Abstract
As in other areas of medicine, the specialty of critical care medicine, which has made important contributions in the pathophysiology of critical illness, is facing challenges that must be recognized and addressed in the current century. In this review, we argue that the skill set required to adequately treat critically ill patients will also require knowledge of molecular biology for better diagnosis and treatment. The foundations of molecular biology and genetics are essential for the understanding of the mechanisms of disease. Incorporating molecular biology techniques in the research arsenal of the intensivist will provide the opportunity to dissect out and define the reversible and irreversible intracellular processes giving rise to the major causes of mortality in intensive care units. Two historical paradigms, the cardiopulmonary resuscitation and polymerase chain reaction, summarize how critical care medicine began, and how it could mature in the years to come.
Keywords: acute respiratory failure, critically ill patients, intensive care medicine, molecular biology, molecular medicine
Introduction
Critical care medicine (CCM) and the intensive care unit (ICU) are essential components of modern health care systems around the globe. CCM has the capability of reversing near-fatal states and of temporarily supporting failing vital functions, systems and organs, while the patient recovers from the underlying disease process. The ICU is not the domain of any specialty; for appropriate care, four major disciplines (anesthesiology, internal medicine, surgery, and pediatrics) have been working collaboratively because patients admitted to ICUs often have multiple system organ diseases. Intensive care physicians have, since the 1970s, been transforming medicine by integrating knowledge derived over generations with modern medical research and information technology.
Over the past three decades, the data set employed by critical care physicians was based largely on physiological and pharmacological concepts, typified by the skills required to perform cardiopulmonary resuscitation (CPR), and was well taught in all training programs. In the current century, we argue that the skill set required to adequately treat critically ill patients will also require knowledge of molecular biology for diagnosis and treatment. Molecular biology holds the promise of transforming medicine; its foundations are essential for the understanding of the mechanisms of life. A metaphor for this knowledge set is the polymerase chain reaction (PCR). The two historical paradigms of CPR and PCR summarize how CCM began, and how it could mature in the years to come to pursue its ultimate goal: to return a critically ill patient to a happy and productive life. Whether they are hereditary or not, many diseases and pathophysiological disturbances have a genetic background. Classic genetics states that the genotype is responsible for the phenotype. It seems clear, however, that the pathogenesis of most diseases is the result of complex interactions among the genotype, the environment, and the nature of the process that leads to cell, tissue, organ, or systemic injury. As the molecular knowledge of normal physiology and disease becomes clearer, we may be able not only to prevent illness, but also to design accurate genetic tests and individualized treatments.
CPR and CCM: a parent and child relationship
CCM is a very young medical specialty. Failure to breathe was, until the early 1950s, considered synonymous with the failure to survive. The introduction of the negative-pressure whole-body ventilator or 'iron lung' during the poliomyelitis epidemic in Europe and North America challenged the concepts of life and death, and began a new page in the history of medicine [1]. Extension of the iron lung concept to other patients with acute respiratory problems, and the emerging development of modern anesthesia and cardiac surgery, soon led to the introduction of a group of life-support techniques. Endotracheal intubation became the optimal method of securing the airway. Although peripheral venous cannulation was often quicker and easier, the central veins became the optimal routes for delivering drugs rapidly into central circulation. For the first time in medical history, physicians were able to reverse terminal states and to revive or resuscitate patients in near-death or clinical death situations [2,3]. By the early 1960s, modern CPR became available not only in hospital emergency departments and operating and recovery rooms [4], but also in the out-of-hospital setting [5]. By 1970, physicians were able to support almost every organ function; death became a neurological concept instead of a cardiological concept, and the term CPR was extended to cardiopulmonary-cerebral resuscitation [6]. All steps in relation to CPR became available when the techniques of 'basic' and 'advanced' life support gave birth to 'prolonged' life support, with the development of the ICU concept. With the introduction of prolonged endotracheal intubation, mechanical ventilation, continuous electrocardiogram monitoring, bedside intravascular catheterization, analysis of respiratory gases in arterial and venous blood, closed-chest cardiac massage and defibrillation, and the development of new anesthetic agents, it was possible to support life and improve the outcome of patients in various surgical states and medical conditions. The first modern ICUs were born [7,8].
A critically ill patient can be defined as a patient with a physiologic disorder who is experiencing an ongoing threat to the integrity of the cardiorespiratory system and who has a high likelihood of requiring aggressive life support. The advances in the treatment of such patients followed advances in other medical and surgical disciplines that were instrumental in the implementation and consolidation of modern ICUs. Much credit has, however, to be given to improvements in monitoring skills, techniques, and equipment. Although well-trained anesthesiol-ogists were the specialists who contributed the most to the birth of modern ICUs [9], many other medical and surgical disciplines soon began to cooperate for care of the seriously ill patient. Because intensive care medicine requires team action by physicians, nurses, and other health professionals with various specialty backgrounds and added expertise in resuscitation, the need for well-trained, full-time 'intensivists' and nurses working in the ICU was followed by the development of education guidelines, fellowship training programs within a multidiscipli-nary approach, and the development of national and international societies of Intensive and Critical Care Medicine in the USA and Europe [10,11,12]. Qualified critical care physicians must be trained to address the complex problems of multiple system organ dysfunction as they possess the requisite knowledge, skill, interest, judgment, and frequency of exposure to treat most problems of critically ill patients directly, and as they are physically present to address them [13].
In the first two decades of the modern history of CCM, emphasis was placed on the needs of patients with acute respiratory failure and multiple system organ failure. The latter is not a specific disease caused by any specific factor, but it is well established that the number of additional organs involved in the disease process, not the organ itself, appears to determine mortality in the critically ill patient requiring prolonged life support [14]. It is currently agreed that multiple system organ failure or dysfunction is the result of an inappropriate response of the host to a variety of challenges, such as severe infection, severe trauma or severe inflammation. Hemodynamic monitoring with bedside catheterization techniques was essential for the understanding, better management and treatment of patients with acute pulmonary edema and shock. The diversity of patients, the implementation of ICUs around the world, the inadequate information on ICU outcome, and the rapid incorporation of new medical discoveries, in conjunction with the use of sophisticated technology, soon prompted the evaluation of different therapeutic protocols and clinical research. Technology forced the stratification of patients by severity and type of illness [15] because expensive equipment could not be widely used in every sick patient. The physiological and pharmacological support of patients in the ICU has, as a result, been very effective to date. Several studies have demonstrated that the input of a critical care specialist to an academically affiliated hospital both lowers the mortality rate and reduces the number of days of ICU and hospital care [16,17,18].
Molecular critical care medicine: from CPR to PCR
It seems probable that all fields of medicine will change more in the next decade than they have in the past 2000 years. The main drivers of this change are molecular biology and information technology. Whether directly involved or merely an interested observer, one cannot fail to recognize that the remarkable progress in understanding disease pathogenesis at the cellular and molecular level has placed us on the threshold of a new, revolutionary era of clinical practice. A decade ago, little was known about how cells communicate or how they transduce stimuli. The development of an ever-expanding armamentarium of technologies for analysis of gene structure and function, referred to as recombinant DNA (deoxyribonucleic acid) technology, has created unprecedented opportunities for significantly improving the treatment and prevention of human diseases. Recombinant DNA technology has made it possible to study the molecular factors modulating cellular responses to metabolic and environmental stresses. The application of molecular biology to elucidating the causes and potential cures of disease has become a major thrust of research at virtually all medical institutions. Incorporating molecular biology techniques in the research arsenal of the critical care physician will provide the opportunity to dissect out and define the reversible and irreversible intracellular processes giving rise to acute respiratory distress syndrome, sepsis, septic shock, and multiple system organ failure (ie the major causes of mortality in most ICUs) [19,20] (Table 1).
Table 1.
Relation of physiological events with clinical presentation and molecular biology in some acute illnesses
| Disease/syndrome | Physiological event | Clinical presentation | Molecular process | Potential new therapy |
| Bacterial pneumonia | Alveolar exudate | Pulmonary dysfunction | Change in cell surface | Inhaled anti-inflammatory |
| receptor affinity for | cytokines | |||
| Inflammatory infiltrates | microorganisms | |||
| Antibiotic therapy based on | ||||
| Cell damage | Immune suppression | bacterial DNA susceptibility | ||
| Shock | Tissue ischemia | Organ hypoperfusion | Upregulation of ROS | Endothelial growth factors |
| Endothelial damage | Reduced NOS synthesis | ROS scavengers | ||
| Ventilator-induced | Alveolar disruption | Respiratory failure | Decreased synthesis and | Lung protective ventilatory |
| lung injury | denaturation of surfactant | strategies | ||
| Alveolar/interstitial edema | ||||
| Upregulation of inflammatory | Inhaled anti-inflammatory | |||
| Capillary stress | cytokines | cytokines | ||
| SIRS | Immune suppression | Multiple organ dysfunction | Upregulation of acute phase | IL-10 gene expression vector |
| proteins and cytokines | ||||
| Sustained low grade | Blocking EBP cellular | |||
| inflammation | receptors |
EBP, Endotoxin binding proteins; IL-10, interleukin-10; NOS, nitric oxide synthase; ROS, reactive oxygen species; SIRS, systemic inflammatory response syndrome.
The specialty of CCM, which has made important contributions in the pathophysiology of critical illness, is facing challenges that must be recognized and addressed as we enter the new century. Compared with other specialties, a parallel momentum for exploiting these technologies has been relatively slow to develop in the field of CCM. Despite the enormous literature pertaining to the molecular biology of the cell that has evolved over the past decade, most intensivists have little understanding of the profound impact of molecular biology in the practice of their own specialty. Discoveries in most biological systems highlight the need to connect physiology with genetics, molecular and cellular biology. Much attention has been focused in recent years on the Human Genome Project, an international effort that has been able to delineate the entire DNA sequence of Homo sapiens [21,22]. The information derived from knowledge of the human genome sequence, when combined with the increasingly sophisticated tools of molecular biology, will certainly radically alter medical practice.
Recombinant DNA techniques allow us to examine a gene's structural integrity and to assess gene expression at the mRNA and protein level. A widely used approach for evaluating gene expression involves use of PCR, a technique that can be applied to the assessment of gene expression using small quantities of tissue. PCR allows us to amplify and identify specific segments of DNA by a simple chemical reaction within a few hours. PCR involves the exponential amplification of a selected DNA segment more than 1 million-fold by the induction of repeated cycles of DNA synthesis from a given DNA template. The strategy is similar to the process of DNA synthesis utilized by all living organisms. PCR has fostered an explosion of research in the diagnosis of genetic and infectious diseases. The molecular changes seen in critically ill tissues and organs are either exaggerations of normal physiology or inappropriate expression of repair patterns. The cellular events involved in mediating organ inflammation, tissue damage, and repair are ultimately controlled at the molecular level and cannot be fully understood without consideration of the functions of the relevant genes and their products. Using PCR and other molecular biology techniques, critical care scientists have evaluated their potential use in the detection of pulmonary and systemic infections [23,24], in systemic and local cytokine expression during the intense host inflammatory response seen in patients with sepsis and acute lung injury [25,26], and to examine the genetic susceptibility to poor outcome during critical illness [27].
Biotrauma: a paradigm for molecular CCM
DNA, mRNA or proteins can be extracted from the lung or any tissue or cell population using standard biochemical techniques. In conjunction with PCR technology, we can assess the relevance of altered gene transcription and translation to given physiological and pathological states. Epithelial and endothelial cells respond to lung injury with acute alterations in mediator generation and surface molecule expression, and appear to act in concert with inflammatory cells to influence the lung tissue response to injury and inflammatory stimuli. These interactions are in turn known to induce the expression of various genes encoding proteins central to coagulation, fibrinolysis, and repair. The influence of these molecules on a particular cell or cell population may be influenced greatly by interactions with cytokines and other types of regulatory factors (Fig. 1).
Figure 1.
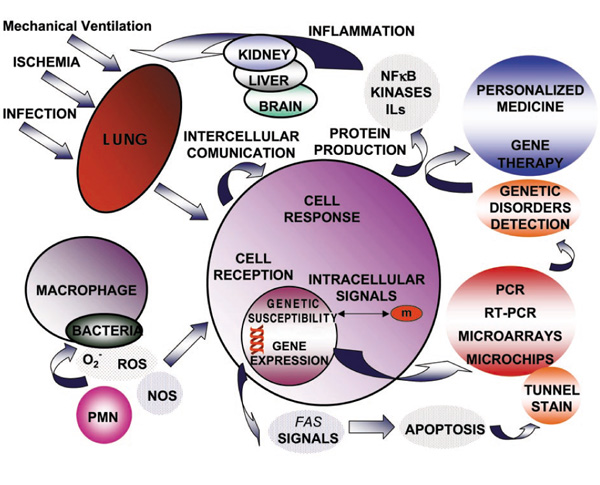
A network of networks between cytokines and other regulatory factors. IL, Interleukin; NF, nuclear factor; NOS, nitric oxide synthase; PMN, neutrophils; ROS, reactive oxygen species; RT-PCR, reverse transcription and polymerase chain reaction.
Research over the past decade has shown that mechanical ventilation per se could lead to a number of serious complications, including the initiation or exacerbation of an underlying lung injury, and might contribute to the development of multiple system organ failure [28]. Recent reports [29,30,31] are instrumental in understanding the inflammatory aspects of the pathogenesis of ventilator-induced lung injury and the influence of different ventilatory patterns on the pulmonary and systemic inflammatory responses. These experimental studies have demonstrated that cyclic mechanical stress imposed on airway epithelium with conventional mechanical ventilation may lead to the activation of alveolar macrophages, which then release pro-inflammatory cytokines from the lungs during less than 3 hours of ventilation. This implies that the activation of intra-alveolar cells occurs very early on, before any signs of lung injury become apparent. Molecular studies of gene expression using PCR technology have revealed that the induction of marked increases in the expression of certain cytokines following lung injury often correlates with the magnitude of lung damage. Although there are various differences between these experimental models and the clinical setting, these reports support the hypothesis that certain current techniques and strategies of mechanical ventilation might contribute to the development of a systemic inflammatory response and multiple system organ failure. A more recent randomized clinical trial has confirmed that pulmonary and systemic cytokine response can be minimized by limiting recruitment or de-recruitment of collapsed lung units, or overdistension of alveolar regions [32]. We have largely underestimated the complexity of the inflammatory and immune systems, and of their interactions in severely ill patients. Propagation of inflammatory signals from the airspace to the vascular space is pivotal in lung inflammation, but mechanisms of inter-compartmental signaling are still not well understood. Tumor necrosis factor-α induces signaling between the alveolar and vascular compartments of the lung; this novel mechanism may be relevant in the alveolar recruitment of leukocytes [33]. Increasing evidence also suggests that pulmonary dysfunction results from acute oxygen toxicity because of the injury and death of lung cells. Altered expression of several apoptotic regulatory proteins, such as p53 and Bcl-2, is associated with hyperoxic cell death and lung injury [34]. The development of an oxidant/antioxidant imbalance during lung inflammation may activate sensitive transcription factors, such as nuclear factor-κB, which regulate the genes for pro-inflammatory mediators and protective antioxidant genes.
From ABC (airway, breathing, circulation) to MGM (molecular and genetic medicine)
From a therapeutic point of view, modern molecular methods are being developed that allow the stable transfer of foreign DNA sequences into human and other mammalian somatic cells. Promising approaches based on the delivery of genes, either as plasmid DNA or by viral vectors, have been extensively evaluated preclinically and in early-phase clinical trials. Plasmids are like viruses living parasitically in bacteria. More than 100 clinical gene therapy studies have actually been currently approved. There is now an impressive range of potential treatments including gene therapy, anti-cytokine and anti-adhesion molecule approaches, and targeting of intracellular signal transduction pathways [35]. Recombinant retrovirus constructs containing a cytokine-cDNA can be used to infect cells in vitro and to obtain information regarding the pathogenesis and treatment of several processes that are relevant in clinical diseases [36]. Blocking cytokine activation or pharmacological effects with specific cytokine-receptor antagonists represents a logical strategy for the treatment or attenuation of sepsis-related inflammation. Strategies that make it possible to selectively downregulate the effects of specific cytokines would be particularly attractive from the therapeutic perspective.
All areas of medical research are being affected by the explosion of knowledge and technology in the fields of cellular and molecular biology. The new intellectual challenge is to reintegrate this information into an understanding of whole tissue, organ and organism function. DNA is certainly part of our destiny [20]. For this reintegration to occur, physicians taking care of patients with critical problems must understand the basic science and technology of molecular biology. As in other specialties, intensive and critical care societies should advocate the need for mandatory training in molecular biology techniques during CCM training programs, not only to provide the transition for clinical application of molecular biological advances, but also to keep the basic science research on track. It is an exciting time for respiratory and critical care medicine.
Abbreviations
CCM = critical care medicine; CPR = cardiopulmonary resuscitation; ICU = intensive care unit; PCR = polymerase chain reaction.
Acknowledgments
Acknowledgements
Supported by Fondo de Investigacion Sanitaria, Spain (#98/1178) and The Medical Research Council of Canada.
References
- Ibsen B. The anesthetist's viewpoint on treatment of respiratory complications in poliomyelitis during the epidemic in Copenhagen, 1952. Proc R Soc Med. 1954;47:72–74. doi: 10.1177/003591575404700120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safar P, Escarraga L, Elam J. A comparison of the mouth-to-mouth and mouth-to-airway methods of artificial respiration with the chest-pressure arm-lift methods. N Engl J Med. 1958;258:671–677. doi: 10.1056/NEJM195804032581401. [DOI] [PubMed] [Google Scholar]
- Kouwenhoven WB, Jude JR, Knickerbocker GG. Closed chest cardiac massage. JAMA. 1960;173:1064–1067. doi: 10.1001/jama.1960.03020280004002. [DOI] [PubMed] [Google Scholar]
- Beck CS, Leighninger DS. Death after a clean bill of health. So-called 'fatal' heart attacks and treatment with resuscitation techniques. JAMA. 1960;174:133–135. doi: 10.1001/jama.1960.03030020021005. [DOI] [PubMed] [Google Scholar]
- Pantridge JF, Geddes JS. A mobile intensive care unit in the management of myocardial infarction. Lancet. 1967;ii:271–273. doi: 10.1016/s0140-6736(67)90110-9. [DOI] [PubMed] [Google Scholar]
- Grenvik A, Safar P. Brain Failure and Resuscitation New York: Churchill Livingstone, 1981.
- Safar P, De Kornfeld TJ, Pearson JW, Redding JS. Intensive care unit. Anaesthesia. 1961;16:275–284. doi: 10.1111/j.1365-2044.1961.tb13827.x. [DOI] [PubMed] [Google Scholar]
- Holmdahl MH. Respiratory intensive care unit. Anesthesiology. 1963;23:559–568. doi: 10.1097/00000542-196207000-00015. [DOI] [PubMed] [Google Scholar]
- Cherniak RM. Recognition and management of respiratory insufficiency. Anesthesiology. 1964;25:209–216. [PubMed] [Google Scholar]
- Safar P, Grenvik A. Critical care medicine, organizing and staffing intensive care units. Chest. 1971;59:535–547. doi: 10.1378/chest.59.5.535. [DOI] [PubMed] [Google Scholar]
- Grenvik A, Leonard JJ, Arens JF, Carey LC, Disney FA. Critical care medicine. Certification as a multidisciplinary subspecialty. Crit Care Med. 1981;9:117–125. doi: 10.1097/00003246-198102000-00012. [DOI] [PubMed] [Google Scholar]
- Kelley MA. Critical care medicine - a new specialty. N Engl J Med. 1988;318:1613–1617. doi: 10.1056/NEJM198806163182410. [DOI] [PubMed] [Google Scholar]
- Marini JJ. Streamlining critical care: responsibilities and cost-effectiveness in intensive care unit organization. Mayo Clin Proc. 1997;72:483–485. doi: 10.1016/S0025-6196(11)64871-4. [DOI] [PubMed] [Google Scholar]
- Villar J, Manzano JJ, Blazquez MA, Quintana J, Lubillo S. Multiple system organ failure in acute respiratory failure. J Crit Care. 1991;6:75–80. [Google Scholar]
- Knaus WA, Zimmerman JE, Wagner DP, Draper EA, Lawrence DE. APACHE - acute physiology and chronic health evaluation: a physiologically based classification system. Crit Care Med. 1981;9:591–597. doi: 10.1097/00003246-198108000-00008. [DOI] [PubMed] [Google Scholar]
- Siegel JH, Cerra FB, Moody EA, Shetye M, Coleman B, Garr L, Shubert M, Keane JS. The effect on survival of critically ill injured patients of an ICU teaching service organized about a computer-based physiologic CARE system. J Trauma. 1980;20:558–579. doi: 10.1097/00005373-198007000-00005. [DOI] [PubMed] [Google Scholar]
- Brown JJ, Sullivan G. Effect on ICU mortality of a full-time critical care specialist. Chest. 1989;96:127–129. doi: 10.1378/chest.96.1.127. [DOI] [PubMed] [Google Scholar]
- Pollack MM, Cuerdon TT, Patel KM, Ruttimann UE, Getson PR, Levetown M. Impact of quality-of-care factors on pediatric intensive care unit mortality. JAMA. 1994;272:941–946. [PubMed] [Google Scholar]
- Liu M, Slutsky AS. Anti-inflammatory therapies application of molecular biology techniques in intensive care medicine. Intens Care Med. 1997;23:718–731. doi: 10.1007/s001340050400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villar J, Siminovitch KA. Molecular intensive care medicine. Intens Care Med. 1999;25:652–661. doi: 10.1007/s001340050926. [DOI] [PubMed] [Google Scholar]
- Watson JD. The human genome project: past, present and future. Science. 1990;248:44–48. doi: 10.1126/science.2181665. [DOI] [PubMed] [Google Scholar]
- Venter JC, Adams MD, Myers EW, Li PW, Mural RJ, Sutton GG, et al. The sequence of the human genome. Science. 2001;291:1304–1351. doi: 10.1126/science.1058040. [DOI] [PubMed] [Google Scholar]
- Schluger NW, Rom WN. The polymerase chain reaction in the diagnosis and evaluation of pulmonary infections. Am J Respir Crit Care Med. 1995;152:11–16. doi: 10.1164/ajrccm.152.1.7599808. [DOI] [PubMed] [Google Scholar]
- Cursons RTM, Jeyerajah E, Sleigh JW. The use of polymerase chain reaction to detect septicemia in critically ill patients. Crit Care Med. 1999;27:937–940. doi: 10.1097/00003246-199905000-00029. [DOI] [PubMed] [Google Scholar]
- Zuckerberg AL, Goldberg LI, Lederman HM. Effects of hypoxia on interleukin-2 mRNA expression by T-lymphocytes. Crit Care Med. 1994;22:197–203. doi: 10.1097/00003246-199402000-00008. [DOI] [PubMed] [Google Scholar]
- Armstrong L, Millar AB. Relative production of tumour necrosis factor α and interleukin 10 in adult respiratory distress syndrome. Thorax. 1997;52:442–446. doi: 10.1136/thx.52.5.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuber F, Petersen M, Bokelmann F, Schade U. A genomic polymorphism within the tumor necrosis factor locus influences plasma tumor necrosis factor-α concentrations and outcome of patients with severe sepsis. Crit Care Med. 1996;24:381–384. doi: 10.1097/00003246-199603000-00004. [DOI] [PubMed] [Google Scholar]
- Slutsky AS, Tremblay LN. Multiple system organ failure. Is mechanical ventilation a contributing factor. Am J Respir Crit Care Med. 1998;157:1721–1725. doi: 10.1164/ajrccm.157.6.9709092. [DOI] [PubMed] [Google Scholar]
- Takata M, Abe J, Tanaka H, Kitano Y, Doi S, Kohsaka T, Miyasaka K. Intraalveolar expression of tumor necrosis factor-α gene during conventional and high-frequency ventilation. Am J Respir Crit Care Med. 1997;156:272–279. doi: 10.1164/ajrccm.156.1.9607072. [DOI] [PubMed] [Google Scholar]
- Tremblay L, Valenza F, Ribeiro SP, Li J, Slutsky AS. Injurious venti-latory strategies increase cytokines and c-fos mRNA expression in an isolated rat lung model. J Clin Invest. 1997;99:944–952. doi: 10.1172/JCI119259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiumello D, Pristine G, Slutsky AS. Mechanical ventilation affects local and systemic cytokines in an animal model of acute respiratory distress syndrome. Am J Respir Crit Care Med. 1999;160:109–116. doi: 10.1164/ajrccm.160.1.9803046. [DOI] [PubMed] [Google Scholar]
- Ranieri VM, Suter PM, Tortorella C, De Tulio R, Dayer JM, Brienza A, Bruno F, Slutsky AS. Effect of mechanical ventilation on inflammatory mediators in patients with acute respiratory distress syndrome: a randomized controlled trial. JAMA. 1999;282:54–61. doi: 10.1001/jama.282.1.54. [DOI] [PubMed] [Google Scholar]
- Kuebler WM, Parthasarathi K, Wang PM, Bhattacharya J. A novel signaling mechanism between gas and blood compartments of the lung. J Clin Invest. 2000;105:905–913. doi: 10.1172/JCI8604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantell LL, Lee PJ. Signal transduction pathways in hyperoxia-induced lung cell death. Mol Genet Metab. 2000;71:359–370. doi: 10.1006/mgme.2000.3046. [DOI] [PubMed] [Google Scholar]
- Curiel DT. Gene-based therapies for inherited and acquired disorders of the lung. Chest. 1997;111(suppl 6):149–152. doi: 10.1378/chest.111.6_supplement.149s-a. [DOI] [PubMed] [Google Scholar]
- Xing Z, Ohkawara Y, Jordana M, Graham FL, Gauldie J. Adenoviral vector-mediated interleukin-10 expression in vivo: intramuscular gene transfer inhibits cytokine responses in endotoxemia. Gene Ther. 1997;4:140–149. doi: 10.1038/sj/gt/3300371. [DOI] [PubMed] [Google Scholar]


