes can spread through the blood and reach numerous target organs, particularly the placenta and the central nervous system. The bacterium first enters via direct contact with the host cells through an 80 kDa protein called internalin, coded by chromosomal gene InlA. Internalin is a protein located at the surface of the bacterium and has a structural organization that is characteristic of numerous Gram-positive bacteria proteins (such as Streptococcus and Staphylococcus spp.). Internalin interacts with receptors of unknown nature (probably integrins) located at the surface of the infected cells. After penetrating the cells, the bacteria are capable of escaping the phagosomes and of multiplying in the cytoplasm. In the infected host, orally ingested bacteria go through the intestine via Peyer's patches and reach the lymphatic ganglions and the blood circulation. The principal target organ is the liver, where the bacteria multiply inside the hepatocytes. Hepatocyte lysis induced by the early recruitment of polynuclear cells triggers a liberation of intercellular bacteria and results in prolonged bacteraemia. At this stage, the disease is often managed well by immunocompetent patients, who may contract an infection that is often totally asymptomatic. This is probably the most frequent scenario if the frequency of exposure to L. monocytogenes is low. However, if the inoculum is massive, in a pregnant woman, or in patients who are immunocompromised (patients with acquired immunode-ficiency syndrome, patients with neutropenia or who are undergoing chemotherapy) or who present with hepatic anomalies (cirrhosis, hemochromatosis), infection of the hepatocytes is not controlled and the bacteria are released into the bloodstream. Metastatic localizations are possible at this stage, particularly in the placenta or the central nervous system [20].
Therapeutic implications
Bacterial meningitis is an infection of a defined space in which the host is compromised because of the absence of complement fractions and the low levels of specific antibodies. For this reason phagocytosis is ineffective and bacterial multiplication is rapid. Optimal antibiotic treatment requires that the selected antibiotic has a bactericidal effect in the CSF.
Passage of antibiotics into the cerebrospinal fluid
Antibiotics that strongly bind to proteins and those with a high molecular weight diffuse poorly into the cerebral parenchyma and the CSF. Meningeal inflammation partially improves this situation by modifying the permeability of the haematomeningeal barrier (increase in vesicular transport, and separation of the tight junctions between the endothelial cells and the meningeal vessels) [21].
Antibacterial synergy in cerebrospinal fluid in vivo
In a model of L. meningitis infection, an ampicillin-gentamicin combination (in vitro synergy) turned out to be more rapidly bactericidal than either when used alone [22].
Antibiotic concentration in the cerebrospinal fluid
Spinal concentrations of antibiotics that are equal to the minimal bacteriostatic concentration of a bacteriostatic drug or to the MIC of a bactericidal drug should be effective. This was not the case in an E. coli meningitis model, however, in which concentrations greater than 10 times the MIC were required to obtain the maximum elimination rate for CSF bacteria. The need to obtain spinal concentrations superior to 10 times the MIC has also been demonstrated with other bacteria and other antibiotics [9]. One explanation could be that infected CSF reduces antibiotic activity. For example, the low pH of infected CSF (between 6.7 and 7.1) reduces the activity of aminoglycosides and the increase in protein concentration decreases the concentration of the active drug, particularly that of the strongly bound β-lactamase (cephalosporins). This implies the use of high doses of antibiotics, especially when the MIC is elevated.
Immunotherapy
Because of the excessive inflammation of the meningeal spaces and its experimental aggravation by bacterial lysis caused by the antibiotics, it can be useful to modify the various players in this immunoinflammatory cascade by attempting to inhibit the polynuclear interactions with the haematomeningeal barrier. Inflammation, however, plays a positive role in the penetration of certain antibiotics at the infection site. The use of methylprednisolone reduces the spinal penetration of ampicillin and gentamicin by approximately half.
Diagnosis
Clinical diagnosis
The infectious syndrome is always present. It has a brutal aspect with a high fever (= 38.5°C), shivers and myalgia, sometimes accompanied by the following: pneumopathy (in cases of pneumococcus infection), purpura and septic shock. At times, it is subtle and is unfortunately diagnosed as an ear-nose-throat infection, which are often degenerated by antibiotic therapy.
The meningeal syndrome is quasi constant. Cephalalgia is diffuse, intense and continuous, and can radiate towards the nape of the neck and the back. It is increased by light, noise and coughing. Vomiting is frequent.Meningeal stiffness corresponds to the extension of the neck muscles during massive flexion of the head. It is responsible for the coiled position. It can sometimes be subtle, especially in an early stage or in elderly patients or in cases of coma. It can be evaluated by means of certain manoeuvres. Kernig's sign is a symptom of meningitis that is demonstrated when patients cannot keep their legs extended while sitting on a bed. In the prone position, the patient cannot raise and extend his arms because flexing occurs at a certain height. Brudzinski's sign is a spontaneous flexion of the thighs and legs during flexion provoked by the head (when the neck is flexed from a supine position). The meningeal syndrome can also include signs of cutaneous hyperesthesia or pyramidal irritation such as in osteotendon hyperreflexia.
Signs of encephalitis are erratic but must be considered as having a poor clinical outcome; they include change in mental status that ranges from mental confusion to coma. They may also include local or generalized convulsions. The factors that should raise suspicion of certain aetiologic diagnoses are listed Table 1.
Table 1.
Aetiologic diagnoses and suggestive features
| Aetiology | Suggestive features and comments | |
| Streptococcus pneumoniae | Severe alcoholism, hypogammaglobulinemia, neutropenia, drepanocytic anemia, splenectomy, polynuclear function deficiency, suppressive immunological therapy, acquired duramatral breach | |
| Neisseria meningitidis | Complement deficiency must be suspected. However, there are no real risk groups. Classically, epidemics occur in closed populations of young adults. | |
| Listeria monocytogenes | Extremes of age, pregnancy, hepatic cirrhosis, haemochromatosis, chronic renal failure, cellular immunodeficiency (Hodgkin's and non-Hodgkin's lymphoma, chronic lymphoid leukaemia, corticotherapy, organ transplantation) | |
Biological diagnosis
Lumbar puncture
When the clinical examination only reveals meningeal and infectious signs (60-70% of cases) lumbar puncture is the first examination to perform [23]. If, on the other hand, onset is progressive and there appears to be signs of deficiency without change in mental status, followed by signs of intracranial hypertension, a cerebral computed tomography (CT) scan must be urgently performed in order to exclude the presence of an expansion process. Funduscopic examination is completely useless.
In cases of comatose meningitis, the relationship between lumbar puncture and herniation is, in any case, very debatable. Such an important examination should therefore not be put off.
Macroscopically, the CSF has an elevated opening pressure, is cloudy (rice water) or purulent, confirming purulent meningitis and the need for subsequent antibiotic therapy (Fig. 1). Cytochemically, the CSF contains numerous polynuclear neutrophils (≥ 1000/mm3); protein concentration is high (≥ 1 g/l); and the CSF glucose concentration is low or even collapsed but, in any case, is less than 70% of the blood glucose concentration (ie the CSF:blood glucose concentration ratio is < 0.3).
Figure 1.
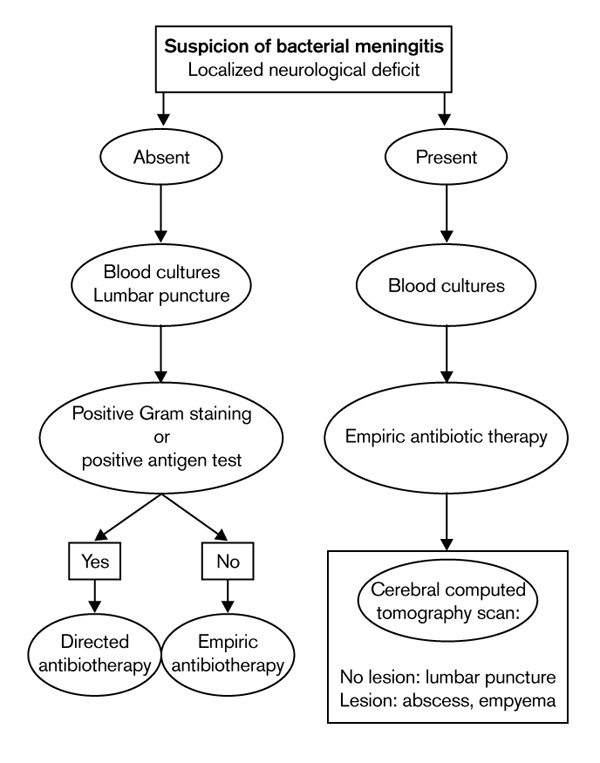
Management algorithm in case of suspicion of bacterial meningitis.
Particular forms can be encountered. The CSF can have minimal cytochemical modifications but be swarming with bacteria in cases of fulminant meningococcus meningitis, necessitating Gram's staining in all cases with purpura, whatever the cytological results of the CSF. Moreover, an intense cellular reaction without micro-organisms on direct examination is possible, with bacteriologic confirmation by culture of CSF.
Lymphocytosis is possible if lumbar puncture is performed very early, but this condition above all indicates Listeria meningoencephalitis [24,25].
Blood cultures
Blood cultures provide an indispensable diagnostic complement. Nonspecific examination results such as polynucleosis and elevated C-reactive protein may be suggestive of a bacterial aetiology.
Bacteriology
Gram's staining will provide an aetiologic diagnosis in 60–90% of the cases of bacteriologic meningitis. The percentage of positive direct examinations is highest for S. pneumoniae and H. influenzae, is lower for N. meningitidis, and is especially low for L. monocytogenes. Culturing is systematic and allows for isolation and identification of the causative bacteria. An antibiogram may be directly performed with the CSF if it is sufficiently rich in bacteria. The results obtained after 18–24 h must always be confirmed by classic study performed after the culture. In cases of pneumococcus infection, resistance to β-lactamases is sought by means of an oxacillin disk. Detection is completed by parallel determination of the MIC, performed either using classic dilution methods or using E-test with penicillin G and the β-lactams habitually used for the treatment of pneumococcus meningitis.
In patients with H. influenzae infection it is essential to look for β-lactamase production. Some strains of N. meningitidis with reduced sensitivity to penicillin G have been observed in France and Spain, but without therapeutic consequences (at the time of writing).
The interest of soluble antigen exploration has been subject to diverse evaluations. Performed using a particle agglutination of latex sensitivity technique, it can reinforce a diagnosis when the Gram's staining is questionable, but is of little use when the Gram's staining is negative [23]. Polymerase chain reaction can be a better means to detect bacteria in the CSF. Intraspinal lactate concentration alone does not have sufficient diagnostic value [26].
During the initial work-up, the diagnosis of bacterial meningitis is confirmed if the bacteriologic examinations are positive. In the case of negative results on direct examination, the medical probabilities should be determined in order to differentiate the bacterial or viral nature of an episode of meningitis. This can be achieved by using valid decision models that take several cytological and biochemical parameters of the CSF into account, in addition to the clinical data. Subsequent biological examinations are dictated by the clinical evolution and the initial bacterial data. A possible second lumbar puncture is performed 36–48 h after treatment, enabling the clinician to judge the therapeutic efficacy of CSF sterilization, as well as the rise in glycorrhachia. The other cytological and biochemical parameters of the CSF are of no diagnostic or prognostic value. In cases of therapeutic failure, an antibiotic may be administered in the CSF.
Radiological examinations
The indications for scanning or imaging should be very limited when a patient with purulent meningitis is admitted. Performing a CT scan before the lumbar puncture exposes the patient to the risks of delaying an antibiotic therapy that has reliable results. Lumbar puncture must therefore precede a CT scan, even in cases of coma. This diagnostic approach should only be changed if there are localized neurological signs that suggest another diagnosis or if there is a risk of an intracranial complication, possibly making lumbar puncture dangerous. Haemocultures and antibiotic therapy must therefore be immediately undertaken before imaging results are obtained (Fig. 1). In an emergency situation, cerebral CT scan enables the clinician to resolve most diagnostic problems. Magnetic resonance imaging is more accurate than CT, but it is less often available and does not provide decisive supplementary information.
Once antibiotic therapy has been started, the diagnosis of intracranial complications will depend on imaging. Cerebral CT scan is adequate for most intracranial complications (hydrocephalus, abscess, empyema, cerebral infarct, haemorrhage, ventriculitis). However, in cases of cerebral thrombophlebitis CT scanning does not reveal direct signs and an angiography is required. Magnetic resonance imaging is the examination of choice when it is available and will provide a diagnosis of all intracranial complications with sensitivity and specificity that are superior to those of CT scanning, particularly for the diagnosis of cerebral thrombophlebitis. In infants, transfontanel ultrasonography is easy to perform and enables the clinician to detect abscess, ventriculitis and hydrocephalus.
A search for a portal of entry is especially justified in pneumococcal meningitis. Portals of entry are often ear–nose–throat-related in cases of both acute and chronic pathology. Imaging relies on CT scanning, which has better bone definition than MRI. It is not indicated for clinically diagnosed acute otitis. On the other hand, it is justified when mastoiditis or an unsatisfactory evolution of meningitis is suspected.
Imaging is indispensable for the preoperative work-up of all chronic pathologies and for the diagnosis of most cases of acute sinusitis.
The existence of an osteodural breach must be considered in cases of post-traumatic pneumococcal meningitis and recurring meningitis. In rare cases, the breach can be a congenital ear and petrosa malformation that is clearly revealed by CT scan, or an anterior meningoencephalocele or lumbosacral dermic sinus, which are better explored using magnetic resonance imaging. More often, the breach is secondary to a recent or old head trauma or to surgery at the base of the skull. At the level of the ear, exploration for a breach must be performed by CT scanning in frontal and axial millimeter sections. For the anterior floor, the CT scan should be used to investigate for a lack of continuity, essentially at the level of the lamina cribrosa and the roof of the ethmoid bone. CT scanning is the technique of choice because it best examines the small bone dehiscences. Isotropic transit of the CSF is no longer an indication [23].
Differential diagnosis
Atypical cerebrospinal fluid
It can be difficult to differentiate between bacterial meningitis "beheaded" by an antibiotic treatment and viral meningitis which is diagnosed early due to the context, briefness of the signs and normal glycorrhachia, reinforced by the disappearance of polynucleosis and the appearance of lymphocytosis in the CSF sampled 24–48 h later.
In the absence of bacterial meningitis, the presence or even the predominance of polynuclear cells in the CSF can be encountered in several situations: bacterial pathologies suppurated from the brain or parameningeal spaces (subdural empyema, brain abscess), fungal infections (cryptococcosis, candidiasis, histoplasmosis, coccidsioidomycosis), rare cases of amoebic meningitis, neoplastic meningitis, chemical meningitis (co-trimoxazole, nonsteroidal anti-inflammatory drugs, azathioprine), systemic recurrent aseptic puriform meningitis, disseminated lupus erythematosus, sarcoidosis, Behçet's disease. Rupture of an epidermoid cyst in the arachnoidea space and Mollaret's benign recurrent pleocytic meningitis are very rare and their diagnosis is favoured by recurrence with a sudden onset, rapidly favourable evolution and the polymorphic character of the CSF.
Acute clear cerebrospinal fluid meningitis
Acute clear CSF meningitis is benign in most cases. It is due to a great number of viruses and evolves spontaneously towards resolution. Measles and German measles are in very distinct decline due to systematic vaccination.
The major problem is the possibility of a herpetic aetiology leading to emergency empiric treatment with acyclovir. The other emergency diagnostic hypothesis is Listeria neuromeningeal infection, which also requires emergency empiric antibiotic treatment. The possibility of a tuberculous infection should not be ignored, but therapy is often less urgent. The various diagnostic hypotheses are often formulated from the epidemiological data gathered from the context, because the microbiological results often arrive too late for emergency treatment [9].
Herpetic meningoencephalitis
Herpetic meningitis is usually due to herpes simplex virus I. In 30% of the cases, the encephalitis is primary, but in 70% it is preceded by a past history of benign herpes. Such pathology represents direct viral aggression located at the frontal and temporal level and leading to necrosis of the affected tissue. The clinical picture is not very specific, but the patient will present with increasingly severe mental confusion, convulsions, cranial nerve impairment and olfactory hallucinations. However, because these symptoms will appear too late for effective treatment, herpetic aetiology must be presumed for encephalitis. If untreated, death occurs in 60–80% of the cases with very severe neurological sequelae in the surviving patients.
Biological diagnosis is difficult to perform: the CSF is lymphocytic and serum antibodies are of little use for an emergency diagnosis because antibody intrathecal secretion (estimated by comparing its level with serum level) is specific but inconsistently detected. The electroencephalograph is more reliable with pseudoperiodic rhythmicity in the frontotemporal regions. However, this assessment is often delayed in comparison with therapy, the need for which is urgent; its absence must not delay antiviral treatment. The same is true for the cerebral CT scan with iodine injection. The images are typically hypodense zones with peripheral contrast in the temporal and frontal regions. Such images are often bilateral and symmetrical.
The currently accepted treatment for herpetic encephalitis has considerably improved outcome: acyclovir, 10 mg/kg every 8 h by intravenous infusions of approximately 1 h. The duration of treatment is classically 10 days, but there have been reports in the literature of rare relapses due to a short duration of therapy, and consequently 3 weeks of treatment are often recommended.
Listeria meningoencephalitis
Listeria meningoencephalitis is certainly the most frequently encountered at present. The clinical signs are not very specific. It is, however, possible to stress the frequent association of a straightforward meningeal syndrome and impairment of one or several cranial nerves. Diagnosis is confirmed by isolation of the bacterium in blood cultures and/or CSF culture. Given the small size of this Gram-positive bacillus and the low inoculum, direct examination of the CSF is very often negative. The cytorachia is typically mixed with polynuclear cells and lymphocytes, but a predominantly lymphocytic composition is observed just as fequently. Cerebral CT scan will reveal diverse features ranging from simple oedema to necrosis. On the other hand, a temporal lesion location is suggestive. Encephalitis is diffuse when there are intracerebral abscesses predominantly located in the rhombencephalon with multiple and necrotic abscesses. More rarely, there can be pure encephalitic forms without meningitis, which must be treated as an emergency (see the section on antibiotic therapy for L. monocytogenes, below).
Such impairment of the central nervous system is singular. Although it is frequently observed with a virus, it is rarely encountered with pathogenic bacteria, except Mycobacterium tuberculosis meningoencephalitis and rare neuromeningeal infections with Nocardia asteroides, or Brucella or Leptospira spp. Certain parasites such as Toxoplasm gondii are also capable of triggering very severe encephalitis, especially in acquired immunodeficiency patients.
Tuberculous meningoencephalitis
Neurological locations account for 0.5% of the cases of tuberculosis. It should nevertheless be considered when the predisposing factors suggest it (disadvantaged social background, recent immigrant, tuberculosis in family circle) or when neurological impairment is not sensitive to antibiotic or antiviral therapies. Diagnosis is difficult: the CSF reveals a mixed composition (but could be lymphocytic), hypoglycorrhachia and hypochlorurorachia (but can be normal), hyperproteinorachia (which only expresses the chronic inflammatory aspect); the growth of M. tuberculosis (Koch's bacillus) is slow and cannot support a rapid diagnosis; and direct examination of the CSF by Ziehl staining is exceptionally positive. There is hope with the detection of bacterial antigens in the CSF (using a gene amplification technique, polymerase chain reaction), but this is still in the research stage and is not routine. Cerebral imaging is only useful in cases with localized lesions: abscess or evolved tuberculoma, or granulomas that are difficult to detect. Apart from these cases, the images are less specific and show inflammation: oedema or inflammatory filling of the cisterna. More often than not, the clinician is therefore reduced to prescribing a presumptive therapy following a series of arguments, which can easily lead to therapeutic failure.
Evolution and prognosis
In the review by Durand et al [3] of 493 cases of bacterial meningitis in adults the mortality rate was 25%, and in a recent study of Gram-negative meningitis 61% of the children had neurological sequelae [27]. Predisposing factors and signs of severity for meningitis caused by several aetiologic agents are outlined in Table 2.
Table 2.
Predisposing factors and signs of severity
| Aetiologic agent | Predisposing factors | |
| Streptococcus pneumoniae | Previous history of head injury, surgery at the base of the skull, meningitis, rhinorrhea, sudden onset, neurological signs, otitis, sinusitis or associated pneumopathy, asplenia, human immunodeficiency virus infection | |
| Neisseria meningitidis | Possible epidemic, purpura | |
| Listeria monocytogenes | Immunodepression, signs of rhomboencephalitis, cloudy cerebrospinal fluid with mixed composition | |
| Haemophilus influenzae | Age <5 years, absence of vaccination. | |
| Principal signs of severity | Purpura fulminans, deep coma (Glasgow coma score <8), cardiorespiratory failure | |
Pneumococcal meningitis
Prognosis depends on the severity of both encephalitic impairment and associated systemic signs. Mortality is high (16–33%) with otoneurological sequelae (17–29%) [10].
Meningococcal meningitis
In such cases severity is indicated by the infectious signs of purpura fulminans (6-13% of meningococcal infections, of which there is a 50% mortality) than to neurological signs, which have a low mortality rate (3–10%) and rare sequelae (6-10%) including deafness, impairment of another cranial nerve, hydrocephalus and epilepsy.
Listeria meningitis
The prognosis is poor, with 33% mortality and 33% sequelae including upper function, tonic and deglutination disorders.
Treatment
Antibiotic therapy
There is no justification for delaying antibiotic therapy (Tables 3 and 4), because early treatment is associated with better outcome in cases of meningococcal and pneumococcal meningitis. Treatment must therefore be started immediately after the lumbar puncture and the initial blood culture, and sometimes before if the CSF examination has been deferred (patient far from a hospital, decision to perform a CT scan) and/or if the symptoms appear to be fulminant (fever at 40°C, meningeal syndrome and worsening level of consciousness in < 24 h or purpura; Fig. 1) [23].
Table 3.
Initial treatment of purulent meningitis with negative direct examination, absence of predisposing factors and signs of severity [1]
| Antibiotic | Posology (mg/kg per day) | Administration route | |
| Child =3 months | Cefotaxime | 200-300 | Four infusions |
| or ceftriaxone | 70-100* | One or two intravenous injections | |
| Adult | Amoxicillin | 200 | Four to six infusions |
| or cefotaxime | 200-300 | Four infusions | |
| or ceftriaxone | 70-100 | One or two intravenous injections | |
*Maximum 4 g/day
Table 4.
Initial treatment of purulent meningitis with negative direct examination, according to predisposing factors and/or signs of severity [1]
| Patient | Suspected organisms | Treatment (posology) |
| Child | Neisseria meningitidis | Amoxicillin or 3-GC |
| Streptococcus pneumoniae | 3-GC + vancomycin | |
| Haemophilus influenzae | 40-60 mg/kg per day. Four infusions = 1 h or continuous infusion | |
| (load: 15 mg/kg) 3-GC | ||
| Adult | Streptococcus pneumoniae | Preference 3-GC |
| If suspicion of penicillin-resistant pneumococci and/or signs of severity | 3-GC + vancomycin 40-60 mg/kg per day. Four infusions = 1 h or continuous infusion (load: 15 mg/kg) | |
| Listeria monocytogenes | Amoxicillin is indispensable in association with gentamicin or co trimoxazole | |
| Neisseria meningitidis | Amoxicillin or 3-GC | |
| Child and adult | Absence of predisposing factors and signs of severity | Amoxicillin + 3-GC |
3-GC, cefotaxime or ceftriaxone (see Table 1 for posologies).
Neisseria meningitidis
N. meningitidis is sensitive to penicillins and third-generation cephalosporins. Amoxicillin (200 mg/kg per day), cefotaxime (200mg/kg per day), or ceftriaxone (70–100 mg/kg per day) can be used for 7–10 days [28].
Listeria monocytogenes
The reference treatment for L. monocytogenes is amoxicillin (200 mg/kg per day) plus gentamicin (6 mg/kg per day), which, despite the weak meningeal diffusion of these compounds, is justified because of their in-vitro synergistic effect and the frequency of initial bacteraemic forms. Another excellent possibility, supported by its diffusion into the CSF, is co-trimoxazole: 960 mg every 12 h intravenously for 2–3 weeks [29].
Haemophilus influenzae
H. influenzae meningitis is treated by cefotaxime (200 mg/kg per day) or ceftriaxone (70-100mg/kg per day).
Streptococcus pneumoniae
Antibiotic therapy for S. pneumoniae is confronted with two difficulties: the virulence of the micro-organism and increasing resistance to the antibiotics usually prescribed for purulent community-acquired meningitis. The major antibiotics that have the best in-vitro activity on PRP are cefotaxime, ceftriaxone, vancomycin, amoxicillin and imipenem. Rapid determination of the MIC for the above β-lactams is indispensable in order to best adjust the treatment. The therapy is administered by the intravenous route for 10–14days and is prolonged in cases of slow response and/or a strain with reduced sensitivity. The choice of antibiotic therapy is governed by the age of the patient, the presence of signs of severity and/or risk factors for PRP [23,30,31].
In children under 3 months of age or in adults with risk factors for PRP and/or presenting with signs of severity, initial treatment must include a third-generation cephalosporin such as cefotaxime (300 mg/kg per day in four infusions) or ceftriaxone (70–100 mg/kg per day in one or two injections, maximum 4 g/day), and vancomycin (60 mg/kg per day either in four infusions of at least 1 h or in continuous infusion with an initial load of 15 mg/kg) [32]. Re-evaluation is performed at 36–48 h and is based on the clinical data and lumbar puncture.
If the evolution is favourable, further treatment will depend on the MIC of the cephalosporin that is used. If the MIC is inferior to 0.5 mg/l, one can discontinue the vancomycin and possibly reduce the dosage of the third-generation cephalosporin (to 200 mg/kg per day) or prescribe amoxicillin (150–200 mg/kg per day) if the amoxicillin MIC is inferior to 0.12 mg/l.
If the third-generation cephalosporin MIC is superior or equal to 0.5 mg/l, initial treatment must be continued. In case of clinical and/or microbiological failure, treatment must be modified by taking into account the results of the second lumbar puncture, the MIC of the antibiotics and the concentration of vancomycin in the CSF [21,23]. The drug combination should be decided with the microbiologist and chosen among a list of antibiotics for which the MIC are known: imipenem, meropenem, rifampin and fosfomycin.
The initial treatment for an adult without a risk factor for PRP or signs of severity is cefotaxime (200–300 mg/kg per day in four infusions), but amoxicillin remains an option at a dose of 200 mg/kg per day in four to six infusions, especially in regions where the prevalence of PRP is low. Clinical re-evaluation is performed at 36–48 h. For subsequent treatment, if the evolution is favourable and if the strain has normal sensitivity to these β-lactams, one can reduce the dose of the third-generation cephalosporin or prescribe amoxicillin (150–200 mg/kg per day). When the MIC of the third-generation cephalosporin is superior or equal to 0.5 mg/l, lumbar puncture is indispensable to confirm an improvement in the CSF; such improvement mandates continuation of the initial treatment. In cases of clinical and/or microbiological failure, the treatment must be modified by adding vancomycin to the initial treatment (see doses above).
It is recommended to treat H. influenzae and meningococcal meningitis for 7–10 days, but longer periods of 10–21 days are required for other pathogens: 10–14 days for S. pneumoniae; 14–21 days for L. monocytogenes and group B streptococci; and 21 days for Gram-negative bacilli, except for H. influenzae.
Empirical choice of an antibiotic therapy
When lumbar puncture is delayed or direct examination of the CSF is not helpful, empiric treatment is essential. A broad-spectrum cephalosporin (cefotaxime or ceftriaxone) is recommended for most patients, combined with ampicillin in children under 3 months and adults over 50 years (two populations in which S. agalactiae and L. monocytogenes are frequently found). In patients with recent head trauma or who have undergone neurosurgery, the clinician must choose a broad-spectrum antibiotic treatment that is active on Gram-positive and Gram-negative bacteria, such as the combination of ceftazidime and vancomycin. In immunocompromised patients (chemotherapy, corticotherapy, haematologic tumours), treatment must include ampicillin (for possible listeriosis) and a cephalosporin, such as ceftazidime, which is active on Gram-negative bacilli.
Intensive care
Given the number of cases of life-threatening secondary or associated visceral impairment, it is important to treat respiratory failure, septic shock, intracranial hypertension or disseminated intravascular coagulation. As for the latter syndrome, a recent study has demonstrated the interest of administering concentrated Protein C for purpura fulminans in cases of meningococcemia.
Treatment of the portal of entry
Otorhinologic treatment of otitis and sinusitis is an emergency that can be put off for a few days if the clinical evolution during the first few days is favourable. It can include atticomastoidectomy, exeresis of a cholesteatoma or sinus drainage. Detection of traumatic osteodural breaches and their treatment are not considered as emergencies.
Immunotherapy
Corticotherapy
The use of anti-inflammatory agents, particularly dexamethasone, makes it possible to reduce local inflammatory reactions that are responsible for cerebral oedema and thereby reduce the morbidity and mortality in purulent meningitis. Several recent studies [23,33,34], notably in children, have investigated neurosensory sequelae and mortality of a short corticoid treatment administered in the initial phase of bacterial meningitis. These studies have demonstrated a possible reduction in mortality that has, however, only been found in open studies performed in populations of patients whose clinical presentation was immediately severe with treatment often delayed. A reduction in auditory sequelae, even neuromotor sequelae, in children treated with dexamethasone (double-blind studies), especially in children with H. influenzae purulent meningitis, has been noted; there is probably a similar effect for pneumococcal meningitis, but cannot be evaluated from the available data for meningococcal meningitis. In adults there is only partial information on the efficacy of initial corticotherapy, but there are potential disadvantages of corticosteroid use. Through the rapid symptomatic modifications that they can trigger, corticosteroids can interfere with assessment of evolution and lead to possible errors in clinical interpretation. Consequently, they must not be used for a meningitis that is insufficiently documented. Complications due to corticotherapy when used in this context are very rare. On the other hand, one can observe a return of fever when corticosteroid treatment is stopped. The use of dexamethasone in animal models has demonstrated a reduction in the penetration of certain antibiotics, including vancomycin, into the CSF. This could create a problem for treatment with certain antibiotics with relatively weak meningeal diffusion in cases of PRP infection.
On the whole, the favourable benefit:risk ratio for early corticoid therapy would advocate the prescription of dexamethasone at the beginning of treatment of purulent meningitis in children when it is probably due to the usual community bacteria. For adults, however, additional clinical studies will be necessary to draw conclusions on this point. The adults who could benefit the most are those who present with a high bacterial concentration in the CSF and signs of intracranial hypertension [35].
When it is used, corticotherapy must be instituted early. It is usually administered a few minutes before the first dose of antibiotic [ie, dexamethasone intravenously (0.6 mg/kg per day in two to four injections)]. The duration of treatment is debatable, but a 2-day treatment would appear to be as effective as a 4-day treatment. If dexamethasone is chosen, it is better to associate it with rifampicin rather than vancomycin in cases of PRP.
Antimediators
The use of agents that block the action of polynuclear cells or cytokines has been promising in experimental meningitis, but has yet to be validated in humans. A recent clinical study would seem to have found an interesting effect of a protein that increases bacterial permeability (rBPI21) in 26 children aged from 1 to 18 years and presenting with severe meningococcaemia.
Prophylaxis
Pneumococcal meningitis
Continuous cyclic chemoprophylaxis with penicillin G is used in certain adults (following splenectomy, osteodural breach victims), but is not based on well established data [36]. The emergence of resistant strains should put this practice into question.
Vaccination is recommended for patients at risk. It is debatable for patients aged over 65 years or under certain circumstances for elderly or handicapped patients living in groups. Its efficacy has not been demonstrated, especially given the poor vaccination response in groups at risk. Vaccination is ineffective before the age of 2 years and not advisable in pregnant women. A booster vaccination is performed after 5 years or earlier in immunocompromised patients.
Meningococcal meningitis
In France, a Public Health Service declaration is obligatory once a case has been identified. Prophylaxis involves those in contact with the patient before diagnosis (living under the same roof in the 10 days before hospitalization, close friends, immediate neighbours in class, cafeteria, room) and after curative treatment, as well as clinical staff exposed to air contamination (mouth-to-mouth resuscitation, intubation). It is extended to an entire school class if several cases have occurred in the same class, and to an entire school if three or more cases have occurred in different classes in less than a month. Casual contacts (coworkers, other students who were not close to the patient, health care personnel in general) are not involved. Except for secondary cases, measures must be taken within 8 days of the diagnosis and are organized by the family doctor (in household cases) or, in group cases, by the Public Health Service and the doctor responsible for the group. Contact subjects and contacts from the same group as the patient must be informed about the disease and the measures to be taken. Medical observation of the contact subjects must be undertaken for 2weeks after the institution of prophylactic measures. Rhinopharyngeal disinfection and sampling are not useful. Suspension from school or isolation of the contact subjects is not recommended. Given the fragility of meningococcus, disinfection or closure of an establishment, including a school, are measures that are unnecessary and unjustified.
Chemoprophylaxis consists of rifampicin per os for 2 days: in adults, 600 mg twice a day; in children aged 1 month to 12 years, 10 mg/kg three times a day; in children aged under 1 month, 5 mg/kg twice a day. The wearing of soft contact lenses must be stopped during treatment due to the risk of irreversible dyeing. Moreover, subjects taking oral contraception must be given another contraceptive solution due to the risk of enzymatic induction.
The contraindications are pregnancy, severe hepatic disease, porphyrias and hypersensitivity to rifampicin. In the latter situation, use spiramycin per os for 5days: in adults, 3 million IU twice a day; in children, 75000 IU/kg twice a day.
Vaccination (meningococcal vaccine A+C) is recommended in addition to chemoprophylaxis, beginning at the age of 3months in the case of meningococcus A and from 1year of age in the case of meningococcus C. It can also be offered to those who have stayed in a region with an epidemic: peace corps workers and members of medical expeditions in the sahel. Professional or academic suspension of contact subjects and disinfection of premises are not useful.
Haemophilus influenzae type B meningitis
The probability of contracting meningitis is markedly increased in children aged under 4years living in the same domicile as an index case. Prophylaxis relies on the administration of rifampicin (30 mg/kg per day) for 4days from the age of 1month. It provides an eradication rate of approximately 95% for carriers of H. influenzae. The emergence of type B strains that are resistant to rifampicin is rare (0.2% in France). H. influenzae type B vaccination and protective and immunogenic vaccines in very young children have greatly modified the epidemiology of meningitis in countries where these vaccines are used on a large scale. In France, according to the National Reference Center data, 635 strains of H. influenzae were isolated in CSF between 1984 and 1992, 71 strains in 1993, and 34 strains in 1994.
Listeria monocytogenes meningitis
The prevention of listeriosis essentially depends on food hygiene. This applies to pregnant women, immunocompromised subjects and the elderly.
References
- Sigurdardottir B, Bjornsson OM, Jonsdottir KE, Erlendsdottir H, Gudmundsson S. Acute bacterial meningitis in adults. A 20-year overview. Arch Intern Med. 1997;157:425–430. doi: 10.1001/archinte.1997.00440250077009. [DOI] [PubMed] [Google Scholar]
- Miller LG, Choi C. Meningitis in older patients: how to diagnose and treat a deadly infection. Geriatrics. 1997;52:43–55, 47-50, 55. [PubMed] [Google Scholar]
- Durand ML, Calderwood SB, Weber DJ, et al. Acute bacterial meningitis in adults. N Engl J Med. 1993;328:21–28. doi: 10.1056/NEJM199301073280104. [DOI] [PubMed] [Google Scholar]
- Schaad UB. Current concepts of bacterial meningitis. Eur J Pediatr. 1995;Suppl 4:S20–S22. doi: 10.1007/BF02191499. [DOI] [PubMed] [Google Scholar]
- Richardson M. Bacterial meningitis. Br J Hosp Med. 1996;55:685–688. [PubMed] [Google Scholar]
- Segreti J, Harris AA. Acute bacterial meningitis. Infect Dis Clin North Am. 1996;10:797–809. doi: 10.1016/s0891-5520(05)70327-5. [DOI] [PubMed] [Google Scholar]
- Grebe T, Hakenbeck R. Penicillin-binding proteins 2b and 2x of Streptococcus pneumoniae are primary resistance determinants for different classes of β- lactam antibiotics . Antimicrob Agents Chemother. 1996;40:829–834. doi: 10.1128/aac.40.4.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booy R, Kroll JS. Bacterial meningitis and meningococcal infection. Curr Opin Pediatr. 1998;10:13–18. doi: 10.1097/00008480-199802000-00004. [DOI] [PubMed] [Google Scholar]
- Infections cérébro-méningées in Monographies [in French]. La Revue du Praticien. 1994;44:2145–2224. [Google Scholar]
- Kragsbjerg P, Källman J, Olcén P. Pneumococcal meningitis in adults. Scand J Infect Dis. 1994;26:659–666. doi: 10.3109/00365549409008633. [DOI] [PubMed] [Google Scholar]
- de Saint-Martin L, Nassif X. Physiopathologie des méningites bac-tériennes aigües purulentes [in French]. Méd Thér. 1995;1:527–532. [Google Scholar]
- Waage A, Halstensen A, Shalaby R, Brandtzaeg P, Kierulf P, Espevik T. Local production of tumor necrosis factor α, interleukin 1 and interleukin 6 in meningococcal meningitis. J Exp Med. 1989;170:1859–1867. doi: 10.1084/jem.170.6.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornelisse RF, Savelkoul HF, Mulder PH, et al. Interleukin-10 and soluble tumor necrosis factor receptors in cerebrospinal fluid of children with bacterial meningitis. J Infect Dis. 1996;173:1498–1502. doi: 10.1093/infdis/173.6.1498. [DOI] [PubMed] [Google Scholar]
- Kornelisse RF, Hack CE, Savelkoul HF, et al. Intrathecal production of interleukin-12 and gamma interferon in patients with bacterial meningitis. Infect Immun . 1997;65:877–881. doi: 10.1128/iai.65.3.877-881.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fassbender K, Ries S, Schminke U, Schneider S, Hennerici M. Inflammatory cytokines in CSF in bacterial meningitis: association with altered blood flow velocities in basal cerebral arteries. J Neurol Neurosurg Psychiatry. 1996;61:57–61. doi: 10.1136/jnnp.61.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprenger H, Rosler A, Tonn P, et al. Chemokines in the cerebrospinal fluid of patients with meningitis. Clin Immunol Immunopathol. 1996;80:155–161. doi: 10.1006/clin.1996.0109. [DOI] [PubMed] [Google Scholar]
- Fassbender K, Schminke U, Ries S, et al. Endothelial-derived adhesion molecules in bacterial meningitis: association to cytokine release and intrathecal leukocyte recruitment. . J Neuroimmunol. 1997;74:130–134. doi: 10.1016/s0165-5728(96)00214-7. [DOI] [PubMed] [Google Scholar]
- van Deuren M, van der Ven Jongekrijg J, Vannier E, et al. The pattern of interleukin-1beta (IL-1beta) and its modulating agents IL-1 receptor antagonist and IL-1 soluble receptor type II in acute meningococcal infections. Blood. 1997;90:1101–1108. [PubMed] [Google Scholar]
- Ernst T, Spath PJ, Aebi C, Schaad UB, Bianchetti MG. Screening for complement deficiency in bacterial meningitis. Acta Paediatr . 1997;86:1009–1010. doi: 10.1111/j.1651-2227.1997.tb15190.x. [DOI] [PubMed] [Google Scholar]
- Calder JA. Listeria meningitis in adults. . Lancet. 1997;350:307–308. doi: 10.1016/S0140-6736(05)63384-3. [DOI] [PubMed] [Google Scholar]
- Paris MM, Ramilo O, McCracken GH., Jr Management of meningitis caused by penicillin-resistant Streptococcus pneumoniae . Antimicrob Agents Chemother. 1995;39:2171–2175. doi: 10.1128/aac.39.10.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheld WM, Fletcher DD, Fink FN, Sande MA. Response to therapy in an experimental model of meningitis due to Listeria monocytogenes . J Infect Dis. 1979;140:287–294. doi: 10.1093/infdis/140.3.287. [DOI] [PubMed] [Google Scholar]
- 9º Conference de Consensus en Therapeutique Anti-infectieuse Les méningites purulentes communautaires [in French]. Méd Mal Infect. 1996;26 Spécial:1–8. [Google Scholar]
- Garty BZ, Berliner S, Liberman E, Danon YL. Cerebrospinal fluid leukocyte aggregation in meningitis. Pediatr Infect Dis. 1997;16:647–651. doi: 10.1097/00006454-199707000-00005. [DOI] [PubMed] [Google Scholar]
- Azuma H, Tsuda N, Sasaki K, Okuno A. Clinical significance of cytokine measurement for detection of meningitis. J Pediatr. 1997;131:463–465. doi: 10.1016/s0022-3476(97)80079-0. [DOI] [PubMed] [Google Scholar]
- Pavese P, François P, Lafond JL, Kayemba-Kay SS, Bosson JL. Dosage de l'acide lactique dans le liquide céphalorachidien pour le diagnostic des méningites bactériennes. Stratégies pour le choix du seuildiscriminant [in French]. Presse Med. 1997;26:551–554. [PubMed] [Google Scholar]
- Grimwood K, Nolan TM, Bond L, et al. Risk factors for adverse outcomes of bacterial meningitis. J Paediatr Child Health . 1996;32:457–462. doi: 10.1111/j.1440-1754.1996.tb00949.x. [DOI] [PubMed] [Google Scholar]
- Kennedy NJ, Duncan AW. Acute meningococcaemia: recent advances in management. Anaesth Intens Care. 1996;24:197–216. doi: 10.1177/0310057X9602400212. [DOI] [PubMed] [Google Scholar]
- Hof H, Nichterlein T, Kretschmar M. Management of listeriosis. . Clin Microbiol Rev. 1997;10:345–357. doi: 10.1128/cmr.10.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astruc J. Méningites à pneumocoque de l'enfant. Propositions thérapeutiques [in French]. Méd Mal Infect. 1997;24 Spécial:982–985. [Google Scholar]
- Decazes J-M, Casin I, Kitzis M-D, et al. Méningite à pneumocoque résistant à la pénicilline: bases expérimentales de la thérapeutique [in French]. Infections à Pneumocoque de Sensibilité Diminuée aux Béta-lactamines. Edited by Carbon C, Chastang C, Decazes J-M. Springer-Verlag, Paris, 1993.
- Viladrich PF, Cabellos C, Pallares R, et al. High doses of cefotaxime in treatment of adult meningitis due to Streptococcus pneumoniae with decreased susceptibilites to broad-spectrum cephalosporins. Antimicrob Agents Chemother. 1996;40:218–220. doi: 10.1128/aac.40.1.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quagliarello VJ, Scheld WM. Treatment of bacterial meningitis. N Engl J Med. 1997;336:708–716. doi: 10.1056/NEJM199703063361007. [DOI] [PubMed] [Google Scholar]
- Lauritsen A, Oberg B. Adjunctive corticosteroid therapy in bacterial meningitis. Scand J Infect Dis. 1995;27:431–434. doi: 10.3109/00365549509047040. [DOI] [PubMed] [Google Scholar]
- Townsend GC, Scheld WM. The use of corticosteroids in the management of bacterial meningitis in adults. J Antimicrob Chemother. 1996;37:1051–1061. doi: 10.1093/jac/37.6.1051. [DOI] [PubMed] [Google Scholar]
- Hristea A, Chiotan M, Tudose M, Mihalcu F. The combined value of chemoprophylaxis and pneumococcal vaccine in the prevention of recurrent pneumococcal meningitis. J Infect. 1997;34:265–267. doi: 10.1016/s0163-4453(97)94451-0. [DOI] [PubMed] [Google Scholar]


