Abstract
Hypothermia as a protectant of neurologic function in the treatment of cardiac arrest patients, although not a new concept, is now supported by two recent randomized, prospective clinical trials. The basic science research in support of the effects of hypothermia at the cellular and animal levels is extensive. The process of cooling for cerebral protection holds potential promise for human resuscitation efforts in multiple realms. It appears that, at least, those patients who suffer a witnessed cardiac arrest with ventricular fibrillation and early restoration of spontaneous circulation, such as those who were included in the European and Australian trials (discussed here), should be considered for hypothermic therapy.
Keywords: cardiac arrest, cerebral protection, cooling, hypothermia, resuscitation
Introduction
In two recent issues of New England Journal of Medicine, studies using hypothermia in patients following cardiac arrest (CA) to improve neurologic outcome were presented and debated [1,2,3,4,5,6,7,8]. Not a new issue, having first surfaced in the 1950s [9,10], hypothermia as a treatment strategy is potentially promising as a mechanism to curtail neurologic injury in specific, although not fully defined, patient situations. As resuscitative measures have expanded, the need for options to improve neurologic function after CA is of paramount importance. Two recent trials reported by Holzer and colleagues [1] (conducted in Europe) and Bernard and coworkers [2] (conducted in Australia) yielded statistically significant, positive outcomes (Table 1). Our goals in the present commentary are to affirm the viability of hypothermia as a therapeutic intervention, to evaluate the European and Australian trials, and to explore the potential of hypothermia as a treatment modality.
Table 1.
Comparison of two recent trials of hypothermia in cardiac arrest
| Trial | ||||
| Study information and statistical significance | European [1] | Australian [2] | ||
| Type of study | Randomized: normothermia versus hypothermia | Randomized: normothermia versus hypothermia | ||
| Multicentered, with nine centers in five countries | Four accepting emergency departments | |||
| Blinded outcome | Not blinded for treatment or outcome | |||
| Number of patients | 275 Total | 77 Total | ||
| 138 Normothermia | 34 Normothermia | |||
| 137 Hypothermia | 43 Hypothermia | |||
| Criteria | Inclusion | Witnessed arrest | Initial rhythm VF | |
| Arrest secondary to VF | Continued coma after ROSC | |||
| Age 18–75 years | Age: women >50 years; men >18 years | |||
| <60 min to ROSC | ||||
| Exclusion | Temp <30°C | Cardiogenic shock (SBP <90 mmHg despite epinephrine) | ||
| Coagulopathy | ||||
| Pregnant | Pregnant | |||
| Awake before randomization | Other causes of coma | |||
| MAP <60 mmHg for >30 min | ICU bed unavailable | |||
| Hypoxemia for >15 min | ||||
| Terminal illness | ||||
| Unavailable for follow-up | ||||
| Enrolment in other study | ||||
| Comparability of hypothermia and normothermia groups | The normothermia group had higher rates of coronary artery disease and diabetes mellitus | The normothermia group had a higher percentage of bystander-performed cardiopulmonary resuscitation | ||
| Cooling | Temperature used | 32–34°C (bladder temperature) | 33°C | |
| Mechanism | Cool air circulating device and ice packs | Ice packs | ||
| Time to start | Mean 105 min | Cooling began prehospital at a rate of 0.9°C/hour | ||
| Duration | 24 hours | 12 hours | ||
| Rewarming | Passive over 8 hours | Passive | ||
| Side effects | No statistical difference between the two groups | No statistical difference between the two groups | ||
| End-points | Primary | Favorable neurologic outcome at 6 months after arrest | Discharge to home or rehabilitation | |
| Secondary | (1) Mortality within 6 months | Side effects of hemodynamic, biochemical, or hematological instability | ||
| (2) Complications within 7 days | ||||
| Outcomes | Hypothermia: favorable outcome in 75 patients (55%) | Hypothermia: favorable outcome in 21 patients (49%) | ||
| Normothermia: favorable outcome in 54 patients (39%) | Normothermia: favorable outcome in 9 patients (26%) | |||
| Statistical significance of the outcomes | P = 0.009 | P = 0.046 | ||
The table summarizes some of the features of the two recent studies that examined the neuroprotective advantage of hypothermia in treatment of cardiac arrest. ICU, intensive care unit; ROSC, restoration of spontaneous circulation; VF, ventricular fibrillation.
Hypothermia: the science
Data support the contention that hypothermia is not only biologically plausible as a therapy but also improves neurologic outcome in animals and, now, in humans [1,2,11]. The timing and duration of treatment, as well as the degree of hypothermia, were shown to impact on efficacy and outcome [12,13]. Given different mechanistic etiologies for neurologic injury, different diseases have been shown to respond variably to treatment with hypothermia [11,14]. Finally, use of mild hypothermia has refuted the previously expected side-effect profile of hypothermia [6,7,11].
The basis for the use of hypothermia in cerebral protection (i.e. to attenuate the effects of cerebral ischemia) is supported by animal studies. Cerebral ischemia causes early and late effects. Energy failure, ion pump failure, and release of free radicals and excitotoxic agents occur early, whereas inflammatory mechanisms and release of stress-related proteins progress over hours after reperfusion. Excitotoxicity and free radical formation promote cell damage in ischemic tissue early after reperfusion. Glutamate levels – a major component of the excitotoxic response – decrease during hypothermic treatment of ischemia in rabbits [15]. By attenuating release of glutamate, the 'death funnel' of N-methyl-D-aspartate receptor stimulation (opening ion channels, allowing influx of calcium, and thereby producing the cascade of second messengers that activate kinases and proteases, leading to cellular destruction) is lessened [16].
Later responses to reperfusion following ischemia include the production of stress-related proteins, such as heat shock proteins. These proteins, in turn, are believed to influence other gene products. Elevations in heat shock protein-70 in the hippocampus are blunted by hypothermia [17]. Additionally, arachidonic acid products may be involved in an inflammatory cascade, affecting cell survival. In gerbils, hypothermia decreases levels of leukotriene B4 [18] – a substance linked to cerebral edema.
Safar and Leonov, along with their research groups, conducted elegent animal studies in the 1980s and 1990s [11], verifying improvement in outcome when hypothermia is used as treatment in cardiac arrest models, both with functional ratings and electromyographic improvement. Weinrauch and colleagues [19] demonstrated that hypothermia to 30° and 34°C, achieved by a combination of partial bypass flow and surface cooling performed immediately after cardiac arrest, improves both neurologic deficit score and histologic damage scores in dogs [19].
Hypothermia begun hours after the initial insult is not likely to affect the initial ischemic processes. Thus, early hypothermia is likely to be more effective than delayed hypothermia. In a rat model, delays of 15 min and 30 min preserved the beneficial effect of hypothermia, but with delays of 45 min there was no attenuation of infarct volume [12]. In dogs, delaying hypothermia by 15 min obscured the benefit in functional outcome as compared with that with immediate hypothermia [13]; however, it might have attenuated tissue damage, as detected histologically. In addition to initiation timing, duration has been investigated. Increasing the duration of hypothermia appears to decrease infarct size [12]. In a rat model, hypothermia with durations of 3 and 4 hours was superior to 2 hours in terms of effect on infarct volume, whereas 1 hour appeared ineffective. An early reported human series by Williams and Spence at Johns Hopkins in 1959 [10] employed durations from 24 to 72 hours, with good outcomes at both extremes.
Hypothermia: the current state
Although many models of neuroprotection in traumatic brain injury have shown a positive correlation between hypothermia and outcome, several studies in humans have failed to affirm this. In a recent meta-analysis of seven clinical trials conducted from 1993 to 2001 [14], it was concluded that hypothermia is not beneficial in the management of severe head injury. Those authors did, however, concede that further studies are 'justified and urgently needed'.
Design problems exist in both of the two new trials of hypothermia for CA [1,2], namely potential bias (the treating physicians were unblinded), the sample sizes were small, and some of the treatment protocol aspects were different (such as the time of initiation of hypothermia [in the field versus hospital] and duration of hypothermia [12 versus 24 hours]). Critics of those studies have expressed concerns over several issues [3,4,5]: the hypothermia and normothermia groups may not have been well matched; the sample sizes were small; the subgroups of patients with CA analyzed were small percentages of the total number of CAs (13–19%); and side effects of hypothermia can be extensive.
The responses of the principal investigators to those issues indicate that both groups were well matched, with median Glasgow Coma Scale scores of 3 in both groups and interquartile ranges from 3 to 4 or 5 [6]. They also point out that, although the subgroup of CA in their studies was a small percentage of the whole CA population, future studies may show that hypothermia could confer a benefit in other subgroups [6,7]. Finally, the two clinical trials from Europe [1] and Australia [2] showed no statistical difference in side-effect profiles between the normothermic and the hypothermic groups. Potential side effects of hypothermia such as arrhythmias, coagulopathies, infection, electrolyte disturbance, and hypothermia-induced polyuria were previously reported in the literature [5,11,16]. In mild hypothermia, it appears that the incidence and severity of side effects is diminished. For example, the development of arrhythmia is temperature related; temperatures below 30°C are more likely to cause serious arrhythmias such as ventricular fibrillation [9]. Additionally, some of the complications experienced in early studies of hypothermia can be negated using modern intensive care monitoring and treatment plans.
Despite the study flaws described above, outcomes show agreement with the relative percentages presented in other studies. In Fig. 1 the numbers of favorable outcomes from the normothermia arms of the two trials can be seen to approximate closely those of other studies over time. Also, despite the small size of the samples, both studies achieved statistical significance (Table 1).
Figure 1.
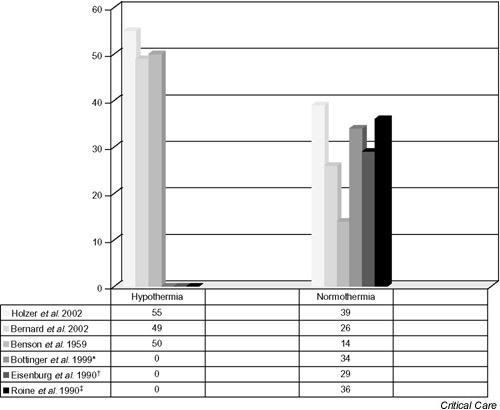
Difference in hypothermia versus normothermia: study comparisons. Shown is the percentage favorable outcome, or survival to discharge, compared among the two recent studies of hypothermia as treatment following cardiac arrest [1,2], a small series from 1959 (27 patients, 12 treated with hypothermia) [9], and three nonhypothermic series [20,21,22]. The right-most three bars are zero within the hypothermia group because they represent studies that were not designed to test hypothermia as an intervention [20,21,22]. Visual comparison reveals the closeness of new data from the two trials [1,2] with respect to the other studies [9,20,21,22]. *, †, ‡These studies were not hypothermic trials; rather, they are included here to give a perspective on relative discharge statistics following cardiac arrest from other series.
Hypothermia: our opinion
The evidence suggests that hypothermia reduces neurologic injury in animals and humans through several intricate biochemical and physiologic mechanisms, most of which we are only beginning to understand. The European [1] and Australian [2] trials both show statistically significant and clinically relevant improvement. Thus, we believe that the time has come to conduct extensive, large, multicenter trials using hypothermia to provide neurologic protection after cardiac arrest. The trials should include broader populations of CA patients (i.e. not limited to ventricular fibrillation arrest) and larger study populations, should explore a quicker method of cooling (such as intravascular cooling catheters), and should attempt to establish an effective duration of therapy (12 versus 24 hours versus other durations).
Given the high incidence of CA and the speed with which patients typically come to medical attention, the numbers of patients available for recruitment should allow a reasonable study completion time. Additionally, if, as expected, the larger trials support the findings of the recent smaller trials, then this will provide the impetus to examine other causes of hypoxic/ischemic injury, such as acute ischemic stroke.
Until such larger trials are conducted, it is our opinion that the evidence, provided in prior feasibility/safety studies as well as in the combined European and Australian trials reported earlier this year, supports employing mild hypothermic therapy in the patient populations studied (those who have suffered witnessed ventricular fibrillation arrest, restoration of spontaneous circulation, etc.).
Competing interests
TLS and TPB have previously conducted trials involving temperature control for neurologic conditions other than cardiac arrest.
Abbreviations
CA = cardiac arrest.
References
- Holzer M, The Hypothermia after Cardiac Arrest Study Group Mild therapeutic hypothermia to improve neurologic outcome after cardiac arrest. N Engl J Med. 2002;346:549–556. doi: 10.1056/NEJMoa012689. [DOI] [PubMed] [Google Scholar]
- Bernard SA, Gray TW, Buist MD, Jones BM, Silvester W, Gut-teridge G, Smith K. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;346:557–563. doi: 10.1056/NEJMoa003289. [DOI] [PubMed] [Google Scholar]
- Darby JM. Therapeutic hypothermia after cardiac arrest [correspondence]. N Engl J Med. 2002;347:63–65. doi: 10.1056/NEJM200207043470114. [DOI] [PubMed] [Google Scholar]
- Padosch SA, Kern KB, Bottiger BW. Therapeutic hypothermia after cardiac arrest [correspondence]. N Engl J Med. 2002;347:63–65. doi: 10.1056/NEJM200207043470114. [DOI] [PubMed] [Google Scholar]
- Polderman KH, Girbes AR. Therapeutic hypothermia after cardiac arrest [correspondence]. N Engl J Med. 2002;347:63–65. doi: 10.1056/NEJM200207043470114. [DOI] [PubMed] [Google Scholar]
- Holzer M. Therapeutic hypothermia after cardiac arrest [correspondence]. N Engl J Med. 2002;347:63–65. doi: 10.1056/NEJM200207043470114. [DOI] [Google Scholar]
- Bernard SA, Buist MD. Therapeutic hypothermia after cardiac arrest [correspondence]. N Engl J Med. 2002;347:63–65. doi: 10.1056/NEJM200207043470114. [DOI] [Google Scholar]
- Safar P, Kochanek PM. Therapeutic hypothermia after cardiac arrest [correspondence]. N Engl J Med. 2002;347:63–65. doi: 10.1056/NEJM200207043470114. [DOI] [PubMed] [Google Scholar]
- Benson DW, Williams GR, Spencer FC, Yates AJ. The use of hypothermia after cardiac arrest. Anesth Analg. 1959;38:423–428. [PubMed] [Google Scholar]
- Williams GR, Spence FC. The clinical use of hypothermia following cardiac arrest. Ann Surg. 1958;148:462–468. doi: 10.1097/00000658-195809000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marion DW, Leonov Y, Ginsberg M, Katz LM, Kochanek PM, Lechleuthner A, Nemoto EM, Obrist W, Safar P, Sterz F, Tisher-man SA, White RJ, Xiao F, Zar H. Resusitative hypothermia. Crit Care Med. 1996;24(suppl):S81–S89. [PubMed] [Google Scholar]
- Markarian GZ, Lee YH, Stein DJ, Hong SC. Mild hypothermia: therapeutic window after experimental cerebral ischemia. Neurosurgery. 1996;38:542–551. doi: 10.1097/00006123-199603000-00024. [DOI] [PubMed] [Google Scholar]
- Kuboyama K, Safar P, Radovsky A, Tisherman SA, Stezoski SW, Alexander H. Delay in cooling negates the beneficial effect of mild resuscitative cerebral hypothermia after cardiac arrest in dogs: a prospective, randomized study. Crit Care Med. 1993;21:1348–1358. doi: 10.1097/00003246-199309000-00019. [DOI] [PubMed] [Google Scholar]
- Harris OA, Colford JM, Good MC, Matz PG. The role of hypothermia in the management of severe brain injury. Arch Neurol. 2002;59:1077–1083. doi: 10.1001/archneur.59.7.1077. [DOI] [PubMed] [Google Scholar]
- Illievich UM, Zornow MH, Choi KT, Scheller MS, Strnat MA. Effects of hypothermic metabolic suppression on hippocam-pal glutamate concentrations after transient global cerebral ischemia. Anesth Analg. 1994;78:905–911. doi: 10.1213/00000539-199405000-00012. [DOI] [PubMed] [Google Scholar]
- Vaagenes P, Ginsberg M, Ebmeyer U, Ernster L, Fischer M, Gisvold SE, Gurvitch A, Hossmann KA, Nemoto EM, Radovsky A, Severinghaus KA, Safar P, Schlichtig R, Sterz F, Tonnessen T, White RJ, Xiao F, Zhou Y. Cerebral resuscitation from cardiac arrest: pathophysiologic mechanisms. Crit Care Med. 1996;24(suppl):S57–S68. [PubMed] [Google Scholar]
- Hicks SD, DeFranco DB, Callaway CW. Hypothermia during reperfusion after asphyxial cardiac arrest improves functional recovery and selectively alters stress-induced protein expression. J Cereb Blood Flow Metab. 2000;20:520–530. doi: 10.1097/00004647-200003000-00011. [DOI] [PubMed] [Google Scholar]
- Dempsey RJ, Combs DJ, Maley ME, Cowen BS, Roy MW, Don-aldson DL. Moderate hypothermia reduces postischemic edema developemnt and leukotriene production. Neuro-surgery. 1987;21:177–181. doi: 10.1227/00006123-198708000-00007. [DOI] [PubMed] [Google Scholar]
- Weinrauch V, Safar P, Tisherman S, Kazutoshi K, Radovsky A. Beneficial effect of mild hypothermia and detrimental effect of deep hypothermia after cardiac arrest in dogs. Stroke. 1992;23:1454–1462. doi: 10.1161/01.str.23.10.1454. [DOI] [PubMed] [Google Scholar]
- Bottiger BW, Grabner C, Bauer H, Bode C, Weber T, Motsch J, Martin E. Long term outcome after out-of-hospital cardiac arrest with physician staffed emergency medical services: the Utstein style applied to a midsize urban/suburban area. Heart. 1999;82:674–679. doi: 10.1136/hrt.82.6.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg MS, Horwood BT, Cummins RO, Reynolds-Haertle R, Hearne TR. Cardiac arrest and resuscitation: a tale of 29 cities. Ann Emer Med. 1990;19:179–186. doi: 10.1016/s0196-0644(05)81805-0. [DOI] [PubMed] [Google Scholar]
- Roine RO, Kaste M, Kinnunen A, Nikki P, Sarna S, Kajaste S. Nimodipine after resuscitation from out-of-hospital ventricular fibrillation. A placebo-controlled, double-blind, randomized trial. JAMA. 1990;264:3171–3177. [PubMed] [Google Scholar]


