Abstract
Inadequate splanchnic perfusion is associated with increased morbidity and mortality, particularly if liver dysfunction coexists. Heart failure, increased intra-abdominal pressure, haemodialysis and the presence of obstructive sleep apnoea are among the multiple clinical conditions that are associated with impaired splanchnic perfusion in critically ill patients. Total liver blood flow is believed to be relatively protected when gut blood flow decreases, because hepatic arterial flow increases when portal venous flow decreases (the hepatic arterial buffer response [HABR]). However, there is evidence that the HABR is diminished or even abolished during endotoxaemia and when gut blood flow becomes very low. Unfortunately, no drugs are yet available that increase total hepato-splanchnic blood flow selectively and to a clinically relevant extent. The present review discusses old and new concepts of splanchnic vasoregulation from both experimental and clinical viewpoints. Recently published trials in this field are discussed.
Keywords: gastric mucosal pH, hepatic arterial buffer response, lactate, splanchnic blood flow
Introduction
Under conditions of low systemic blood flow or haemorrhage, perfusion of vital organs is maintained at the expense of perfusion of visceral organs [1,2,3]. If blood flow to the splanchnic tissues is sufficiently low, ischaemia and (if it is prolonged) tissue damage and necrosis may occur. As a result of splanchnic ischaemia the gut may become permeable, and endotoxin and other bacterial products can pass through the gut wall into lymph nodes and blood vessels [4,5], thereby causing injury to local and distant organs [6,7]. The splanchnic organs may also be at risk in septic shock, even when splanchnic blood flow is normal or elevated, because of a major increase in metabolic demand [8,9].
There are only a few methods with which to measure splanchnic perfusion in the clinical setting, and interpretation of the obtained results can be difficult. Once detected, the treatment of splanchnic ischaemia is not straightforward [10]. There is no drug available that selectively improves splanchnic perfusion in a clinically significant way. On the other hand, a number of drugs may actually worsen splanchnic perfusion and/or metabolism [11,12].
The present review discusses important pathophysiological aspects of splanchnic vasoregulation and presents recently published experimental and clinical trials in the field of impaired splanchnic blood flow and metabolism.
Splanchnic perfusion in low-flow states and mechanisms of impairment
A number of studies have demonstrated disproportionately impaired perfusion of the gut and colon in low-flow states [1,2,3,13,14,15]. However, redistribution of blood flow away from the splanchnic organs has been demonstrated mainly in experimental haemorrhagic shock. We [16] and others [17] have provided evidence that blood flow to the splanchnic region is reduced in proportion to systemic blood flow under different conditions of low cardiac output. Varying study conditions and consequent pathophysiological reactions may explain these inconsistent findings. Nevertheless, there is evidence that even a reduction in splanchnic blood flow in proportion to other regional flows may have severe consequences.
The vasoconstrictive response to circulatory shock is mediated by the sympathetic nervous system, the renin–angiotensin system and vasopressin [14]. When α-adrenergic receptors on postcapillary mesenteric venules and veins are stimulated, the resulting autotransfusion will improve the performance of the heart by increasing cardiac filling. Selective vasoconstriction of the afferent mesenteric arterioles serves to sustain systemic vascular resistance and therefore to maintain arterial blood pressure. This response is dependent to a limited degree on the sympathetic nervous system, but is mainly mediated by the renin–angiotensin axis and vasopressin [14]. This has recently been demonstrated in pigs, in which sustained periods of cardiac tamponade after mild haemorrhage followed by resuscitation were associated with selective splanchnic vasospasm and ischaemic hepatic injury [13]. These manifestations of splanchnic vasoconstriction and the resulting biochemical and histological signs of postischaemic liver injury were not ameliorated by α-adrenergic blockade, but were attenuated either by prior nephrectomy or by angiotensin-converting enzyme inhibition. Similar results were reported during graded haemorrhagic shock [15].
Mechanisms of inadequate splanchnic blood flow in septic states
Splanchnic tissue oxygenation may also be at risk in septic shock, even though total hepato-splanchnic blood flow may be normal or elevated. This is due to a major increase in metabolic demand, reflected by increased tissue oxygen consumption and impaired oxygen extraction [8,9]. The increase in oxygen consumption has been related to cytokine production [18] and to diversion of oxygen to the generation of reactive oxygen species [19]. It has been proposed that the relative hypoxia of mesenteric organs during sepsis may actually account for a large proportion of the vasodilatation that is seen in this condition.
Dangers associated with inadequate splanchnic blood flow
Low blood flow to the gut with and without reperfusion is associated with increased permeability of the gut wall [20], endotoxaemia, presence of bacteria in abdominal lymph nodes and the thoracic duct [4,5], and possibly bacteraemia [21]. Furthermore, leucocyte-activating factors are released during ischaemia and reperfusion of splanchnic organs [22]. Inadequate splanchnic perfusion is associated with multiple organ failure and death [23,24]. However, studies have failed to demonstrate this complete sequence of events in humans. Nevertheless, low gastric mucosal pH is clearly associated with increased morbidity and mortality in critically ill patients [24,25,26,27].
Another line of evidence for the deleterious effects of inadequate splanchnic perfusion comes from sepsis trials. Liver dysfunction was associated with a markedly higher mortality rate [28], and the ability to increase splanchnic oxygen delivery in septic conditions correlated with a lower mortality rate [29].
When is the splanchnic region at risk for inadequate perfusion?
Compromised cardiac function is a main reason why blood flow becomes inadequate in critically ill patients. This may be particularly true when the metabolic demands are increased, for example after cardiac surgery. Such patients may already exhibit signs of tissue hypoxia when they arrive in the intensive care unit after cardiopulmonary bypass (CPB). A recent retrospective study attempted to identify risk factors for peri-operative hyperlactataemia in 124 patients after elective cardiac surgery with extracorporeal bypass [30]. Patients with increased postoperative lactate concentrations had longer CPB times and lower blood pressures in the initial phase of extracorporeal circulation. Hyperlactataemia in such patients may have been the result of increased lactate production, decreased lactate uptake by the liver and other organs, or a combination of both. In animals subjected to low cardiac output [16] mesenteric, but not prehepatic, lactate exchange increased; this suggests that splanchnic tissue supplied by the coeliac trunk (e.g. spleen, pancreas, duodenum) is able to utilize or store lactate. Hyperlactataemia during low systemic perfusion was found to be a result of both an increased lactate production and a lack of ability to increase hepatic lactate uptake [16].
Tissue hypoxia in patients during CPB may be induced by low blood pressure or by insufficient tissue perfusion due to limited pump flow, or both. The relative importance of flow versus pressure in splanchnic perfusion during CPB was recently studied in rabbits [31]. Simultaneous measurements of tissue blood flow in four different splanchnic areas (gastric, jejunum, ileum, liver) were taken using laser Doppler flow-metry before and during CPB. Blood pressure and flow were maintained high or low in random order. The flow was better preserved in all organs but the liver when CPB flow was high, and this was independent of the pressure. Hence, it seems reasonable to focus more on preserving CPB flow rather than pressure to avoid splanchnic ischaemia during CPB. In humans undergoing elective coronary artery bypass grafting, jejunal mucosal perfusion appeared to be well maintained during mild hypothermic CPB, when pump flow was maintained at around 2.5 l/min per m2 [32].
Blood flow may become insufficient after cardiac surgery because of increasing metabolic demands in combination with compromised myocardial function. We measured systemic, splanchnic and femoral blood flows, metabolism, and markers of the adequacy of tissue perfusion in 17 patients after elective cardiac surgery, from arrival in the intensive care unit until extubation [33]. Cardiac output and femoral blood flows increased by 12% and 28%, respectively, whereas the fraction of cardiac output distributed to the splanchnic region decreased by 20%. At the same time, splanchnic oxygen extraction increased by 16%. In certain patients splanchnic oxygen extraction was in the range at which signs of organ dysfunction or damage are likely to occur [34,35]. Hepatic venous lactate:pyruvate ratio was high after admission to the intensive care unit and decreased subsequently (Fig. 1). The high splanchnic lactate : pyruvate ratios at admission to the intensive care unit resulted from low pyruvate rather than high lactate levels, and are therefore unlikely to indicate anaerobic metabolism. On the other hand, only excessive anaerobic mesenteric metabolism or frank liver hypoxia will result in systemic hyperlactataemia because of the high lactate extraction capability of the liver. Initially high concentrations of glu-tathione transferase-α decreased during the postoperative period, and indocyanine green extraction was well preserved. It is likely that the observed increase in splanchnic oxygen extraction was sufficient to compensate for the lack of increase in blood flow and to maintain aerobic metabolism and cellular integrity in the splanchnic region in these patients with a normal cardiac reserve.
Figure 1.
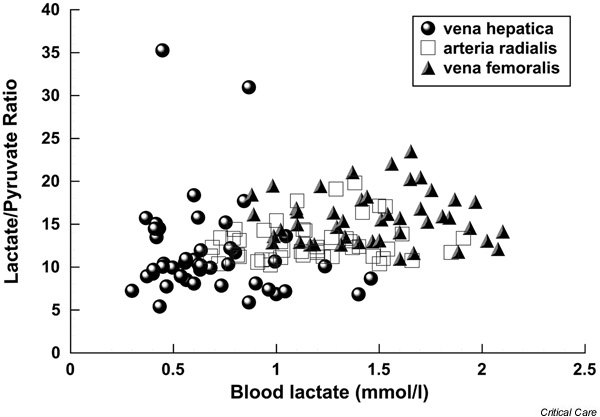
Lactate:pyruvate ratio in relation to lactate concentrations in 17 patients after cardiac surgery. Data points are pooled values from three different time points from arrival to the intensive care unit until extubation. (Adapted from [33].)
Surgical, medical and nursing procedures in critically ill patients may also interfere with the ability to maintain adequate systemic and regional oxygen delivery. We recently showed that haemodialysis with ultrafiltration is associated with a significant reduction in systemic, splanchnic and femoral blood flows [36]. Nine patients with acute renal failure and stable systemic haemodynamics were studied. The need for extracorporeal renal replacement therapy was defined as excess of extracellular water and insufficient urinary output despite the use of diuretic drugs; serum urea concentration greater than 20 mmol/l; and creatinine concentration greater than 400 mol/l. Haemodialysis was performed according to standard clinical practice, and hypotension (systolic blood pressure <90 mmHg) was treated by reducing the filtration rate and infusion of 100–200 ml Ringers acetate if necessary. Systemic, hepato-splanchnic and femoral haemodynamics and oxygen transport, and gastric mucosal partial carbon dioxide tension were measured before haemodialysis, 2 hours after initiating a 4-hour period of haemodialysis, and again 2 hours after completion of haemodialysis.
The median amount of fluid removed during 4 hours of haemodialysis was 2000 ml (range 300–2500 ml) [36]. Haemodialysis was associated with a parallel decrease in cardiac output, stroke volume and splanchnic blood flow despite stable arterial blood pressure. All flows returned to baseline values after dialysis without therapeutic interventions, suggesting that this mode of renal replacement therapy induces acute but only temporary reduction in splanchnic perfusion in intensive care patients with stable haemodynam-ics. In such patients haemodialysis can therefore still be recommended. However, the systemic and regional response to acute intermittent haemodialysis may be different in patients who are hypotensive or who need vasoactive drugs to maintain a sufficient blood pressure. These patients may not tolerate intermittent haemodialysis.
The abdominal compartment syndrome is another clinical condition during which splanchnic blood flow is at risk (recently reviewed by Morken and West [37]). It may occur under various surgical and medical conditions, such as blunt and penetrating abdominal trauma, retroperitoneal haemorrhage, ruptured abdominal aortic aneurysm, peritonitis and pancreatitis, among others. Inadequate gastric mucosal perfusion has recently also been described in patients with obstructive sleep apnoea [38] and during periods of weaning from mechanical ventilation [39,40].
Mechanisms to preserve hepato-splanchnic blood flow
In contrast to the gut, the liver is believed to be relatively well protected against hypoperfusion because of the HABR [41]. The HABR describes the hydrodynamic interaction between portal venous and hepatic arterial blood flow. Table 1 summarizes important findings of studies in HABR [41,42,43,44,45,46,47]. When mesenteric and, consequently, portal venous blood flow decreases, hepatic arterial blood flow increases. The compensation of hepatic arterial blood flow for the decreased portal venous blood flow is in the range 20–30% [41,46,48]. Compensation in terms of oxygen delivery is substantially higher because of the much greater oxygen content in the hepatic artery as compared with the portal vein. The current concept is the adenosine washout hypothesis; namely, adenosine in the Mall's space is washed out when portal venous blood flow is normal but not when it is low. Under these circumstances, adenosine causes hepatic arterial vasodilatation [41]. It has been shown that liver oxygen supply is maintained during haemorrhage until the blood loss exceeds 30% [49]. The HABR is abolished early during endotoxaemia and recovers partially after several hours [47,50,51,52].
Table 1.
Important studies on hepatic arterial buffer response, with main findings
| Reference | Species | Main finding |
| Lautt (1985) [41] | Cat | Antagonism of HABR by the adenosine antagonist 8-phenyltheophylline |
| Lautt et al. (1988) [42] | Cat | Hepatic arterial vascular response to intravenous drugs dependent on direct action of the drug on hepatic artery and on indirect effects of drug-induced changes in portal venous blood flow |
| Lautt and McQuaker (1989) [43] | Cat | Protective dilatation of hepatic artery during haemorrhage is mediated by adenosine |
| Lautt et al. (1990) [44] | Cat | During high portal venous blood flow, hepatic artery is nearly fully constricted; during low portal venous blood flow, hepatic artery is nearly fully dilated |
| Henderson et al. (1992) [45] | Human | Intact HABR in liver transplant patients |
| Ayuse et al. (1994) [46] | Pig | Change in portal venous blood flow alters hepatic arterial resistance upstream from the site of a constant arterial back pressure |
| Ayuse et al. (1995) [47] | Pig | HABR is abolished during endotoxaemia independently of nitric oxide or α-adrenergic receptor antagonists |
HABR = hepatic arterial buffer response. Adapted from Jakob [10].
We tested the HABR under conditions of low mesenteric blood flow in pigs [53]. A total of 14 animals were randomized either to partial superior mesenteric artery occlusion or to serve as controls. Superior mesenteric artery flow was reduced by a clamp to a median flow of 2 ml/kg per min (range 1–3 ml/kg per min) for 120 min in ischaemic animals. After 2 hours the clamp was released and the measurements were continued for another 60 min. The HABR was assessed four times at hourly intervals during ischaemia and reperfusion by acute and intermittent reduction in portal vein blood flow. The absolute increase in hepatic arterial blood flow in response to portal vein occlusion decreased significantly during ischaemia. A decrease in the efficiency but not an exhaustion of the HABR was also seen in control animals. During reperfu-sion, hepatic arterial blood flow changes induced by portal vein occlusion increased again. We hypothesize that the repeated ischaemia/reperfusion events caused by testing the buffer response may have interfered with hepatic adenosine production or transport. Alternatively, an effect of the surgical procedure and anaesthesia may have contributed to the observed changes in both groups of animals.
The exhaustion of the HABR has important clinical implications. If a reduction in splanchnic blood flow has already resulted in a compensatory increase in hepatic arterial blood flow, then an acute further decrease in splanchnic blood flow may no longer be compensated for by the HABR, especially when systemic blood flow and/or pressure decrease concomitantly.
Our experimental design, which is similar to that employed by most investigators to test the HABR, may modify both quantitative and qualitative aspects of the HABR elicited under conditions of low systemic blood flow. This is because clamping the superior mesenteric artery results in increased systemic vascular resistance and increased perfusion pressure across the coeliac trunk–hepatic artery axis. If the systemic blood flow is low, then a low coeliac trunk blood flow may not increase sufficiently to compensate for an acute decrease in portal venous blood flow.
In order to address this question, we designed a study in which systemic blood flow was decreased in two steps by cardiac tamponade [16]. At baseline and every 30 min thereafter, the portal vein was intermittently occluded. Cardiac output decreased by 21% and 55% and systemic arterial blood pressure by 40% and 64% during the first stage (moderate) and second stage (severe) of tamponade, respectively, and it remained constant in a control group. During moderate tamponade, hepatic arterial blood flow increased in both groups by approximately 50%. During severe tamponade hepatic arterial blood flow decreased in tamponade animals. Nevertheless, fractional splanchnic blood flow was preserved. The acute compensation of hepatic arterial blood flow for a decrease in portal venous flow decreased during moderate tamponade and disappeared during severe tamponade. These data demonstrate that the liver is only initially protected during low systemic perfusion. Later, the HABR is exhausted. In the same experiment, we demonstrated that the splanchnic organs are not among the first to produce lactate because fractional splanchnic blood flow is preserved. In contrast, the capacity of the liver to increase lactate uptake was exhausted early.
Is splanchnic blood flow impaired in low blood pressure?
It has been proposed that decreasing arterial blood pressure is associated with impaired perfusion of splanchnic organs and disturbed cellular integrity [54]. Andel and coworkers [55] tested the effect of deliberate hypotension on splanchnic perfusion balance with the use of either isoflurane or a combination of esmolol and nitroglycerin. Sixteen anaesthetized patients undergoing elective maxillofacial surgery were randomly allocated to one of the two drug regimens. Systolic blood pressure was decreased to approximately 30% below preoperative values while maintaining mean arterial blood pressure at levels greater than 50 mmHg. Gastric tonometers were used to assess the adequacy of mucosal perfusion, and arterial lactate was measured in order to assess the overall adequacy of oxygen delivery. Those investigators found that neither method that had been used to decrease blood pressure compromised splanchnic tissue oxygen balance in these patients. Overall organ perfusion was sufficient in both groups because none of the patients demonstrated an increase in blood lactate concentration. Maintaining arterial blood pressure above 50 mmHg in defined patient groups therefore appears to be safe in terms of splanchnic tissue oxygenation if hypovolaemia is prevented.
Gastric mucosal pH in early goal-directed therapy for critically ill patients
In 1992 Gutierrez and coworkers [56] proposed the use of treatment titrated against gastric intramucosal pH in the management of critically ill patients. However, three consecutive studies did not confirm these findings [57,58,59]. The trial by Gutierrez and coworkers has been criticized because of the unexplained high mortality in the control group, in which no attempts were made to standardize the treatment. It is important to realize in this context that changes in gastric intramucosal pH do not necessarily reflect similar changes in hepato-splanchnic blood flow.
How can the splanchnic blood flow be increased?
Recently, the haemodynamic effects of fenoldopam (a dopamine-1 receptor agonist) were studied before and after induction of splanchnic ischaemia by haemorrhage [60]. After haemorrhage, this drug restored portal vein blood flow to near baseline, maintained the splanchnic fraction of cardiac output, and attenuated the rise in gut mucosal partial carbon dioxide tension. Fenoldopam also redistributed the blood flow away from the serosal to the mucosal layer both at baseline and during haemorrhage. Whether this drug also exerts its beneficial effects under clinical conditions of low splanchnic blood flow has yet to be demonstrated.
In patients with septic shock, dobutamine was compared with the phosphodiesterase inhibitor enoximone [61]. In 48 patients either one of the drugs was infused randomly after fluid resuscitation. Liver blood flow was estimated using the continuous indocyanine green infusion technique and hepatic venous catheterization. Liver function was assessed using monoethylglycine xylidide formation after lidocaine injection, and inflammation was quantified by release of hepatic tumour necrosis factor-α. Cardiac output and total hepato-splanchnic blood flow increased in both groups after 12 and 48 hours of the respective drug infusions. The fractional hepato-splanchnic blood flow decreased slightly in dobutamine-treated patients and remained unchanged in the enoximone group. Hepato-splanchnic oxygen consumption and release of tumour necrosis factor-α were increased in both groups after 12 hours of vasoactive drug infusion, but arterial monoethylglycine xylidide concentrations increased only in the enoxi-mone group. Because of methodological problems in that study (lack of control patients, incomplete assessment of monoethylglycine xylidide kinetics and multiple comparisons without correction), it is hard to conclude whether one drug is superior to the other in terms of preserved liver function or attenuation of inflammation.
Even relatively small increases in intra-abdominal pressure during carbon dioxide laparoscopy are associated with impaired systemic, portal venous and hepatic arterial blood flow [62]. Although small doses of dobutamine appear to restore gut mucosal perfusion and improve hepatic arterial blood flow in this setting, total hepato-splanchnic blood flow cannot be maintained with either dobutamine or dopamine [62].
Dopamine is used to support cardiac output and blood pressure in patients with cardiac failure and septic shock [63,64]. Dopamine at a low rate is still frequently used in patients with renal failure, with the aim of increasing both renal perfusion and urinary output, although there is a lack of data demonstrating subsequent improvement in renal function [65,66]. We measured the effects of dopamine on systemic and splanchnic blood flow and metabolism in septic and cardiac surgery patients [12]. Dopamine infusion was started at a dose of 1 μg/kg per min and then gradually increased until the thermodilution cardiac output was 25% higher than at baseline. Dopamine infusion (1–9 g/kg per min) caused a parallel increase in systemic and splanchnic blood flow. Although systemic oxygen consumption did not change in either group, splanchnic oxygen consumption decreased significantly during dopamine infusion in septic patients and increased in cardiac surgery patients.
A decrease in splanchnic oxygen consumption during dopamine infusion has recently been reported in patients with acute hepatic failure [67]. A reduction in hepato-splanchnic oxygen uptake despite an increase in regional perfusion may indicate blood flow redistribution. Alternatively, some metabolic functions of the hepato-splanchnic region may have been impaired. Dopamine may directly inhibit isoenzymes of the cytochrome P450 complex [68]. The different effects of dopamine infusion on splanchnic oxygen consumption in the two patient groups could possibly be explained by different baseline activities of P450 isoenzymes. The indications and safety of dopamine in sepsis should therefore be re-evaluated.
Conclusion
Inadequate splanchnic perfusion in critically ill patients is associated with increased morbidity and mortality. The underlying pathophysiological mechanisms are still not well understood. Splanchnic blood flow may become insufficient as a result of a multitude of different diseases and treatment modalities. Splanchnic vasoregulation is complex and is also altered by disease and treatment. Unfortunately, many of the available monitoring tools for hepato-splanchnic blood flow and metabolism are difficult to apply in the clinical setting, and interpretation of the results obtained is not straightforward. Thus, concepts of splanchnic resuscitation are not established. Future research projects should focus on the interplay between the physiological regulatory mechanisms in splanchnic organs, disease and treatment.
Competing interests
None declared.
Abbreviations
CPB = cardiopulmonary bypass; HABR = hepatic arterial buffer response.
See related Commentary: http://ccforum.com/content/6/4/282
References
- Bulkley GB, Kvietys PR, Perry MA, Granger DN. Effects of cardiac tamponade on colonic hemodynamics and oxygen uptake. Am J Physiol. 1983;244:G604–G612. doi: 10.1152/ajpgi.1983.244.6.G604. [DOI] [PubMed] [Google Scholar]
- Schlichtig RA, Kramer DJ, Pinsky MR. Flow redistribution during progressive hemorrhage is a determinant of critical O2 delivery. J Appl Physiol. 1992;70:169–178. doi: 10.1152/jappl.1991.70.1.169. [DOI] [PubMed] [Google Scholar]
- Vatner SF. Effects of hemorrhage on regional blood flow distribution in dogs and primates. J Clin Invest. 1974;54:225–235. doi: 10.1172/JCI107757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grotz MR, Ding J, Guo W, Huang Q, Deitch EA. Comparison of plasma cytokine levels in rats subjected to superior mesenteric artery occlusion or hemorrhagic shock. Shock. 1995;3:362–368. [PubMed] [Google Scholar]
- Ljungdahl M, Lundholm M, Katouli M, Rasmussen I, Engstrand L, Haglund U. Bacterial translocation in experimental shock is dependent on the strains in the intestinal flora. Scand J Gastroenterol. 2000;35:389–397. doi: 10.1080/003655200750023958. [DOI] [PubMed] [Google Scholar]
- Aranow JS, Fink MP. Determinants of intestinal barrier failure in critical illness. Br J Anaesth. 1996;77:71–81. doi: 10.1093/bja/77.1.71. [DOI] [PubMed] [Google Scholar]
- Baker JW, Deitch EA, Li M, Berg RD, Specian RD. Hemorrhagic shock induces bacterial translocation from the gut. J Trauma. 1988;28:896–906. doi: 10.1097/00005373-198807000-00002. [DOI] [PubMed] [Google Scholar]
- Arvidsson D, Rasmussen I, Almqvist P, Niklasson F, Haglund U. Splanchnic oxygen consumption in septic and hemorrhagic shock. Surgery. 1991;109:190–197. [PubMed] [Google Scholar]
- Dahn MS, Lange P, Lobdell K, Hans B, Jacobs LA, Mitchell RA. Splanchnic and total body oxygen consumption differences in septic and injured patients. Surgery. 1987;101:69–80. [PubMed] [Google Scholar]
- Jakob SM. Splanchnic vasoregulation and metabolism: new insights into physiology [PhD thesis]. Kuopio, Finland: Kuopio University Publications D, Medical Sciences 240. 2001.
- Reinelt H, Radermacher P, Kiefer P, Fischer G, Wachter U, Vogt J, Georgieff M. Impact of exogenous beta-adrenergic receptor stimulation on hepatosplanchnic oxygen kinetics and metabolic activity in septic shock. Crit Care Med. 1999;27:325–331. doi: 10.1097/00003246-199902000-00039. [DOI] [PubMed] [Google Scholar]
- Jakob SM, Ruokonen E, Takala J. Effects of dopamine on systemic and regional blood flow and metabolism in septic and cardiac surgery patients. Shock. 2002. [DOI] [PubMed]
- Bailey RW, Bregman ML, Fuh KC, Hamilton SR, Herlong HF, Bulkley GB. Hemodynamic pathogenesis of ischemic hepatic injury following cardiogenic shock/resuscitation. Shock. 2000;14:451–459. doi: 10.1097/00024382-200014040-00006. [DOI] [PubMed] [Google Scholar]
- Reilly PM, Wilkins KB, Fuh KC, Haglund U, Bulkley GB. The mesenteric hemodynamic response to circulatory shock: an overview. Shock. 2001;15:329–343. doi: 10.1097/00024382-200115050-00001. [DOI] [PubMed] [Google Scholar]
- Toung T, Reilly PM, Fuh KC, Ferris R, Bulkley GB. Mesenteric vasoconstriction in response to hemorrhagic shock. Shock. 2000;13:267–273. doi: 10.1097/00024382-200004000-00003. [DOI] [PubMed] [Google Scholar]
- Jakob SM, Tenhunen JJ, Laitinen S, Heino A, Alhava E, Takala J. Effects of systemic arterial hypoperfusion on splanchnic hemodynamics and hepatic arterial buffer response in pigs. Am J Physiol Gastrointest Liver Physiol. 2001;280:G819–G827. doi: 10.1152/ajpgi.2001.280.5.G819. [DOI] [PubMed] [Google Scholar]
- Riddez L, Hahn RG, Brismar B, Strandberg A, Svensen C, Hedenstierna G. Central and regional hemodynamics during acute hypovolemia and volume substitution in volunteers. Crit Care Med. 1997;25:635–640. doi: 10.1097/00003246-199704000-00013. [DOI] [PubMed] [Google Scholar]
- Fong Y, Marano MA, Moldawer LL, Wei H, Calvano SE, Kenney JS, Allison AC, Cerami A, Shires GT, Lowry SF. The acute splanchnic and peripheral tissue metabolic response to endotoxin in humans. J Clin Invest. 1990;85:1896–1904. doi: 10.1172/JCI114651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor DE, Kantrow SP, Piantadosi CA. Mitochondrial respiration after sepsis and prolonged hypoxia. Am J Physiol. 1998;275:L139–L144. doi: 10.1152/ajplung.1998.275.1.L139. [DOI] [PubMed] [Google Scholar]
- Khanna A, Rossman JE, Fung H-L, Caty MG. Intestinal and hemodynamic impairment following mesenteric ischemia/ reperfusion. J Surg Res. 2001;99:114–119. doi: 10.1006/jsre.2001.6103. [DOI] [PubMed] [Google Scholar]
- Bone RC. Toward an epidemiology and natural history of SIRS. JAMA. 1992;268:3452–3455. [PubMed] [Google Scholar]
- Kistler EB, Lefer AM, Hugli TE, Schmid-Schönbein GW. Plasma activation during splanchnic arterial occlusion shock. Shock. 2000;14:30–34. doi: 10.1097/00024382-200014010-00006. [DOI] [PubMed] [Google Scholar]
- Kirton OC, Windsor J, Wedderburn R, Hudson-Civetta J, Shatz DV, Mataragas NR, Civetta JM. Failure of splanchnic resuscitation in the acutely injured trauma patient correlates with multiple organ system failure and length of stay in the ICU. Chest. 1998;113:1064–1069. doi: 10.1378/chest.113.4.1064. [DOI] [PubMed] [Google Scholar]
- Doglio GR, Pusajo JF, Egurrola MA, Bonfigli GC, Parra C, Vetere L, Hernandez MS, Fernandez S, Palizas F, Gutierrez G. Gastric mucosal pH as a prognostic index of mortality in critically ill patients. Crit Care Med. 1991;19:1037–1040. doi: 10.1097/00003246-199108000-00011. [DOI] [PubMed] [Google Scholar]
- Maynard N, Bihari D, Beale R, Smithies M, Baldock G, Mason R, McColl I. Assessment of splanchnic oxygenation by gastric tonometry in patients with acute circulatory failure. JAMA. 1993;270:1203–1210. [PubMed] [Google Scholar]
- Theodoropoulos G, Lloyd LR, Cousins G, Pieper D. Intraoperative and early postoperative gastric intramucosal pH predicts morbidity and mortality after major abdominal surgery. Am Surg. 2001;67:303–308. [PubMed] [Google Scholar]
- Poeze M, Takala J, Greve JW, Ramsay G. Pre-operative tonome-try is predictive for mortality and morbidity in high-risk surgical patients. Intensive Care Med. 2000;26:1272–1281. doi: 10.1007/s001340000604. [DOI] [PubMed] [Google Scholar]
- Norton L, Moore G, Eiseman B. Liver failure in the postoperative patient: the role of sepsis and immunologic deficiency. Surgery. 1975;78:6–13. [PubMed] [Google Scholar]
- Imamura M, Clowes GHA. Hepatic blood flow and oxygen consumption in starvation, sepsis, and septic shock. Surg Gynecol Obstet. 1975;141:27–34. [PubMed] [Google Scholar]
- Inoue S, Kuro M, Furuya H. What factors are associated with hyperlactatemia after cardiac surgery characterized by well-maintained oxygen delivery and a normal postoperative course?A retrospective study. Eur J Anesthesiol. 2001;18:576–584. doi: 10.1046/j.1365-2346.2001.00893.x. [DOI] [PubMed] [Google Scholar]
- Bastien O, Piriou V, Aouifi A, Flamens C, Evans R, Lehot JJ. Relative importance of flow versus pressure in splanchnic perfusion during cardiopulmonary bypass in rabbits. Anesthesiology. 2000;92:457–464. doi: 10.1097/00000542-200002000-00028. [DOI] [PubMed] [Google Scholar]
- Thorén A, Elam M, Ricksten S-E. Jejunal mucosal perfusion is well-maintained during mild hypothermic cardiopulmonary bypass in humans. Anesth Analg. 2001;92:5–11. doi: 10.1097/00000539-200101000-00003. [DOI] [PubMed] [Google Scholar]
- Jakob S, Ruokonen E, Takala J. Assessment of the adequacy of systemic and regional perfusion after cardiac surgery. Br J Anaesth. 2000;84:571–577. doi: 10.1093/bja/84.5.571. [DOI] [PubMed] [Google Scholar]
- Rowell LB, Blackmon JR, Kenny MA, Escourrou P. Splanchnic vasomotor and metabolic adjustments to hypoxia and exercise in humans. Am J Physiol. 1984;247:H251–H258. doi: 10.1152/ajpheart.1984.247.2.H251. [DOI] [PubMed] [Google Scholar]
- Nelson DP, Samsel RW, Wood LDH, Schumacker PT. Pathological supply dependence on systemic and intestinal O2 uptake during endotoxemia. J Appl Physiol. 1988;64:2410–2419. doi: 10.1152/jappl.1988.64.6.2410. [DOI] [PubMed] [Google Scholar]
- Jakob SM, Ruokonen E, Vuolteenaho O, Lampainen E, Takala J. Splanchnic perfusion during hemodialysis: evidence for marginal tissue perfusion. Crit Care Med. 2001;29:1393–1398. doi: 10.1097/00003246-200107000-00015. [DOI] [PubMed] [Google Scholar]
- Morken J, West M. Abdominal compartment syndrome in the intensive care unit. Curr Opin Crit Care. 2001;7:268–274. doi: 10.1097/00075198-200108000-00010. [DOI] [PubMed] [Google Scholar]
- Epstein LJ, Jervis OJ, Jr, Henderson JH, II, Sullivan M, Mohsenifar Z. Measurement of gastric P(CO2) as an index of tissue hypoxia during obstructive sleep apnea. Respiration. 2001;68:28–34. doi: 10.1159/000050459. [DOI] [PubMed] [Google Scholar]
- Hurtado FJ, Beron M, Olivera W, Garrido R, Silva J, Caragna E, Rivara D. Gastric intramucosal pH and intraluminal PCO2 during weaning from mechanical ventilation. Crit Care Med. 2001;29:70–76. doi: 10.1097/00003246-200101000-00017. [DOI] [PubMed] [Google Scholar]
- Uusaro A, Chittock DR, Russell JA, Walley KR. Stress test and gastric-arterial PCO2 measurement improve prediction of successful extubation. Crit Care Med. 2000;28:2313–2319. doi: 10.1097/00003246-200007000-00022. [DOI] [PubMed] [Google Scholar]
- Lautt WW. Mechanism and role of intrinsic regulation of hepatic arterial blood flow: the hepatic arterial buffer response. Am J Physiol. 1985;249:G549–G556. doi: 10.1152/ajpgi.1985.249.5.G549. [DOI] [PubMed] [Google Scholar]
- Lautt WW, D'Almeida MS, McQuaker J, D'Aleo L. Impact of the hepatic arterial buffer response on splanchnic vascular responses to intravenous adenosine, isoproterenol, and glucagon. Can J Physiol Pharmacol. 1988;66:807–813. doi: 10.1139/y88-128. [DOI] [PubMed] [Google Scholar]
- Lautt WW, McQuaker JE. Maintenance of hepatic arterial blood flow during hemorrhage is mediated by adenosine. Can J Physiol Pharmacol. 1989;67:1023–1028. doi: 10.1139/y89-161. [DOI] [PubMed] [Google Scholar]
- Lautt WW, Legare DJ, Ezzat WR. Quantitation of the hepatic arterial buffer response to graded changes in portal blood flow. Gastroenterology. 1990;98:1024–1028. doi: 10.1016/0016-5085(90)90029-z. [DOI] [PubMed] [Google Scholar]
- Henderson JM, Gilmore GT, Mackay GJ, Galloway JR, Dodson TF, Kutner MH. Hemodynamics during liver transplantation: the interactions between cardiac output and portal venous and hepatic arterial flows. Hepatology. 1992;16:715–718. doi: 10.1002/hep.1840160316. [DOI] [PubMed] [Google Scholar]
- Ayuse T, Brienza N, O'Donnell CP, Robotham JL. Pressure-flow analysis of portal vein and hepatic artery interactions in porcine liver. Am J Physiol. 1994;267:H1233–H1242. doi: 10.1152/ajpheart.1994.267.4.H1233. [DOI] [PubMed] [Google Scholar]
- Ayuse T, Brienza J, Revelly P, Boitnott JK, Robotham JL. Role of nitric oxide in porcine liver circulation under normal and endotoxemic conditions. J Appl Physiol. 1995;78:1319–1329. doi: 10.1152/jappl.1995.78.4.1319. [DOI] [PubMed] [Google Scholar]
- Mathie RT, Blumgart LH. The hepatic haemodynamic response to acute portal venous blood flow reductions in the dog. Pflügers Arch. 1983;399:223–227. doi: 10.1007/BF00656719. [DOI] [PubMed] [Google Scholar]
- Rasmussen A, Skak C, Kristensen M, Ott P, Kirkegaard P, Secher NH. Preserved arterial flow secures hepatic oxygenation during haemorrhage in the pig. J Physiol. 1999;516:539–548. doi: 10.1111/j.1469-7793.1999.0539v.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dziki AJ, Lynch WH, Ramsey CB, Law WR. Beta-adrenergic-dependent and -independent actions of naloxone on perfusion during endotoxin shock. Circ Shock. 1993;39:29–38. [PubMed] [Google Scholar]
- Halvorsen L, Roth R, Gunther RA, Firoozmand E, Buoncristiani AM, Kramer GC. Liver hemodynamics during portal venous endotoxemia in swine. Circ Shock. 1993;41:166–175. [PubMed] [Google Scholar]
- Schiffer ER, Mentha G, Schwieger IM, Morel DR. Sequential changes in the splanchnic circulation during continuous endotoxin infusion in sedated sheep: evidence for a selective increase of hepatic artery blood flow and loss of the hepatic arterial buffer response. Acta Physiol Scand. 1993;147:251–261. doi: 10.1111/j.1748-1716.1993.tb09497.x. [DOI] [PubMed] [Google Scholar]
- Jakob SM, Tenhunen JJ, Heino A, Pradl R, Alhava E, Takala J. Splanchnic vasoregulation, during mesenteric ischemia and reperfusion. Shock. 2002. [DOI] [PubMed]
- Suttner SW, Boldt J, Schmidt CC, Piper SN, Schuster P, Kumle B. The effects of sodium nitroprusside-induced hypotension on splanchnic perfusion and hepatocellular integrity. Anesth Analg. 1999;89:1371–1377. doi: 10.1097/00000539-199912000-00008. [DOI] [PubMed] [Google Scholar]
- Andel D, Andel H, Hörauf K, Felfernig D, Millesi W, Zimpfer M. The influence of deliberate hypotension on splanchnic perfusion balance with use of either isoflurane or esmolol and nitroglycerin. Anesth Analg. 2001;93:1116–1120. doi: 10.1097/00000539-200111000-00009. [DOI] [PubMed] [Google Scholar]
- Gutierrez G, Palizas F, Doglio G, Wainsztein N, Gallesio A, Pacin J, Dubin A, Schiavi E, Jorge M, Pusajo J, et al. Gastric intramucosal pH as a therapeutic index of tissue oxygenation in critically ill patients. Lancet. 1992;339:195–199. doi: 10.1016/0140-6736(92)90002-k. [DOI] [PubMed] [Google Scholar]
- Ivatury RR, Simon RJ, Islam S, Fueg A, Rohman M, Stahl WM. A prospective randomized study of end points of resuscitation after major trauma: global oxygen transport indices versus organ-specific gastric mucosal pH. J Am Coll Surg. 1996;183:145–154. [PubMed] [Google Scholar]
- Pargger H, Hampl KF, Christen P, Staender S, Scheidegger D. Gastric intramucosal pH-guided therapy in patients after elective repair of infrarenal abdominal aneurysms: is it beneficial? Intensive Care Med. 1998;24:769–776. doi: 10.1007/s001340050664. [DOI] [PubMed] [Google Scholar]
- Gomersall CD, Joynt GM, Freebairn RC, Hung V, Buckley TA, Oh TE. Resuscitation of critically ill patients based on the results of gastric tonometry: a prospective, randomized, controlled trial. Crit Care Med. 2000;28:607–614. doi: 10.1097/00003246-200003000-00001. [DOI] [PubMed] [Google Scholar]
- Guzman JA, Rosado AE, Kruse JA. Dopamine-1 receptor stimulation attenuates the vasoconstrictive response to gut ischemia. J Appl Physiol. 2001;91:596–602. doi: 10.1152/jappl.2001.91.2.596. [DOI] [PubMed] [Google Scholar]
- Kern H, Schröder T, Kaulfuss M, Martin M, Kox W, Spies C. Enoximone in contrast to dobutamine improves hepatosplanchnic function in fluid-optimized septic shock patients. Crit Care Med. 2001;29:1519–1525. doi: 10.1097/00003246-200108000-00004. [DOI] [PubMed] [Google Scholar]
- Agusti M, Elizalde JI, Adalia R, Martinez-Palli G, Garcia-Valdecasas JC, Pique JM, Taura P. The effects of vasoactive drugs on hepatic blood flow changes induced by CO2 laparoscopy: an animal study. Anesth Analg. 2001;93:1121–1126. doi: 10.1097/00000539-200111000-00011. [DOI] [PubMed] [Google Scholar]
- Landgarten MJ, Kumar A, Parrillo JE. Cardiovascular dysfunction in sepsis and septic shock. Curr Treatment Options Cardiovasc Med. 2000;2:451–459. doi: 10.1007/s11936-000-0040-z. [DOI] [PubMed] [Google Scholar]
- Day NP, Phu NH, Bethell DP, Mai NT, Chau TT, White NJ. The effects of dopamine and adrenaline infusions on acid-base balance and systemic haemodynamics in severe infection. Lancet. 1996;348:219–223. doi: 10.1016/s0140-6736(96)09096-4. [DOI] [PubMed] [Google Scholar]
- Marik PE, Iglesias J. Low-dose dopamine does not prevent acute renal failure in patients with septic shock and oliguria. NORASEPT II Study Investigators. Am J Med. 1999;107:387–390. doi: 10.1016/s0002-9343(99)00246-6. [DOI] [PubMed] [Google Scholar]
- Bellomo R, Chapman M, Finfer S, Hickling K, Myburgh J. Low-dose dopamine in patients with early renal dysfunction: a placebo-controlled randomised trial. Australian and New Zealand Intensive Care Society (ANZICS) Clinical Trials Group. Lancet. 2000;356:2139–2143. doi: 10.1016/s0140-6736(00)03495-4. [DOI] [PubMed] [Google Scholar]
- Clemmesen JO, Galatius S, Skak C, Dalgaard P, Larsen FS, Ott P. The effect of increasing blood pressure with dopamine on systemic, splanchnic, and lower extremity hemodynamics in patients with acute liver failure. Scand J Gastroenterol. 1999;34:921–927. doi: 10.1080/003655299750025417. [DOI] [PubMed] [Google Scholar]
- Ben-Shachar D, Zuk R, Glinka Y. Dopamine neurotoxicity: inhibition of mitochondrial respiration. J Neurochem. 1995;64:718–723. doi: 10.1046/j.1471-4159.1995.64020718.x. [DOI] [PubMed] [Google Scholar]


