Abstract
Extracorporeal membrane oxygenation (ECMO) is a technique for providing life support, in case the natural lungs are failing and are not able to maintain a sufficient oxygenation of the body's organ systems. ECMO technique was an adaptation of conventional cardiopulmonary bypass technique and introduced into treatment of severe acute respiratory distress syndrome (ARDS) in the 1970s. The intial reports of the use of ECMO in ARDS patients were quite enthusiastic, however, in the following years it became clear that ECMO was only of benefit in newborns with acute respiratory failure. In neonates treated with ECMO, survival rates of 80% could be achieved. In adult patients with ARDS, two large randomized controlled trials (RCTs) published in 1979 and 1994 failed to show an advantage of ECMO over convential treatment, survival rates were only 10% and 33%, respectively, in the ECMO groups. Since then, ECMO technology as well as conventional treatment of adult ARDS have undergone further improvements. In conventional treatment lung-protective ventilation strategies were introduced and ECMO was made safer by applying heparin-coated equipment, membranes and tubings. Many ECMO centres now use these advanced ECMO technology and report survival rates in excess of 50% in uncontrolled data collections. The question, however, of whether the improved ECMO can really challenge the advanced conventional treatment of adult ARDS is unanswered and will need evaluation by a future RCT.
Keywords: acute respiratory distress syndrome, adults, children, complications, extracorporeal membrane oxygenation, follow up, history, lung protective mechanical ventilation, mortality rates, technology
Introduction
Acute respiratory failure, which is defined as the necessity for intubation and mechanical ventilation, occurs with an incidence of 78-88 cases/100 000 inhabitants per year [1,2]. The more severe forms of acute respiratory failure, such as acute lung injury and acute respiratory distress syndrome (ARDS), occur with incidences of 18-70 [1,3,4] and 1.5-13.5/100 000 inhabitants per year [1,2,5,6,7,8], respectively. Patients with ARDS respond favourably to advanced methods of intensive care, which include, but are not limited to, various forms of mechanical ventilation with positive end-expiratory pressure (PEEP) and permissive hypercapnia, positional manoeuvres, sophisticated fluid regimens and inhalational pulmonary vasodilators [9,10,11]. In specialized centers that apply these therapeutic options, encouraging survival rates of greater than 60% have been reported in uncontrolled trials [10,12,13,14,15].
There remains, however, a small number of ARDS patients whose pulmonary gas exchange can not be improved sufficiently by the above mentioned methods, and extracorporeal membrane oxygenation (ECMO) may then be an additional therapeutic option during the acute phase. This review discusses the rationale, history, technique and outcome of this demanding procedure.
Rationale of extracorporeal membrane oxygenation
During the past few decades it has become evident from laboratory and clinical research that, in patients with acute lung injury, mechanical ventilation contributes to the progression of the disease. To describe the nonspecific radiographical, physiological and pathological manifestations of acute lung injury and its complications, the term 'ventilator-induced lung injury' was coined [16]. Ventilator-induced lung injury, for years used synonymously with barotrauma of the lung, is currently viewed as a systemic disease with similar symptoms and macroscopical and microscopical features of experimental acute lung injury, which is not markedly different from the diffuse alveolar damage that is present in human ARDS. It may be associated with pulmonary and systemic infections, multisystem organ dysfunction, volutrauma, barotrauma and increased mortality.
Animal studies [17,18] have shown that mechanical ventilation involving the application of high airway pressures or large tidal volumes (VTs) in combination with too high or too low PEEP may generate shear forces that effect healthy or diseased lungs, and can increase capillary permeability, promote gas leaks and oedema, and initiate inflammation. Results of a study in an isolated rat lung model [19] suggest that mechanical ventilation can induce activation and influx of neutrophil granulocytes and liberation of cytokines, leading to local and systemic inflammatory reactions. These findings were recently reproduced in human ARDS. Ranieri et al [20] found that ARDS patients who were mechanically ventilated with VTs of 11.1 ml/kg body weight and an end-inspiratory plateau pressure of 31.0 cmH2O had significantly higher concentrations of inflammatory mediators than did patients who were mechanically ventilated with smaller VTs of 7.6 ml/kg body weight and an end-inspiratory plateau pressure of 24.6 cmH2O.
These insights were paralleled by implementation of the 'protective ventilation strategy' in critical care of patients with severe acute respiratory failure [9]. This therapeutic concept is aimed at shielding the diseased, ventilated lung by applying only small VTs. Establishing a 'lung-protective ventilation strategy' in severe ARDS may be incompatible with the goal of maintaining sufficient gas exchange. ECMO is able to partly take over oxygenation and carbon dioxide removal, and thereby may allow respirator settings (peak inspiratory pressure, VT, PEEP, respiratory rate, fractional inspired oxygen) to be adjusted to the mechanical and gas exchange properties of the diseased lung. In this way the goals of lung protective mechanical ventilation can be reached, even in severe ARDS.
History
In 1885, von Frey and Gruber [21] developed the first device to oxygenate blood extracorporally for perfusion of isolated organs. Gas exchange was achieved by conducting a continuous flow of oxygen through an inclined rotating cylinder, the inner surface of which was covered with a thin film of blood. Gibbon [22] began developing the heart-lung machine in 1937 in order to allow open heart surgery to be performed. He designed a system in which anticoagulated blood was directly exposed to oxygen ('film' or 'bubble oxygenators'). Due to the direct contact between blood and the gaseous phase, however, severe haemolysis, thrombocytopaenia, haemorrhage and organ failure complicated the treatment and limited the use of this device to a few hours [23]. In 1956, Clowes et al [24] developed an artificial lung that separated the gaseous from the liquid phase by a membrane. This 'membrane oxygenator', with subsequent improvements in materials, provided faster and more efficient blood oxygenation with fewer complications than the 'film' or 'bubble oxygenators', and became practical for cardiopulmonary bypass that lasted longer than a few hours [25]. In 1972, clinical application of ECMO in respiratory failure of newborns and adults was attempted. In that year, Hill et al [26] reported the survival of a 24-year-old polytraumatized patient with ARDS who had been treated with ECMO during the acute phase of the disease. Four years later, Bartlett et al [27] reported on baby Esperanza, the first newborn treated with ECMO, who survived.
These enthusiastic reports nourished the hope that an effective new symptomatic therapy for severe hypoxia in ARDS was available. A large randomized multicenter trial was launched in 1974 to test venoarterial ECMO versus conventional therapy in adult ARDS patients [28]. The study revealed that mortality rates in the ECMO therapy group were as high as 90% and not significantly different from those in the conventionally treated group. These discouraging results in adult ARDS patients dampened previous enthusiasm, and interest in venoarterial ECMO waned in most research groups.
The idea of supporting impaired lung function with extracorporeal gas exchange in adults, however, was subsequently pursued by Kolobow et al [29]. The rationale of their advanced technique was to prevent further damage to the diseased lungs by reducing their motion (pulmonary rest) with application of only a few ventilator breaths with low VT and low peak inspiratory pressures. This lung protective mechanical ventilation strategy became known as low-frequency positive-pressure ventilation (LFPPV) [30]. With this method, oxygen uptake and carbon dioxide removal were dissociated. Oxygenation was primarily accomplished through the nearly motionless natural lung via apneic oxygenation, and carbon dioxide was cleared through the artificial lung [extracorporeal carbon dioxide removal (ECCO2-R)]. The so-called LFPPV-ECCO2-R technique was performed at low extracorporeal blood flows (20-30% of cardiac output), so that a venovenous bypass technique instead of an arteriovenous one sufficed, which turned out to be less detrimental to blood cells, coagulation and internal organs. Using LFPPV-ECCO2-R, Gattinoni et al [30] reported survival rates of up to 49%. In the following years several centres corroborated the promising survival rates of around 50% and higher (Fig. 1); these were uncontrolled observations that needed further confirmation in RCTs.
Figure 1.
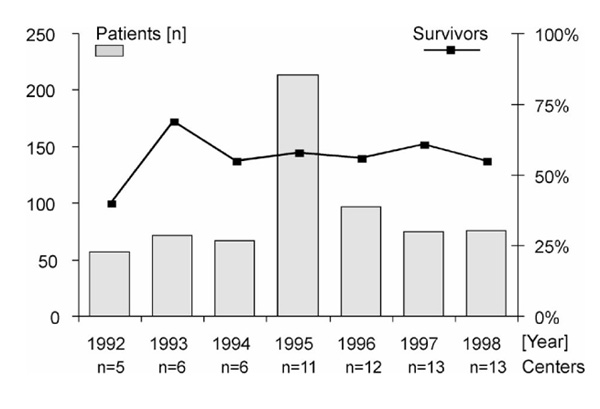
ARDS patients treated with ECMO in European centres from 1992 to 1998. Data were obtained by a yearly fax survey among the centres. In 1995 the number of centres participating in the survey had almost doubled, as did the number of ECMO therapies. Since 1996 the frequency of ECMO therapies in Europe has been decreasing. Survival rates in ECMO patients have remained constant at above 50% during the past 6 years.
These positive results from European centres were noted in the USA, and the refined ECMO technique was again taken into consideration for treatment of severe ARDS in adults. In 1994, Morris et al [31] reported the results of a RCT performed in a pulmonary intensive care unit of the Latter Day Saints Hospital in Salt Lake City, which tested the altered ECMO technique against advanced standard treatment.They compared pressure-controlled inverse ratio ventilation (pcCMV-IRV) followed by LFPPV-ECCO2-R with controlled positive-pressure ventilation in patients with ARDS.They found that survival was not significantly different between the groups (42% conventional therapy versus 33%), although overall survival rate had improved significantly when compared with the results from the US ECMO study of 1974 [28]. The authors recommended that ECMO should not be used for the treatment of adult ARDS.
ARDS treatment centres in Europe, however, believed that the potential of ECMO to improve the severely impaired gas exchange during the acute phase of the disease was an important factor contributing to the good survival rates in excess of 50% that were found in uncontrolled studies. Clinicians wondered why the RCT of Morris et al [31] did not result in better survival rates in the ECMO group. Several explanations have been forwarded. Habashi et al [32] pointed out that the ventilatory management in the ECMO group was not uniform and may have altered patient outcome, because the first half of the patient group had peak airway pressures controlled and the latter half had VTs controlled. Brunet et al [33] commented that the ventilatory management in the ECMO group did not significantly differ from that in the control group, in which peak inspiratory pressures that were higher than the maximum recommended levels [34] were employed. Furthermore, a considerable amount of criticism was directed at the ECMO technique used in the trial by Morris et al [31]. Most reviewers agree that the ECMO methodology was not optimized and did not achieve modern standards [32,33,35,36,37]. The untypically high blood loss complication rate associated with ECMO therapy [32,35,36,37] was considered to be an indicator of antiquated ECMO technology without heparin-coated equipment.
Bleeding due to complete anticoagulation has been reported as the major complication during extracorporeal respiratory support [38,39,40]. In 1983, Larm et al [41] developed a technique in which the heparin molecule is covalently attached to synthetic surfaces, which allowed heparinization of all surfaces of the extracorporeal gas exchange device that come into contact with blood. In 1987, Bindslev et al [42] reported the first long-term application of a surface-heparinized extracorporeal circuit in a 44-year-old woman with severe ARDS. Since that time, nearly all European ECMO centres have switched to the surface-heparinized extracorporeal circulation technique, with no or minimal systemic heparinization [10,43].
Terminology
Several terms have been used to describe the variety of techniques that have been designed to oxygenate blood and remove carbon dioxide extracorporally. When the term 'ECMO' was coined in the 1970s, it was used to refer to a high-flow venoarterial bypass system that was aimed primarily at blood oxygenation [28]. In the 1980s, when Kolobow et al [29] developed their low-flow venovenous bypass technique for extracorporeal gas exchange, the term ECCO2-R instead of ECMO came into use to underscore the importance of elimination of carbon dioxide in this method. For patients with chronic lung diseases, Marcolin et al [44] established a technique termed 'partial extracorporeal carbon dioxide removal' (PECOR). Its goal is to eliminate only part of the body's carbon dioxide load. In 1987, a Japanese working group introduced the term 'extracorporeal lung assist' (ECLA), to describe a venovenous low-flow bypass system that is used in patients who were not endotracheally intubated and mechanically ventilated [45]. In an effort to simplify and reduce terminology to a common denominator, Zwischenberger and Bartlett [46] proposed the term 'extracorporeal life support' (ECLS), to describe prolonged but temporary (1-30 days) support of heart or lung function using mechanical devices.
The typical bypass circuit currently employed in ARDS patients is a low-flow venovenous bypass circuit, but occasionally cardiac support is also an issue, and in such circumstances the intensivist will change to a venoarterial setting. This 'flexible' handling of the extracorporeal circuit incorporates the whole spectrum of advanced bypass techniques, so who decides when exactly PECOR ends and ECCO2-R begins, or when ECCO2-R changes to venovenous ECMO? In part, it was these problems in terminology that allowed the acronym ECMO to survive the changing technologies. What is more, ECMO has become a general byword for the wide range of methods that are in use for extracorporeal blood oxygenation and carbon dioxide removal. More important than the question of which acronym to use, however, should be the exact characterization of the applied extracorporeal circulation and pulmonary support system. Pesenti et al [37] suggested a minimum of three criteria: the vascular access used (venovenous, venoarterial or arteriovenous); the proportion of cardiac output pumped; and the ventilatory regimen of the natural lung.
Extracorporeal membrane oxygenation technique
In the typical low-flow venovenous bypass that is frequently used at present, as in our ECMO centre [10], venous blood is drained through two heparin-coated, 28-gauge, wire-reinforced catheters that are percutaneously inserted from both sides of the groin area into the inferior vena cava and then connected by a Y-piece.The oxygenated blood is returned into the superior vena cava through a 20-gauge, heparin-coated, wire-reinforced catheter, which is advanced percutaneously via the right internal jugular vein. A femoral-jugular venovenous bypass is established using a near-occlusive roller pump and a parallel configuration of two hollow fibre oxygenators. Figure 2 shows an ECMO circuit. All internal surfaces of the extracorporeal system, including the membrane oxygenators, are coated with covalently bound heparin. The system is primed with packed red blood cells and fresh frozen plasma at a ratio of 2:1. The gas phase of the oxygenator is initially flushed with dry pure oxygen. The gas flow rate is set to the required arterial carbon dioxide tension levels. Oxygenation is accomplished through the mechanically ventilated natural lung as well as by arterializing the circulating blood via the membrane oxygenator. The ECMO technique and the setup described above is just one possible approach; some groups use modifications of this circuit.
Figure 2.
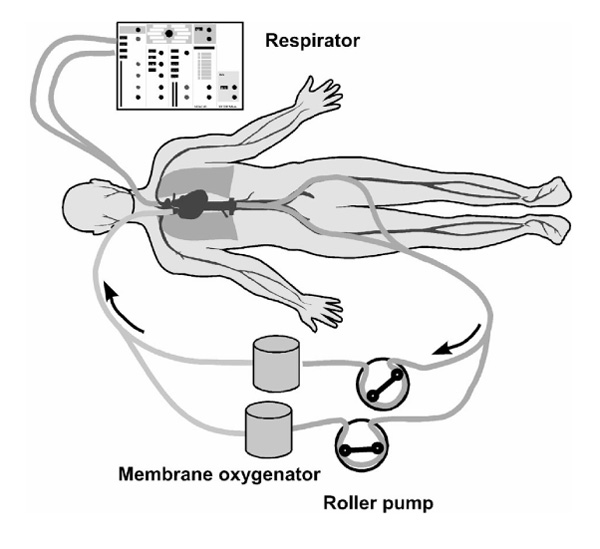
Schematic drawing of a low-flow venovenous ECMO circuit.
Complications associated with extracorporeal membrane oxygenation
Known hazards of the ECMO technique can be classified into mechanical and patient-medical complications. Mechanical complications include oxygenator failure, tubing/circuit disruption, pump or heat exchanger malfunction, and problems associated with cannula placement or removal. Patient-related medical problems are bleeding, neurological complications, additional organ failure (eg renal, cardiovascular, liver), barotrauma, infection and metabolic disorders.
Data regarding the frequency of complications associated with ECMO in adult ARDS patients are provided by the Extracorporeal Life Support Registry [47]. In 95 adult ARDS patients, a total of 68 mechanical and 302 patient-related complications occurred during bypass. Further information regarding technical complications experienced in extracorporeal respiratory support in adult ARDS patients is given by Gattinoni et al [48]. In 22 000 h of bypass, no life-threatening technical complications occurred. During 27 137 h of ECMO bypass, we registered a total of 27 technical complications, among which pump malfunction (n=6), tubing rupture (n=6), and cannula placement or removal problems (n=5) were the most frequent [10]. Available literature, however, suggests that bleeding remains the most frequent complication in adult patients with ARDS who are undergoing extracorporeal gas exchange, and seems partly related to constant intravenous heparinization [38,39,48,49,50,51,52,53]. In a prospective randomized controlled study, Knoch et al [54] suggested that the use of surface-heparinized ECMO circuits and membranes reduces the daily blood loss, the amount of substituted red cells and the necessary intravenous heparin dose. Survival rate was higher than that in patients treated with nonheparinized systems. Pesenti et al [37] also report a significantly reduced requirement of packed red blood cells, which they attributed to the use of a heparinized ECMO circuit.
Patient selection criteria
The criteria for treatment with ECMO in adults vary substantially among centres (Table1). They are usually modifications of the classic fast and slow entry criteria of the US ECMO study [28], and typically include an oxygenation criterion that is assessed at certain time points during the acute phase of the disease at a specific respirator setting, the compliance of the lung, and sometimes extravascular lung water and a pulmonary scoring system. More consensus exists regarding exclusion criteria. Widely accepted exclusion criteria are as follows: contraindication to anticoagulation, irreversible damage to the central nervous system, severe chronic pulmonary disease, extremely poor prognosis due to underlying disease (eg terminal cancer), immunosuppression, multiple organ failure and left ventricular failure. Currently, with increasing use of surface-heparinized ECMO equipment, contraindication to anti-coagluation as an exclusion criterion for ECMO requires reconsideration. Definite clinical testing of the ECMO technique requires a consensus on reasonable, standardized inclusion and exclusion criteria among centres.
Table 1.
Criteria for treatment with extracorporeal gas exchange used in different centres
| Center(s) [Reference] | Fast entry criteria | Slow entry criteria |
| Orange County Medical Center, Peter Bent Brigham Hospital, Hospital of the University of Pennsylvania, Pacific Medical Centre, Latter-day Saints Hospital, Mount Sinai Hospital NYC, University of North Carolina, University of California S.F., Massachusetts General Hospital, USA [92] | PaO2<50 mmHg for >2 h at FiO2 1.0; PEEP≥ 5 cmH20 | PaO2<50 mmHg for >12 h at FiO2 0.6; PEEP ≥5 cmH2O; maximal medical therapy >48 h |
| University of Milan, Italy [30] | Same as [92] | Same as [92] plus QS/QT>30%; CTstat<30 ml/cmH2O |
| Karolinska Hospital, Stockholm, Sweden [43] | Same as [92] | Same as [92] |
| Philipps University, Marburg, Germany [72] | A-aDO2>525 mmHg; CTstat<30 ml/cmH2O; PIP >35 cmH2O; extended infiltrations on chest X-ray; maximal medical therapy for >24 h (No distinction between fast and slow entry criteria) | |
| Charité/Campus Virchow, Humboldt-University Berlin Germany [10]; Heartlink ECMO-Centre, Leicester, UK | PaO2/FiO2<50 mmHg for >2 h; PEEP ≥10 cmH2O | Maximal medical therapy for 24-120 h; PaO2/FiO2<150 mmHg; PEEP ≥10 cmH20; QS/QT≥30% at FiO2 1.0; EVLW ≥15 ml/kg bodyweight; CTstat≤30 ml/cmH2O or recurrent barotrauma |
| Cochin University Hospital, Paris, France [39] | Same as [92] plus Murray score [93] >2.5; failure to improve respiratory parameters with different modes of mechanical ventilation | Same as [92] plus Murray score [93] >2.5; failure to improve respiratory parameters with different modes of mechanical ventilation |
| Freiburg University Hospital, Freiburg, Germany [94] | PaO2≤50 mmHg; FiO21.0; PEEP≥10 cmH2O | FiO2≥0.7; PEEP≥10 cmH2O; maximal medical therapy for >48 h |
| University of Michigan Medical School, Ann Arbor, USA [40] | Optimal conventional therapy; QS/QT>30%; CTstat<0.5 ml/cmH2O/kg bodyweight; diffusely abnormal chest radiography in four quadrants (No distinction between fast and slow entry criteria) | |
| University of Utah, School of Medicine, Salt Lake City, USA [31] | Same as [92] | Same as [92] |
| Ludwigs-Maximilians-University, Munich, Germany [95] | PaO2/FiO2<50 mmHg; at PEEP≥5 cmH2O for >2 h; CTstat≤30 ml/cmH2O | After 48-96 h conventional therapy, 3 out of 4 criteria must be fulfilled: PaO2/FiO2<150 mmHg at PEEP ≥5 cmH2O for >2 h; PaCO2 ≥60 mmHg at VE≥200 ml/kg; PIP ≥40 cmH CTstat≤30 ml/cmH2O and QS/QT≥30% |
| Toronto General Hospital, Toronto, Canada [96] | Patients with combined cardiorespiratory compromise that is life threatening; patients with predominantly respiratory failure that is progressive and with a level of oxygenation thought to be incompatible with life; patients placed on ECMO semielectively to provide support during a procedure (No distinction between fast and slow entry criteria) | |
| University of Vienna, Austria [14] | PaO2/FiO2<70 mmHg at PEEP >10 cmH2O for 96 h | |
A-aDO2, alveolar-arterial oxygen difference; CTstat, total thoracopulmonary compliance; EVLW, extravascular lung water; FiO2, fractional inspired oxygen; PaO2, arterial oxygen tension; PIP,peak inspiratory pressure; QS/QT, intrapulmonary right-to-left shunt; VE, minute ventilation.
Outcome from extracorporeal membrane oxygenation
ARDS was first described by Ashbaugh et al in 1967 [55]. They reported a 58% mortality rate in 12 patients presenting with the syndrome. During the following years, several new mechanical ventilation modes and therapeutic measures, as well as ECMO, were introduced into the therapy for ARDS. Gille and Bagniewski [56] collected data on the first 10 years of use of ECMO in the treatment of acute respiratory insufficiency. From 1966 to 1975, 233 ARDS patients were treated with ECMO by 90 medical teams in seven countries. The cumulative survival rate was 15%. No standardized entry and perfusion criteria were used, there were variations in utilization of cannulation modes, and different membrane oxygenators were employed. The results of the 1974 US ECMO trial [28] confirmed this disappointing survival rate; they showed a survival rate of only 10% in the ECMO group.
Since the 1980s, however, reports have been published that note quite acceptable survival rates of 40-50% in patients treated with the refined ECMO technique, the so called LFPPV-ECCO2-R method. Gattinoni et al [30] found a survival rate of 49% in 1986. Through July 1995, the Extracorporeal Life Support Organization Register in Ann Arbor, Michigan, USA recorded an overall survival rate of 41% in 197 adult ARDS patients treated with extracorporeal respiratory support [57]. In Europe, no such register exists, but since 1992 survival data of ECMO treated ARDS patients have been collected via an annual fax survey (Fig. 1). From 1992 to 1999, more than 850 patients with acute respiratory distress syndrome were treated with ECMO, and survival rates around 50% have been achieved. These survival rates, although coming from uncontrolled data collections, differ greatly from the 10% survival of the 1974 US ECMO trial [28].
The US ECMO trial was only the first of two RCTs that tested ECMO versus standard therapy. The US ECMO trial of 1974 [28] applied the original venoarterial ECMO technique, the second ECMO trial, which was performed from 1987 to 1991 by Morris et al [31], employed the LFPPV-ECCO2-R technique. Although both RCTs did not find significant differences in the survival rates between ECMO and conventionally treated patients, the overall survival rate had improved significantly when comparing the results obtained in 1974 (survival rates of 10 and 8%, respectively) with those from 1994 (survival rates of 33 and 42%, respectively). Improvement in survival rates of conventionally treated ARDS patients was also observed by one US centre [12] and two European centres [13,15]. The studies used different methodologies and reported that survival had improved considerably in recent years compared with historical controls, taking into account severity of illness and adjusting for other variables.
In response to these findings, several editorials and reviews [58,59,60] have expressed an optimistic view toward improvement in survival rates in ARDS. Schuster [61] reviewed 14 major ARDS studies regarding outcome and concluded that mortality rates are decreasing. Krafft et al [62], however, after evaluating 101 peer-reviewed studies published between 1967 and 1994, came to the conclusion that no changes in outcome can be detected. The most recent data on survival in ARDS were offered by Zilberberg and Epstein [63] and Monchi et al [64]; they reported survival rates in ARDS patients of 42 and 35%, respectively. Luhr et al [1] reported the first large multicentre trial, however, in which a survival rate of as high as 59% was assessed.
Figure 3 presents the trends in reported survival rates of ARDS since 1966 in two treatment groups (conventionally and ECMO-treated patients). The reported survival rates in conventionally and ECMO treated ARDS patients appear to have improved and converged over time. My personal belief is that survival rates in conventionally treated ARDS patients and in ECMO-treated ARDS patients have improved since 1967. However, the problem in evaluating potential improvements in survival rates of ARDS derives from the long-standing heterogeneity and lack of definitions for the underlying disease processes, the lack of a uniform definition for ARDS, the various therapies applied, and indistinct definitions of study populations [65].
Figure 3.
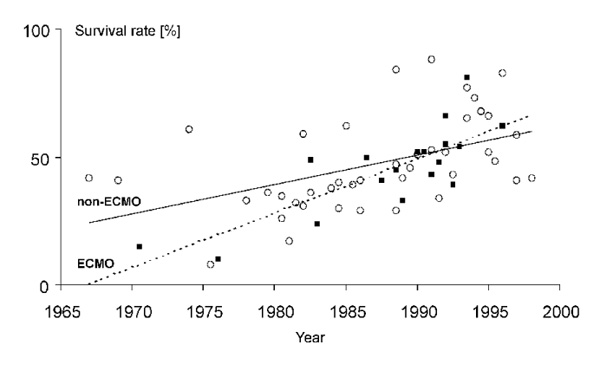
Trends in reported survival rates in conventionally (non-ECMO) and ECMO-treated ARDS patients, including data from 51 clinical studies. Reported survival rates in (clear circle) conventionally treated and (filled square) ECMO-treated ARDS patients. The survival rate reported in each study was assigned to the year representing the median of study period or, if the study period was not stated, to the year of report. The data support the view that survival rates in both treatment groups followed a positive trend. Data have been taken from [1,5,8,9,10,12,13,14,15,30,31,39,43,55, 56,63,64,91,92,95,96,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130].
Long-term follow up of survivors from ARDS who were treated with ECMO
Given the catastrophic degree of respiratory failure, the acute pathological findings (including interstitial fibrosis) [66], the high mortality and the high costs of treating these patients US$120 800 (UK #75 500) per patient for therapy with ECMO, US$97 200 (UK #60 750) per patient for conventional treatment [31], what is the long-term recovery of those who do survive? Regarding conventionally treated patients, most authors agree that the very poor short-term prognosis of ARDS is outweighed by the chance of nearly complete recovery, although different patterns and grades of respiratory dysfunction were found. The most frequently noted disorder is a reduction in the diffusing capacity of carbon monoxide, but in other articles remaining pathological lung volumes, expiratory flow rates and gas exchange impairments are documented [67]. All in all, the results of quality of life investigations appeared satisfactory [68,69,70], until Hopkins et al [71] very recently reported results on cognitive and psychological outcomes in ARDS survivors. They studied 55 consecutive patients and found that, at hospital discharge, 100% exhibited cognitive and affective impairments, as well as problems with health status that affected their quality of life. One year after ARDS, 30% of the patients still showed generalized cognitive decline.
What degree of recovery can be expected in ARDS patients treated with ECMO? One could assume that ARDS patients treated with ECMO have suffered from severe lung injury, and therefore that only incomplete recovery might be likely. On the other hand, patients treated with ECMO may be protected from the damaging effects of mechanical ventilation.
Only few authors have investigated larger populations of ARDS patients who had been treated with extracorporeal respiratory support. Knoch et al [72] investigated lung function and recovery in 38 ARDS survivors whose pulmonary gas exchange had been supported by ECMO. Within the third and sixth month after hospital discharge, the forced expiratory volume in 1 s and arterial oxygen tension levels reached the lower range of normal values. The ratio of residual volume:total lung capacity (a measure of relative lung emphysema) was 167% of normal at the time of discharge from the hospital, and had decreased to 114% of normal after a further 3 months. This was found to reverse during the following months. All patients had an abnormal diffusion capacity with normal transfer coefficients for carbon monoxide 12-20 months after hospital discharge. Impaired diffusion capacity and the reduced forced expiratory flow between 25 and 75% of expiration (62.5 ± 25.0% of predicted at 12-20 months after hospital discharge) indicated residual changes in the small airways. At that time, chest radiography and computed tomography scans of the lungs revealed various minor morphological residuals only. Cardiopulmonary function during spiroergometry showed values normal for untrained individuals. Within 12-20 months after discharge, 36 out of the 38 patients had returned to normal working and social activities.
Stoll et al [73] investigated the health-related quality of life using the Medical Outcomes Study 36-Item Short-Form Health Survey [74] in 14 patients who survived ARDS and had been treated with ECMO. Long-term survivors of ECMO therapy reported significant reductions in physical functioning when compared with other mechanically ventilated ARDS patients or healthy control individuals, and showed a higher incidence of chronic physical pain. There were no differences with regard to the mental health dimensions of the 36-Item Short-Form Health Survey between ECMO patients and control individuals. Of the ECMO group 64.3%, but all patients treated conventionally had full-time employment.
Extracorporeal membrane oxygenation in neonates and children
ECMO technology has been developed and improved during the past 3 decades. In its infancy, in the 1970s, ECMO was most commonly used to support mature newborn infants with acute respiratory failure. In neonates, quite promising survival rates of 56% have been observed since ECMO was introduced into treatment [75], whereas in adults comparable good survival rates were lacking [28]. Bartlett et al [76] attributed the success of ECMO in newborns to the fact that in neonatal respiratory failure the lungs require only a short time for recovery, and extracorporeal techniques in the 1970s could be carried out safely for a few days. In the 1980s, the technology was adapted to paediatric and, later on, in the 1990s, to adult acute respiratory failure and cardiac failure.
In the neonate, persistent pulmonary hypertension (PPHN) is the most frequent cause of acute respiratory failure. The aetiology of PPHN can either be idiopathic or a secondary consequence of a variety of diseases that include, but are not limited to, sepsis, pneumonia, meconium aspiration, diaphragmatic hernia and hyaline membrane disease. PPHN is pathophysiologically characterized by the distinct pulmonary hypertension, pronounced hypoxia and the extrapulmonary monary right-to-left shunt across the patent ductus arteriosus and the foramen ovale. The course of the disease may be complicated by the effects of direct lung damage, leading to additional intrapulmonary right-to-left shunt.
Conventional treatment includes mechanical ventilation with high inspired fractional oxygen, hyperventilation, intravenous bicarbonate and vasodilators, as well as instillation of surfactant. Advanced concepts involve replacing simple intermittent positive pressure ventilation by high-frequency oscillatory ventilation, and adding inhalation of nitric oxide (NO) to the therapeutic armamentarium [77].Additionally, ECMO has been used to relieve hypoxaemia and had become standard practice in many neonatal centres by 1988 [78]. The registry of the Extracorporeal Life Support Organization [79] reported an 80% survival rate in 10 391 patients with neonatal respiratory failure who were treated with ECMO from 1980 to 1995. Bartlett et al [76] recently reviewed the worldwide experience with ECMO, and pointed out that ECMO for severe respiratory failure in neonates has been evaluated in four prospective randomized trials [80,81,82,83] and one uncontrolled study [84].The survival rates reported in those trials are presented in Table2. All neonatal studies showed that survival rates after ECMO were much better than those achieved with conventional therapy.
Table 2.
Survival rates of neonatal ECMO
| Control group | ECMO group | |||||||
| Reference | Year | Study design | Patients total (n) | n | survival rate (%) | n | survival rate (%) | P |
| [80] | 1985 | RCT 'play the winner'* | 12 | 1 | 0 | 11 | 100 | Not reported |
| [82] | 1989 | RCT (phase 1+2) | 39 | 10 | 60 | 29 | 97 | <0.05 |
| [81] | 1993 | RCT | 41 | 19 | 89 | 22 | 91 | NS |
| [83] | 1996 | RCT | 185 | 92 | 41 | 93 | 68 | <0.001 |
| [84] | 1999 | Uncontrolled study | 100 | - | - | 100 | 82 | - |
*Bartlett et al [80] used a special randomized play-the-winner statistical method; the chance of randomly assigning an infant to one treatment or the other is influenced by the outcome of treatment of each patient in the study. If one treatment is more successful, more patients are randomly assigned to that treatment.
In the 1980s, the benefit of ECMO for paediatric patients was subject to discussion [85,86]. Concerns mainly concentrated on the widely unknown mechanisms of pulmonary injury in this patient group and the new, expensive and potentially dangerous technology. Performance of a RCT was considered mandatory before ECMO gained further acceptance in children. Due to ethical considerations, however, this approach was postponed. Instead, the Extracorporeal Life Support Organization registry was founded in 1989, and documented a cumulative survival rate of 53% in 982 cases of paediatric respiratory failure from 1990 to 1995 [79]. In these patients, respiratory failure was caused by various direct and indirect lung injuries such as pneumonia, aspiration, ARDS and others. In 1996, Green et al [87] presented the results of a retrospective multicenter cohort analysis in 331 paediatric patients, evaluating factors associated with survival with a multivariate logistic regression analysis. The authors found ECMO to be associated with a reduction in mortality. In an additional matched-pairs analysis, they found that 74% in the ECMO group (n=29) survived, and 53% in the non-ECMO group (n=53; P<0.01).
The introduction of inhaled NO into the therapy of PPHN has had a fundamental impact on both the therapeutic approach and the mortality rates of neonates. The results of at least four RCTs have been published to date, which all demonstrated a decrease in the frequency of use of ECMO. Details of these studies are given in Table3. The data from the Extracorporeal Life Support Organization [79] also show a decrease in the frequency of use of ECMO beginning in 1993, which, according to the authors (of the registry) may be attributed to the introduction of new treatment options such as high-frequency oscillatory ventilation, inhaled NO, or surfactant into clinical practice. The greatest decrease in case volume coincided with the use of exogenous surfactant, beginning in 1990.
Table 3.
Reduction of ECMO frequency in newborns in four randomized controlled studies
Although the reported survival rates in neonates and children appear to be impressive, ECMO therapy in these patients is subject to specific complications. Major problems that need to be tackled in the near future include neurological deficits that probably arise from cannulation techniques, bleeding and technical complications during running bypass, and hearing loss that possibly results from medication during hospitalization (furosemide, antibiotics). The total incidence of neurological deficits associated with ECMO is 10% for neonates and 2% for children at the time of hospital discharge [76].
Impact of new treatment strategies on the requirement for ECMO
Recent studies suggest that mechanical ventilation with too low or too high levels of PEEP, high airway pressures and large VTs may further damage the injured lung and cause ventilator-induced lung injury [16]. Lung-protective mechanical ventilation attempts to shield the injured lung by applying only small VT [88] and using adjunctive measures such as prone positioning [89], inhalation of NO [11] and early spontaneous breathing [90].
Small VT ventilation was recently compared with large VT ventilation in a RCT by the National Heart Lung and Blood Institute organized ARDS network [88]. The effects of reduced VTs of 6 ml/kg body weight versus large VTs of 12 ml/kg body weight were evaluated with regard to their effects on mortality and other outcome parameters. After inclusion of 841 intubated and mechanically ventilated patients with acute lung injury or ARDS, the trial was stopped because the mortality difference crossed a predetermined threshold. Estimated mortality at hospital discharge was 30.4% in the low VT group and 39.8% in the high VT group (P=0.0054). This trial showed that a low V T ventilator strategy was associated with improved survival in acute lung injury/ARDS patients. The results of other recently conducted studies [9,91] also suggest that avoidance of ventilator-induced lung injury may be associated with increased survival rates.
A reduction in mortality rates in ARDS patients after treatment with inhaled NO or prone positioning has not been documented; however, significant improvements in distinct physiological parameters, such as arterial oxygen tension/fractional inspired oxygen, pulmonary hypertension and intrapulmonary right-to-left shunt, were recorded during the acute phase of the disease [11,89]. The new therapies might therefore interfere with the ECMO entry criteria, which are, in most centres, defined by gas exchange and haemodynamic parameters (Table1). Consequently, ECMO is not considered as an additional measure until the other therapeutic options are exhausted. This approach may delay or exclude the decision for extracorporeal support [36]. In conclusion, the introduction of new therapies and improvements in conventional ARDS therapy have possibly changed the indications for ECMO to include only the most severe cases of ARDS.
Recent data from our ARDS centre support this idea because we have been observing a decreasing frequency of ECMO treatment in adult ARDS patients within the past few years (Fig. 4). Researchers are encouraged to investigate this observation, because no studies in adults have been performed to identify the cause of this phenomenon definitively.
Figure 4.
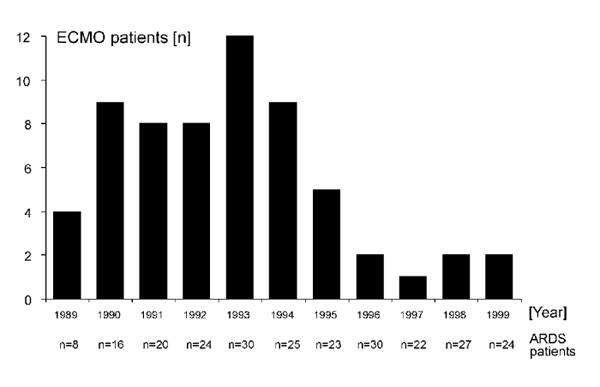
ARDS patients treated with ECMO at the Charité, Virchow Clinic, Berlin. Since 1995/1996 the frequency of ECMO treatment in ARDS patients has been decreasing, although the number of ARDS patients admitted to the centre has remained almost constant.
Conclusion
ARDS is a rare disease; only 1-14 adults in 100 000 develop the syndrome each year. The severely impaired gas exchange that is the main and immediate life-threatening characteristic of ARDS can be improved in most patients by a lung protective therapeutic approach using mechanical ventilation with PEEP and permissive hypercapnia, positional manoeuvres, a cautious fluid regimen and inhalational pulmonary vasodilators. In a few patients the combined application of all these measures fails to improve the gas exchange, however, and continuous severe hypoxia, if not prevented, may cause irreparable organ damage or death. ECMO can symptomatically prevent severe hypoxia and remove carbon dioxide until the patient's lungs are able to resume their gas exchange function. ECMO, in its principal features, has been at our disposal since the 1970s, but almost a quarter of a century was needed before the technology and indications had undergone the refinement that guarantees successful and safe treatment of severe hypoxia in adult ARDS. Today we know that the venovenous bypass techniques are, in most cases, safer and more promising than the arteriovenous methods. We now have modern heparinized tubings and membrane oxygenators at hand, which help us to avoid bleeding complications. European working groups collected data from more than 850 adult ARDS patients treated with ECMO and observed a survival rate of greater than 50% (Fig. 1). These quite promising data are uncontrolled, however. Two RCTs published in 1979 [28] and 1994 [31] failed to show an advantage of ECMO over conventional treatment in adults. ECMO technique has undergone some improvement since these trials, but the question of whether the improved ECMO can really challenge conventional treatment of adult ARDS will need to be answered by a future RCT, which has already been scheduled [76].
In my opinion the expenditure on money, time and personal energy when treating a patient with ECMO is worth the effort, because the majority of the survivors will achieve a satisfying general health status, lung function and quality of life 6-12 months after the acute phase of the disease, and most of them can return to their former social and working lives.
References
- Luhr OR, Antonsen K, Karlsson M, et al. Incidence and mortality after acute respiratory failure and acute respiratory distress syndrome in Sweden, Denmark, and Iceland. Am J Respir Crit Care Med. 1999;159:1849–1861. doi: 10.1164/ajrccm.159.6.9808136. [DOI] [PubMed] [Google Scholar]
- Lewandowski K, Metz J, Deutschmann C, et al. Incidence, severity, and mortality of acute respiratory failure in Berlin, Germany. Am J Respir Crit Care Med. 1995;151:1121–1125. doi: 10.1164/ajrccm.151.4.7697241. [DOI] [PubMed] [Google Scholar]
- Kanazawa M. Acute lung injury: clinical concept and experimental approaches to pathogenesis. Keio J Med. 1996;45:131–139. doi: 10.2302/kjm.45.131. [DOI] [PubMed] [Google Scholar]
- Luce JM. Acute lung injury and the acute respiratory distress syndrome. Crit Care Med. 1998;26:369–376. doi: 10.1097/00003246-199802000-00043. [DOI] [PubMed] [Google Scholar]
- Webster NR, Cohen AT, Nunn JF. Adult respiratory distress syndrome: how many cases in the UK? Anaesthesia. 1988;43:923–926. doi: 10.1111/j.1365-2044.1988.tb05652.x. [DOI] [PubMed] [Google Scholar]
- Villar J, Slutsky AS. The incidence of the adult respiratory distress syndrome. Am Rev Respir Dis. 1989;140:814–816. doi: 10.1164/ajrccm/140.3.814. [DOI] [PubMed] [Google Scholar]
- Thomsen GE, Morris AH. Incidence of the adult respiratory distress syndrome in the State of Utah. Am J Respir Crit Care Med. 1995;152:965–971. doi: 10.1164/ajrccm.152.3.7663811. [DOI] [PubMed] [Google Scholar]
- Nolan S, Burgess K, Hopper L, Braude S. Acute respiratory distress syndrome in a community hospital ICU. Intens Care Med. 1997;23:530–538. doi: 10.1007/s001340050369. [DOI] [PubMed] [Google Scholar]
- Amato MBP, Barbas CSV, Medeiros DM, et al. Effect of a protective-ventilation strategy on mortality in the acute respiratory distress syndrome. N Engl J Med. 1998;338:347–354. doi: 10.1056/NEJM199802053380602. [DOI] [PubMed] [Google Scholar]
- Lewandowski K, Rossaint R, Pappert D, et al. High survival rate in 122 ARDS patients managed according to a clinical algorithm including extracorporeal membrane oxygenation. Intens Care Med. 1997;23:819–835. doi: 10.1007/s001340050418. [DOI] [PubMed] [Google Scholar]
- Rossaint R, Falke KJ, Lopez F, et al. Inhaled nitric oxide for the adult respiratory distress syndrome. N Engl J Med. 1993;328:399–405. doi: 10.1056/NEJM199302113280605. [DOI] [PubMed] [Google Scholar]
- Milberg JA, Davis DR, Steinberg KP, Hudson LD. Improved survival of patients with acute respiratory distress syndrome (ARDS): 1983-1993. JAMA. 1995;273:306–309. [PubMed] [Google Scholar]
- Abel SJC, Finney SJ, Brett SJ, et al. Reduced mortality in association with the acute respiratory distress syndrome (ARDS). Thorax. 1998;53:292–294. doi: 10.1136/thx.53.4.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich R, Lorber C, R??der G, et al. Controlled airway pressure therapy, nitric oxide inhalation, prone position, and extracorporeal membrane oxygenation (ECMO) as components of an integrated approach to ARDS. Anesthesiology. 1999;91:1577–1586. doi: 10.1097/00000542-199912000-00007. [DOI] [PubMed] [Google Scholar]
- Jardin F, Fellahi J-L, Beauchet A, et al. Improved prognosis of acute respiratory distress syndrome 15 years on. Intens Care Med. 1999;25:936–941. doi: 10.1007/s001340050985. [DOI] [PubMed] [Google Scholar]
- Dreyfuss D, Saumon G. Ventilator-induced lung injury: lessons from experimental studies. Am J Respir Crit Care Med. 1998;157:294–323. doi: 10.1164/ajrccm.157.1.9604014. [DOI] [PubMed] [Google Scholar]
- Dreyfuss D, Soler P, Basset G, Saumon G. High inflation pressure pulmonary edema. Respective effects of high airway pressure, high tidal volume, and positive end-expiratory pressure. Am J Respir Crit Care Med. 1988;137:1159–1164. doi: 10.1164/ajrccm/137.5.1159. [DOI] [PubMed] [Google Scholar]
- Webb HH, Tierney DF. Experimental pulmonary edema due to intermittent positive pressure ventilation with high inflation pressures. Protection by positive end-expiratory pressure. Am Rev Respir Dis. 1974;110:556–565. doi: 10.1164/arrd.1974.110.5.556. [DOI] [PubMed] [Google Scholar]
- Tremblay L, Valenza F, Ribeiro SP, Li J, Slutsky AS. Injurious ventilatory strategies increase cytokines and c-fos m-RNA expression in an isolated rat lung model. J Clin Invest. 1997;99:944–952. doi: 10.1172/JCI119259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranieri VM, Suter PM, Tortorella C, et al. Effect of mechanical ventilation on inflammatory mediators in patients with acute respiratory distress syndrome. A randomized controlled trial. JAMA. 1999;282:54–61. doi: 10.1001/jama.282.1.54. [DOI] [PubMed] [Google Scholar]
- Von Frey M, Gruber M. Studies on metabolism of isolated organs. A respiration-apparatus for isolated organs [in German] Virchows. Arch Physiol. 1885. pp. 519–532.
- Gibbon JH. Artificial maintenance of circulation during experimental occlusion of pulmonary artery. Arch Surg. 1937;34:1105–1131. [Google Scholar]
- Lee LH, Krumhaar D, Fonkolsrud EW, Schjeide OA, Maloney JV. Denaturation of plasma proteins as a cause of morbidity and death after intracardiac operations. Surgery. 1961;50:29–37. [PubMed] [Google Scholar]
- Clowes GH, Hopkins AL, Neville WE. An artificial lung dependent upon diffusion of oxygen and carbon dioxide through plastic membranes. J Thorac Cardiovasc Surg. 1956;32:630–637. [PubMed] [Google Scholar]
- Kolobow T, Bowman RL. Construction and evaluation of an alveolar membrane artificial heart-lung. Trans Am Soc Artif Intern Organs. 1963;9:238–243. [PubMed] [Google Scholar]
- Hill JD, O'Brien TG, Murray JJ, et al. Prolonged extracorporeal oxygenation for acute post-traumatic respiratory failure (shock-lung syndrome). N Engl J Med. 1972;286:629–634. doi: 10.1056/NEJM197203232861204. [DOI] [PubMed] [Google Scholar]
- Bartlett RH, Gazzaniga AB, Jefferies MR, et al. Extracorporeal membrane oxygenation (ECMO) cardiopulmonary support in infancy. Trans Am Soc Artif Intern Organs. 1976;22:80–93. [PubMed] [Google Scholar]
- Zapol WM, Snider MT, Hill JD, et al. Extracorporeal membrane oxygenation in severe acute respiratory failure. A randomized prospective study. JAMA. 1979;242:2193–2196. doi: 10.1001/jama.242.20.2193. [DOI] [PubMed] [Google Scholar]
- Kolobow T, Gattinoni L, Tomlinson T, Pierce JE. Control of artificial breathing using an extracorporeal membrane lung. Anesthesiology. 1977;46:138–141. doi: 10.1097/00000542-197702000-00012. [DOI] [PubMed] [Google Scholar]
- Gattinoni L, Pesenti A, Mascheroni D, et al. Low-frequency positive pressure ventilation with extracorporeal CO2-removal in severe acute respiratory failure. JAMA. 1986;256:881–886. [PubMed] [Google Scholar]
- Morris AH, Wallace CJ, Menlove RL, et al. Randomized clinical trial of pressure-controlled inverse ratio ventilation and extracorporeal CO2 removal for adult respiratory distress syndrome. Am J Respir Crit Care Med. 1994;149:295–305. doi: 10.1164/ajrccm.149.2.8306022. [DOI] [PubMed] [Google Scholar]
- Habashi NM, Reynolds HN, Borg U, Cowley RA. Randomized clinical trial of pressure-controlled inverse ratio ventilation and extra corporeal CO2 removal for ARDS. Correspondence to the editor. Am J Respir Crit Care Med. 1995;151:255–256. doi: 10.1164/ajrccm.151.1.7812567. [DOI] [PubMed] [Google Scholar]
- Brunet F, Mira J-P, Dhainaut JF, Dallava-Santussi J. Efficacy of low-frequency positive pressure ventilation-extracorporeal CO2-removal. Correspondence to the editor. Am J Respir Crit Care Med. 1995;151:1269–1270. doi: 10.1164/ajrccm/151.4.1269-a. [DOI] [PubMed] [Google Scholar]
- Marini J, Kelsen SG. Re-targeting ventilatory objectives in adult respiratory distress syndrome. Am Rev Respir Dis. 1992;146:2–3. doi: 10.1164/ajrccm/146.1.2. [DOI] [PubMed] [Google Scholar]
- Falke K. Randomized clinical trial of pressure-controlled inverse ratio ventilation and extracorporeal CO2 removal for adult respiratory distress syndrome. Correspondence to the editor. Am J Respir Crit Care Med. 1997;156:1016–1017. [PubMed] [Google Scholar]
- Pesenti A, Fumagalli R. Extracorporeal respiratory support. Update in Intensive Care and Emergency Medicine 30. Acute Lung Injury. Edited by Marini JJ, Evans TW. Berlin, Heidelberg, New York: Springer. 1997. pp. 319–328.
- Pesenti A, Bombino M, Gattinoni L. Extracorporeal support of gas exchange. Physiological Basis of Ventilatory Support. Edited by Marini JJ, Slutsky AS. New York: Marcel Dekker. 1998. pp. 997–1020.
- Uziel L, Cugno M, Fabrizi I, et al. Physiopathology and management of coagulation during long term extracorporeal respiratory assistance. Int J Artif Organs. 1990;13:280–287. [PubMed] [Google Scholar]
- Brunet F, Belghith M, Mira J-P, et al. Extracorporeal carbon dioxide removal and low-frequency positive-pressure ventilation. Improvement in arterial oxygenation with reduction of risk of pulmonary barotrauma in patients with adult respiratory distress syndrome. Chest. 1993;104:889–898. doi: 10.1378/chest.104.3.889. [DOI] [PubMed] [Google Scholar]
- Anderson H, Steimle C, Shapiro M, et al. Extracorporeal life support for adult cardiorespiratory failure. Surgery. 1993;114:161–173. [PubMed] [Google Scholar]
- Larm O, Larsson R, Olsson P. A new thrombogenic surface prepared by selective covalent binding of heparin via a modified reducing terminal residue. Biomater Med Device Artif Organs. 1983;2:161–173. doi: 10.3109/10731198309118804. [DOI] [PubMed] [Google Scholar]
- Bindslev L, Eklund J, Norlander O, et al. Treatment of acute respiratory failure by extracorporeal carbon dioxide elimination performed with a surface heparinized artificial lung. Anesthesiology. 1987;67:117–120. doi: 10.1097/00000542-198707000-00024. [DOI] [PubMed] [Google Scholar]
- Bindslev L, B??hm C, Jolin A, et al. Extracorporeal carbon dioxide removal performed with surface-heparinized equipment in patients with ARDS. Acta Anaesthesiol Scand. 1991;35:125–131. doi: 10.1111/j.1399-6576.1991.tb03409.x. [DOI] [PubMed] [Google Scholar]
- Marcolin R, Mascheroni D, Pesenti A, Bombino M, Gattinoni L. Ventilatory impact of partial extracorporeal CO2 removal (PECOR) in ARF patients. Trans Am Soc Artif Intern Organs. 1986;32:508–510. doi: 10.1097/00002480-198609000-00025. [DOI] [PubMed] [Google Scholar]
- Terasaki H, Nogami T, Saito Y, Otsu T, Morioka T. Extracorporeal lung assist without endotracheal intubation and mechanical ventilation [letter]. Crit Care Med. 1987;15:84–85. doi: 10.1097/00003246-198701000-00019. [DOI] [PubMed] [Google Scholar]
- Zwischenberger JB, Bartlett RH. An introduction to extracorporeal life support. In ECMO Extracorporeal Cardiopulmonary Support in Critical Care Edited by Zwischenberger JB, Bartlett RH Ann Arbor, Michigan: Extracorporeal Life Support Organisation. 1995. pp. 11–13.
- Extracorporeal Life Support Registry. Ann Arbor, Michigan: Extracorporeal Life Support Organization. 1993 [Google Scholar]
- Gattinoni L, Pesenti A, Bombino M, Pelosi P, Brazzi L. Role of extracorporeal circulation in adult respiratory distress syndrome mangement. New Horizons. 1993;1:603–612. [PubMed] [Google Scholar]
- Wetterberg T, Steen S. Total extracorporeal lung assist: a new clinical approach. Intens Care Med. 1991;17:73–77. doi: 10.1007/BF01691426. [DOI] [PubMed] [Google Scholar]
- Fjalldal O, Torfason B, ??nundarson PT, et al. Prolonged total extracorporeal lung assistance without systemic heparinization. Acta Anaesthesiol Scand. 1993;37:115–120. doi: 10.1111/j.1399-6576.1993.tb03611.x. [DOI] [PubMed] [Google Scholar]
- Brunet F, Mira JP, Belghith M, et al. Effects of aprotinin on hemorrhagic complications in ARDS patients during prolonged extracorporeal CO2 removal. Intensive Care Med. 1992;18:364–367. doi: 10.1007/BF01694366. [DOI] [PubMed] [Google Scholar]
- McCoy-Pardington D, Judd WJ, Knafl P, et al. Blood use during extracorporeal membrane oxygenation. Transfusion. 1990;30:307–309. doi: 10.1046/j.1537-2995.1990.30490273436.x. [DOI] [PubMed] [Google Scholar]
- Gattinoni L, Pesenti A, Marcolin R, et al. Extracorporeal support in acute respiratory failure. Adult Respiratory Distress Syndrome. Edited by Artigas A, Lemaire F, Suter PM, Zapol WM. Edinburgh: Churchill Livingstone. 1992:469–475. [Google Scholar]
- Knoch M, Kollen B, Dietrich G, et al. Progress in veno-venous long-term bypass techniques for the treatment of ARDS. Controlled clinical trial with the heparin-coated bypass circuit. Int J Artif Organs. 1992;15:103–108. [PubMed] [Google Scholar]
- Ashbaugh DG, Bigelow DB, Petty TL, Levine BE. Acute respiratory distress in adults. Lancet. 1967;2:319–323. [Google Scholar]
- Gille JP, Bagniewski AM. Ten years of use of extracorporeal membrane oxygenation (ECMO) in the treatment of acute respiratory insufficiency (ARI). Trans Am Soc Artif Intern Organs. 1976;22:102–108. [PubMed] [Google Scholar]
- Extracorporeal Life Support Registry. Ann Arbor, Michigan: Extracorporeal Life Support Organization. 1995.
- Lemaire F. The prognosis of ARDS: appropriate optimism? Intens Care Med. 1996;2:371–373. doi: 10.1007/BF01712150. [DOI] [PubMed] [Google Scholar]
- Steltzer H, Krafft P. Prognosis of ARDS patients: light at the end of the tunnel? Intens Care Med. 1997;23:803–805. doi: 10.1007/s001340050414. [DOI] [PubMed] [Google Scholar]
- Schuster DP. The changing prognosis of ARDS. Schweiz Med Wochenschr. 1997;127:1018–1022. [PubMed] [Google Scholar]
- Schuster DP. What is acute lung injury? What is ARDS? Chest. 1995;107:1721–1726. doi: 10.1378/chest.107.6.1721. [DOI] [PubMed] [Google Scholar]
- Krafft P, Fridrich P, Pernerstorfer T, et al. The acute respiratory distress syndrome: definitions, severity and clinical outcome: an analysis of 101 clinical investigations. Intens Care Med. 1996;22:519–529. doi: 10.1007/BF01708091. [DOI] [PubMed] [Google Scholar]
- Zilberberg MD, Epstein SK. Acute lung injury in the medical ICU. Comorbid conditions, age, etiology, and hospital outcome. Am J Respir Crit Care Med. 1998;157:1159–1164. doi: 10.1164/ajrccm.157.4.9704088. [DOI] [PubMed] [Google Scholar]
- Monchi M, Bellenfant F, Cariou A, et al. Early predictive factors of survival in the acute respiratory distress syndrome. A multivariate analysis. Am J Respir Crit Care Med. 1998;158:1076–1081. doi: 10.1164/ajrccm.158.4.9802009. [DOI] [PubMed] [Google Scholar]
- Bernard GR, Artigas A, Brigham KL, et al. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994;149:818–824. doi: 10.1164/ajrccm.149.3.7509706. [DOI] [PubMed] [Google Scholar]
- Suchyta MR, Elliott CG, Colby T, et al. Open lung biopsy does not correlate with pulmonary function after the adult respiratory distress syndrome. Chest. 1991;99:1232–1237. doi: 10.1378/chest.99.5.1232. [DOI] [PubMed] [Google Scholar]
- Lewandowski K, Lewandowski M, Pappert D, Falke KJ. Outcome and follow up of adults following extracorporeal life support. In ECMO: Extracorporeal Cardiopulmonary Support in Critical Care Edited by Zwischenberger JB, Bartlett RH Ann Arbor, Michigan: Extracorporeal Life Support Organization. 1995. pp. 415–444.
- McHugh LG, Milberg JA, Whitcomb ME, et al. Recovery of function in survivors of the acute respiratory distress syndrome. Am J Respir Crit Care Med. 1994;150:90–94. doi: 10.1164/ajrccm.150.1.8025779. [DOI] [PubMed] [Google Scholar]
- Musthafa AA, Angus AC, Linde-Zwirble WT, et al. The first year after ARDS: results of a multi-center study [abstract]. Crit Care Med. 1999;27:A37. [Google Scholar]
- Schelling G, Stoll C, Haller M, et al. Health-related quality of life and posttraumatic stress disorder in survivors of the acute respiratory distress syndrome. Crit Care Med. 1998;26:651–659. doi: 10.1097/00003246-199804000-00011. [DOI] [PubMed] [Google Scholar]
- Hopkins RO, Weaver LK, Pope D, et al. Neuropsychological sequelae and impaired health status in survivors of severe acute respiratory distress syndrome. Am J Respir Crit Care Med. 1999;160:50–56. doi: 10.1164/ajrccm.160.1.9708059. [DOI] [PubMed] [Google Scholar]
- Knoch M, Kukule I, M??ller E, H??ltermann W. Lung function one year after extracorporeal lung assist (ELA). Long-term follow-up of patients with most severe adult respiratory distress syndrome. [in German]. An??sthesiol Intensivmed Notfallmed Schmerzther. 1992;27:477–482. doi: 10.1055/s-2007-1000342. [DOI] [PubMed] [Google Scholar]
- Stoll C, Haller M, Briegel J, et al. Health-related quality of life. Long-term survival in patients with ARDS following extracorporeal membrane oxygenation (ECMO). Anaesthesist. 1998;47:24–29. doi: 10.1007/s001010050518. [DOI] [PubMed] [Google Scholar]
- Ware JE, Sherbourne CD. The MOS-36-Item Short-Form Health Survey (SF-36): I. Conceptional framework and item selection. Med Care. 1992;30:473–484. [PubMed] [Google Scholar]
- Bartlett RH, Andrews AF, Toomasian JM, Haiduc NJ, Gazzaniga AB. Extracorporeal membrane oxygenation (ECMO) for newborn respiratory failure: forty-five cases. Surgery. 1982;92:425–433. [PubMed] [Google Scholar]
- Bartlett RH, Roloff DW, Custer JR, Younger JG, Hirschl RB. Extracorporeal life support. The University of Michigan experience. JAMA. 2000;283:904–908. [PubMed] [Google Scholar]
- Kinsella JP, Truog WE, Walsh WF, et al. Randomized multicenter trial of inhaled nitric oxide and high-frequency oscillatory ventilation in severe, persistent pulmonary hypertension of the newborn. J Pediatr. 1997;131:55–62. doi: 10.1016/s0022-3476(97)70124-0. [DOI] [PubMed] [Google Scholar]
- Toomasian JM, Snedecor SM, Cornell RG, Cilley RE, Bartlett RH. National experience with extracorporeal membrane oxygenation for newborn respiratory failure. ASAIO Trans. 1988;34:140–147. doi: 10.1097/00002480-198804000-00011. [DOI] [PubMed] [Google Scholar]
- Tracy TF, Delosh TN, Stolar CJH. The Registry of the Extracorporeal Life Support Organization. 2000 http://www.med.umich.edu/ecmo/ELSO_reg.html [Google Scholar]
- Bartlett RH, Roloff DW, Cornell RG, et al. Extracorporeal circulation in neonatal respiratory failure: a prospective randomized study. Pediatrics. 1985;76:479–487. [PubMed] [Google Scholar]
- Schumacher RE, Roloff DW, Chapman R, Snedecor S, Bartlett RH. Extracorporeal membrane oxygenation in term newborns: a prospective cost benefit analysis. ASAIO J. 1993;39:873–879. [PubMed] [Google Scholar]
- O'Rourke PP, Crone R, Vacanti J, et al. Extracorporeal membrane oxygenation and conventional medical therapy in neonates with persistent pulmonary hypertension of the newborn: a prospective randomized study. Pediatrics. 1989;84:957–963. [PubMed] [Google Scholar]
- UK Neonatal EMCO Trial Group UK collaborative randomized trial of neonatal extracorporeal membrane oxygenation. Lancet. 1996;348:75–82. [PubMed] [Google Scholar]
- Van Heijst AF, van der Staak FH, Geven WB, et al. Results of extracorporeal membrane oxygenation in 100 newborns with cardiorespiratory insufficiency. Ned Tijdschr Geneeskd. 1999;143:356–360. [PubMed] [Google Scholar]
- O'Rourke PP, Crone RK. Pediatric applications of extracorporeal membrane oxygenation. J Pediatr. 1990;116:393–394. doi: 10.1016/s0022-3476(05)82827-6. [DOI] [PubMed] [Google Scholar]
- Vernon DD, Dean JM, McGough EC. Pediatric extracorporeal membrane oxygenation. The time for anecdotes is over. Am J Dis Child. 1990;144:855–856. doi: 10.1001/archpedi.1990.02150320017015. [DOI] [PubMed] [Google Scholar]
- Green TP, Timmons OD, Fackler JC, et al. The impact of extracorporeal membrane oxygenation on survival in pediatric patients with acute respiratory failure. Pediatric Critical Care Study Group. Crit Care Med. 1996;24:323–329. doi: 10.1097/00003246-199602000-00023. [DOI] [PubMed] [Google Scholar]
- Thompson BT. US ARDS Network: results of the study on mechanical ventilation. Abstract book of the proceedings of the Berlin ARDS-Symposium. Acute Respiratory Distress Syndrome: History, New Pathological Insights and their Implications for Therapeutic Strategies. Edited by Lewandowski K, Falke KJ. Berlin: 1999;Abstract Book:65–69. [Google Scholar]
- Chatte G, Sab JM, Dubois JM, et al. Prone position in mechanically ventilated patients with severe acute respiratory failure. Am J Respir Crit Care Med. 1997;155:473–478. doi: 10.1164/ajrccm.155.2.9032181. [DOI] [PubMed] [Google Scholar]
- Putensen C, Mutz NJ, Putensen-Himmer G, Zinserling J. Spontaneous breathing during ventilatory support improves ventilation-perfusion distributions in patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. 1999;159:1241–1248. doi: 10.1164/ajrccm.159.4.9806077. [DOI] [PubMed] [Google Scholar]
- Hickling KG, Walsh J, Henderson S, Jackson R. Low mortality rate in adult respiratory distress syndrome using low-volume, pressure-limited ventilation with permissive hypercapnia: a prospective study. Crit Care Med. 1994;22:1568–1578. doi: 10.1097/00003246-199422100-00011. [DOI] [PubMed] [Google Scholar]
- Extracorporeal Support for Respiratory Insufficiency. A Collaborative Study in Response to RFP-NHLI-73-20. National Heart, Lung, and Blood Institute, Division of Lung Diseases. 1979 [Google Scholar]
- Murray JF, Matthay MA, Luce JM, Flick MR. An expanded definition of the adult respiratory distress syndrome. Am Rev Respir Dis. 1988;138:720–723. doi: 10.1164/ajrccm/138.3.720. [DOI] [PubMed] [Google Scholar]
- Brieschal T, Benzing A, Geiger K. Therapeutic strategy for ARDS [in German]. Intensivmed. 1993;30:312–317. [Google Scholar]
- Manert W, Haller M, Briegel J, et al. Venovenous extracorporeal membrane oxygenation (ECMO) with a heparin-lock bypass system. An effective addition in the treatment of acute respiratory failure (ARDS). Anaesthesist. 1996;45:437–448. doi: 10.1007/s001010050278. [DOI] [PubMed] [Google Scholar]
- Egan T, Duffin J, Glynn M, et al. Ten-year experience with extracorporeal membrane oxygenation for severe respiratory failure. Chest. 1988;94:681–687. doi: 10.1378/chest.94.4.681. [DOI] [PubMed] [Google Scholar]
- Roberts JD, Fineman JR, Morin FC, et al. Inhaled nitric oxide and persistent pulmonary hypertension of the newborn. The Inhaled Nitric Oxide Study Group. N Engl J Med. 1997;336:605–610. doi: 10.1056/NEJM199702273360902. [DOI] [PubMed] [Google Scholar]
- Inhaled nitric oxide in full-term and nearly full-term infants with hypoxic respiratory failure. N Engl J Med. 1998;336:597–604. doi: 10.1056/NEJM199702273360901. [DOI] [PubMed] [Google Scholar]
- Davidson D, Barefield ES, Kattwinkel J, et al. Inhaled nitric oxide for the early treatment of persistent pulmonary hypertension of the term newborn: a randomized, double-masked, placebo-controlled, dose-response, multicenter study. Pediatrics. 1998;101:325–334. doi: 10.1542/peds.101.3.325. [DOI] [PubMed] [Google Scholar]
- Clark RH, Kueser TJ, Walker MW, et al. Low-dose nitric oxide therapy for the persistent pulmonary hypertension of the newborn. N Engl J Med. 2000;342:469–474. doi: 10.1056/NEJM200002173420704. [DOI] [PubMed] [Google Scholar]
- Squara P, Dhainaut J-FA, Artigas A, Carlet J. Hemodynamic profile in severe ARDS: results of the European Collaborative ARDS Study. Intens Care Med. 1998;24:1018–1028. doi: 10.1007/s001340050710. [DOI] [PubMed] [Google Scholar]
- Hickling KG, Henderson SJ, Jackson R. Low mortality associated with low volume pressure limited ventilation with permissive hypercapnia in severe adult respiratory distress syndrome. Intens Care Med. 1990;16:372–377. doi: 10.1007/BF01735174. [DOI] [PubMed] [Google Scholar]
- Bone RC. Treatment of adult respiratory distress syndrome with diuretics, dialysis, and positive end-expiratory pressure. Crit Care Med. 1978;6:136–139. doi: 10.1097/00003246-197805000-00002. [DOI] [PubMed] [Google Scholar]
- Montgomery B, Stager MA, Carrio CJ, Hudson LD. Causes of mortality in patients with adult respiratory distress syndrome. Am Rev Respir Dis. 1985;132:485–489. doi: 10.1164/arrd.1985.132.3.485. [DOI] [PubMed] [Google Scholar]
- Kirby RR, Downs JB, Civetta JM, et al. High level positive end expiratory pressure (PEEP) in acute respiratory insufficiency. Chest. 1975;67:156–163. doi: 10.1378/chest.67.2.156. [DOI] [PubMed] [Google Scholar]
- Petty TL. editor's perspective. Semin Respir Med. 1983;4:321–326. [Google Scholar]
- Bell RC, Coalson JJ, Smith JD, Johanson WG. Multiple organ system failure and infection in ARDS. Ann Intern Med. 1983;99:293–298. doi: 10.7326/0003-4819-99-3-293. [DOI] [PubMed] [Google Scholar]
- Fowler AA, Hamman RF, Good JT, et al. Adult respiratory distress syndrome: risk with common predispositions. Ann Intern Med. 1983;98:593–597. doi: 10.7326/0003-4819-98-5-593. [DOI] [PubMed] [Google Scholar]
- Frikker MJ, Lynch K, Pontoppidan H, Wilson RS, Rie MA, Zapol WM. The adult respiratory distress syndrome: aetiology, progression and survival. Adult Respiratory Distress Syndrome. Edited by Artigas A, Lemaire F, Suter PM, Zapol WM. Edinburgh: Churchill Livingstone. 1992:3–9. [Google Scholar]
- Mancebo J, Artigas A. A clinical study of the adult respiratory distress syndrome. Crit Care Med. 1987;15:243–246. doi: 10.1097/00003246-198703000-00013. [DOI] [PubMed] [Google Scholar]
- Pepe PE, Potkin RT, Reus DH, Hudson LD, Carrico CJ. Clinical predictors of the adult respiratory distress syndrome. Am J Surg. 1982;144:124–130. doi: 10.1016/0002-9610(82)90612-2. [DOI] [PubMed] [Google Scholar]
- Baumann WR, Jung RC, Koss M, Boylen T, Navarro L, Sharma OP. Incidence and mortality of adult respiratory distress syndrome: a prospective analysis from a large metropolitan hospital. Crit Care Med. 1986;14:1–4. doi: 10.1097/00003246-198601000-00001. [DOI] [PubMed] [Google Scholar]
- Bernard GR, Luce JM, Sprung CL, et al. High-dose corticosteroids in patients with the adult respiratory distress syndrome. N Engl J Med. 1987;317:1565–1570. doi: 10.1056/NEJM198712173172504. [DOI] [PubMed] [Google Scholar]
- Hudson LD. Survival data in patients with acute and chronic lung disease requiring mechanical ventilation. Am Rev Respir Dis. 1989;140:S19–S24. doi: 10.1164/ajrccm/140.2_Pt_2.S19. [DOI] [PubMed] [Google Scholar]
- Villar J, Blazquez MA, Lubillo S, Quintana J, Manzano J. Pulmonary hypertension in acute respiratory failure. Crit Care Med. 1989;17:523–526. doi: 10.1097/00003246-198906000-00007. [DOI] [PubMed] [Google Scholar]
- Rinaldo JE. The prognosis of the adult respiratory distress syndrome. Inappropriate pessimism? Chest. 1986;90:470–471. doi: 10.1378/chest.90.4.470. [DOI] [PubMed] [Google Scholar]
- Ronco JJ, Phang T, Walley KR, Fenwick JC, Wiggs B, Russel JA. Oxygen consumption is independent of changes in oxygen delivery in severe adult respiratory distress syndrome. Am Rev Respir Dis. 1991;143:1267–1273. doi: 10.1164/ajrccm/143.6.1267. [DOI] [PubMed] [Google Scholar]
- Suchyta MR, Clemmer TP, Elliott CG, et al. Increased mortality of older patients with acute respiratory distress syndrome. Chest. 1997;111:1334–1339. doi: 10.1378/chest.111.5.1334. [DOI] [PubMed] [Google Scholar]
- Sloane PJ, Gee M, Gottlieb JE, et al. A multicenter registry of patients with acute respiratory distress syndrome. Am Rev Respir Dis. 1992;146:419–426. doi: 10.1164/ajrccm/146.2.419. [DOI] [PubMed] [Google Scholar]
- Gee MH, Gottlieb JE, Albertine KH, Kubis JM, Peters SP, Fish JE. Physiology of aging related to outcome of the adult respiratory distress syndrome. J Appl Physiol. 1990;69:822–829. doi: 10.1152/jappl.1990.69.3.822. [DOI] [PubMed] [Google Scholar]
- Bone RC, Balk R, Slotman G, et al. Adult respiratory distress syndrome: sequence and importance of development of multiple organ failure. Chest. 1992;101:320–326. doi: 10.1378/chest.101.2.320. [DOI] [PubMed] [Google Scholar]
- Wagner P, Knoch M, Sangmeister C, M??ller E, Lennartz H. Extracorporeal gas exchange in adult respiratory distress syndrome: associated morbidity and its surgical treatment. Br J Surg. 1990;77:1395–1398. doi: 10.1002/bjs.1800771224. [DOI] [PubMed] [Google Scholar]
- Pesenti A, Bombino M, D'Andrea L, Gattinoni L. Present status of extracorporeal support in patients with ARDS. Abstract book of the 2nd European Congress on Extracorporeal Lung Support. Edited by Lennartz H. Marburg, Germany: Philipps-University, Department of Anesthesiology and Intensive Care Therapy:IL-3. 1992 [Google Scholar]
- M??ller E, Knoch M, Holtermann W, Wagner P, Lennartz H. Extracorporeal support in patients with ARDS: the results and experiences of the Marburg group. Abstract book of the 2nd European Congress on Extracorporeal Lung Support. Edited by Lennartz H. Marburg, Germany: Philipps-University, Department of Anesthesiology and Intensive Care Therapy:IL-3. 1992 [Google Scholar]
- Benzing A, Geiger K. Extracorporeal membrane oxygenation in Freiburg, Germany [in German]. Schriftenreihe Intensivmedizin, Notfallmedizin, An??sthesiologie. Band 84 Intensivmedizin: Organdysfunktionen. Edited by Peter K, Lawin P, Briegel J. Stuttgart: Thieme. 1994:139–142. [Google Scholar]
- Peek GJ, Moore HM, Moore N, Sosnowski AW, Firmin RK. Extracorporeal membrane oxygenation for adult respiratory failure. Chest. 1997;112:759–764. doi: 10.1378/chest.112.3.759. [DOI] [PubMed] [Google Scholar]
- Macha M, Griffith BP, Keenan R, et al. ECMO support for adult patients with acute respiratory failure. ASAIO J. 1996;42:M841–M844. doi: 10.1097/00002480-199609000-00109. [DOI] [PubMed] [Google Scholar]
- Kolla S, Awad SS, Rich PB, Schreiner RJ, Hirschl RB, Bartlett RH. Extracorporeal life support for 100 adult patients with severe respiratory failure. Ann Surg. 1997;226:544–566. doi: 10.1097/00000658-199710000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brower RG, Shanholtz CB, Fessler HE, et al. Prospective, randomized, controlled clinical trial comparing traditional versus reduced tidal volume ventilation in acute respiratory distress syndrome patients. Crit Care Med. 1999;27:1492–1498. doi: 10.1097/00003246-199908000-00015. [DOI] [PubMed] [Google Scholar]
- Stewart TE, Meade MO, Cook DJ, et al. Evaluation of a ventilation strategy to prevent barotrauma in patients at high risk for acute respiratory distress syndrome. N Engl J Med. 1998;338:355–361. doi: 10.1056/NEJM199802053380603. [DOI] [PubMed] [Google Scholar]


