Abstract
Background
Eradication of Helicobacter pylori is an important objective in overcoming gastric diseases. Many regimens are currently available but none of them could achieve 100% success in eradication. Eugenol and cinnamaldehyde that are commonly used in various food preparations are known to possess antimicrobial activity against a wide spectrum of bacteria.
Aim
The present study was performed to assess the in vitro effects of eugenol and cinnamaldehyde against indigenous and standard H. pylori strains, their minimum inhibitory concentrations (MICs) and time course lethal effects at various pH.
Methods
A total of 31 strains (29 indigenous and one standard strain of H. pylori ATCC 26695, one strain of E. coli NCIM 2089) were screened. Agar dilution method was used for the determination of drug sensitivity patterns of isolates to the commonly used antibiotics and broth dilution method for the test compounds.
Results
Eugenol and cinnamaldehyde inhibited the growth of all the 30 H. pylori strains tested, at a concentration of 2 μg/ml, in the 9th and 12th hours of incubation respectively. At acidic pH, increased activity was observed for both the compounds. Furthermore, the organism did not develop any resistance towards these compounds even after 10 passages grown at sub-inhibitory concentrations.
Conclusion
These results indicate that the two bioactive compounds we tested may prevent H. pylori growth in vitro, without acquiring any resistance.
Introduction
Helicobacter pylori was first discovered in 1982 and was subsequently found to be an important etiological agent for gastritis [1], peptic ulcers [2] and gastric malignancy [3]. It is estimated that one-half of the world's population is infected with H. pylori [4]. Numerous clinical evidences reveal that eradication of H. pylori results in improvement of gastritis and drastically decreases the rate of relapse of gastric and duodenal ulcers [5,6]. H. pylori carriage rates are about 80–90% in developing countries [7], with a high risk of gastric cancer and antibiotic regimens against H. pylori are frequently ineffective in such populations [8]. Besides this, undesirable side effects [9], noncompliance among the patients [10] and the cost of the antibiotic regimens [11] are few other factors for their ineffectiveness. Hence there is a need to develop alternative approaches to suppress/cure the infection.
Since ancient times, spices and condiments have been considered indispensable in the culinary arts, as they are used to flavor foods. Also these spices were recognized for their physiological and medicinal properties. It is presumed that the broad-spectrum effectiveness of these spices [12-14] may provide a suitable basis for new anti H. pylori therapies. Some of the epidemiological studies suggest an inverse relationship between the occurence of cancer of stomach (for which H. pylori infection is a known risk factor) and the intake of the allium vegetables [15,16]. There are reports on in vitro antimicrobial activity of many spice extracts (and not their active principles) on H. pylori [17,18]. But active principles of garlic and garlic components have been tried [11,19].
Eugenol, a phenolic compound is the main component of clove (Eugenia caryophillis) oil. Cinnamaldehyde is an aromatic aldehyde and main component of bark extract of cinnamon (Cinnamomum verum). Both these compounds are present in essential oils of many plants and are proved to be active against many pathogenic bacteria [20,21], fungi [21,22] and viruses [14]. There is a paucity of information on the effects of these bioactive compounds on H. pylori. Therefore, the present study was undertaken to evaluate the efficacy of eugenol and cinnamaldehyde as anti H. pylori agents.
Materials and methods
H. pylori strains
Thirty H. pylori strains and one E. coli strain (NCIM2089) were used in the study. Twenty-nine strains that were designated as MS-1 to MS-29 were isolated from gastric biopsy specimens (15 from duodenal ulcer, 10 from non ulcer dyspepsia and 4 from gastric ulcer subjects), after informed consents from patients who have undergone upper gastrointestinal endoscopy at Deccan College of Medical Sciences, Hyderabad, India. One strain of H. pylori (ATCC26695) was also included.
Primary isolation was performed on Brucella agar (Becton, Dickinson & Co, Sparks, USA), supplemented with 7% (V/V) sheep blood and antibiotics (Vancomycin, 6 mg/L; Amphoteracin B, 3 mg/L; Polymixin B, 2500 IU/L; Hi-Media, Mumbai, India). Final pH of the media was adjusted to 7.2. H. pylori was identified by its typical colony morphology, Gram staining, microaerophilic growth at 37°C and by other biochemical tests such as oxidase, catalase and urease. These strains were preserved at -80°C in brucella broth (Becton, Dickinson & Co, Sparks, USA) containing 10% (V/V) fetal calf serum (FCS) and 20% (V/V) glycerol.
Bioactive compounds
Commercial preparations of bioactive compounds such as eugenol and cinnamaldehyde were used in our experiments. All the strains were seeded on Brucella agar supplemented with 7% sheep blood and grown for 48 hours under microaerobic conditions. Bacterial growth was harvested from the plates and resuspended in sterile saline. The inoculum was prepared to contain ~108 CFU/ml by adjusting the suspension to match the McFarland No-4 turbidity standard [23].
Antibacterial activity of standard antibiotics
Minimum inhibitory concentrations (MICs) for antibiotics like metronidazole, amoxycillin and clarithromycin were determined by agar dilution method, using two fold increments (0.016 to 256 μg/ml) on Mueller Hinton agar (MHA) plates supplemented with 7% sheep blood and incubated for 3 days at 37°C in a microaerophilic cabinet. MICs were determined as the lowest concentration of the antibiotics inhibiting visible growth [24] using ~106 CFU of inoculum.
Antibacterial activity of eugenol and cinnamaldehyde
Primary screening
The Kirby-Bauer disk diffusion susceptibility test was used to check the activity of the bioactive compounds on H. pylori. The inoculum prepared as described previously was inoculated onto MHA supplemented with 7% sheep blood and bioactive compound discs (prepared by loading the respective compounds in variable concentration on to the sterile filter paper discs) were placed on to the plates with the sterile dispenser and incubated in microaerobic atmosphere at 37°C for 3 days.
Minimum inhibitory concentration (MIC) of bioactive compounds
As there was no established method to determine the antibacterial activity of bioactive compounds against H. pylori, serial two fold dilutions of eugenol and cinnamaldehyde were made in a 5 ml Brucella broth preparation (pH-7) with 0.1% dimethyl sulfoxide (DMSO; that enhances compound solubility) and supplemented with 2.5% fetal calf serum (FCS). Cotton-wool plugged 25 ml conical flasks of Brucella broth prepared as above, were inoculated with H. pylori (~106 CFU/ml)[11]. The flasks were incubated for 3–5 days in a microaerophilic cabinet with shaking at ~150 rpm. Following incubation, 100 μl aliquots of the medium were plated onto brucella agar plates supplemented with 7% sheep blood to determine the viable counts.
Time Course
Time course viability studies of the bioactive compounds were set up in the same way as the above MIC tests, but the broths were sampled hourly over the first 4 h and then again at 6, 9, 12, 24, 48 and 72 h to check the viable count. H. pylori ATCC26695 was used as the inoculum for the study.
For comparison, known antibiotic such as amoxycillin was used and E. coli (NCIM2089) was used as a standard bacterial control other than H. pylori.
Effects of bioactive compounds on H. pylori morphology
To check the effect of bioactive compounds on the morphology of H. pylori, slides were prepared from the above samples of first four hours, stained with Giemsa and observed under oil immersion.
Measurement of bactericidal activity at acidic pH
The effects of pH on the antibacterial activities of eugenol and cinnamaldehyde were done as reported previously [25,26]. The pH of the buffer was adjusted to 4.00 using 100 mM citrate buffer (pH 5.2) supplemented with 10 mM urea and the pH was adjusted to 7.00 using 10 mM phosphate buffer (pH 6.8). H. pylori ATCC26695 (~107 CFU/ml) was inoculated into 5 ml of the medium with 0.1% DMSO to which various concentrations of the compounds were added. All the flasks were incubated at 37°C. Twenty μL of the samples were taken at 0, 15, 30 and 60 minutes after inoculation to enumerate the number of viable cells.
Test for development of resistance to bioactive compounds
To investigate the development of resistance, five strains i.e. ATCC26695, MS-27 (sensitive to all the three antibiotics), MS-1 (resistant to all the three antibiotics), MS-8 (resistant to metronidazole, clarithromycin and sensitive to amoxycillin), and MS-15 (resistant only to metronidazole), were grown on agar at sub inhibitory concentrations (0.25 and 0.5 μg/ml) of eugenol and cinnamaldehyde for 10 passages. All the experiments stated above were repeated three times.
Results
Antibacterial activity against H. pylori
MICs of antibiotics for H. pylori were determined. Strain ATCC26695 was sensitive (MIC < 0.016 μg/ml) to all the three drugs tested. Twenty (66.7%) clinical isolates (MS1-8, 10, 12, 14 – 17, 20, 23 – 26 and MS28) from our laboratory were resistant to metronidazole (MICs > 8 μg/ml), 2 (6.7%) clinical isolates (MS1 & 8) were resistant to clarithromycin (MICs > 2 μg/ml) and 1 (3.3%) clinical isolate (MS1) was resistant to amoxycillin (MIC > 0.5 μg/ml) [27] (Table 1).
Table 1.
Sensitivity of H. pylori strains (n = 30) to different antibiotics
| Antibiotic | Sensitive (%) | Resistance (%) | Range (μg/ml) | MIC27 (μg/ml) |
| Metronidazole | 10 (33.3) | 20 (66.7) | <0.5 – >256 | >8 |
| Clarithromycin | 28 (93.3) | 2 (6.7) | <0.5 – >256 | >2 |
| Amoxycillin | 29 (96.7) | 1 (3.3) | <0.5 – >256 | >0.5 |
In the preliminary screening, both the compounds gave approximately 15 mm inhibition zones to all the clinical isolates and standard strain (H. pylori ATCC26695). This in vitro antimicrobial effect of the bioactive compounds demonstrated that both the compounds were capable of completely inhibiting the growth of all the strains (both sensitive & resistant) at a concentration of 2 μg/ml. For E. coli (NCIM-2089), MIC of eugenol and cinnamaldehyde was 1 μg/ml.
Time course
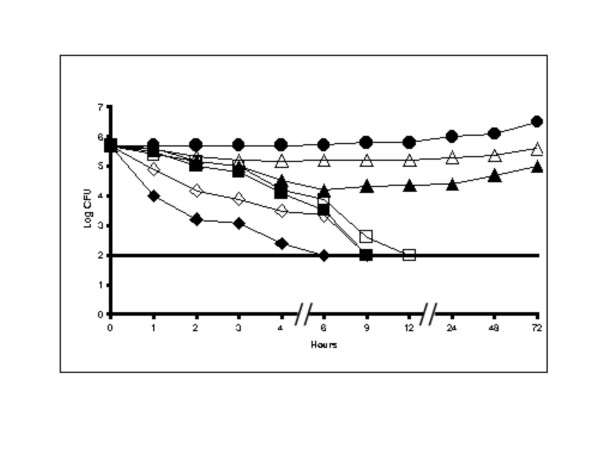
As both the compounds were active at a concentration of 2 μg/ml, we examined the killing curve time course of both the compounds at different concentrations i.e. 1 μg/ml, 2 μg/ml and 4 μg/ml. Eugenol completely inhibited the growth of H. pylori ATCC 26695 at 2 μg/ml concentration in 9 hours of incubation. The growth was completely inhibited in only 6 hours when the MIC was doubled (4 μg/ml). Some inhibition of growth was observed at the MIC of 1 μg/ml, where up to 6th hour a decrease of ~1.6 Log was observed then afterwards there was no effect of the compound on the bacterium (Figure 1). Cinnamaldehyde inhibited ATCC26695 totally at 2 μg/ml in 12 hours and in 9 hours at double the MIC (4 μg/ml). However, at half of the MIC (1 μg/ml), a little effect was observed up to the 3rd hour and there was a decrease of 0.6 Log with no subsequent inhibitory effect (Figure 1).
Figure 1.
Time course viability studies of eugenol and cinnamaldehyde with H. pylori ATCC26695. ATCC26695 was exposed to eugenol at concentrations of (◆) 4 μg/ml, (■) 2 μg/ml, (▴) 1 μg/ml, (●) control, and to cinnamaldehyde (◊) 4 μg/ml, (▫) 2 μg/ml, (△) 1 μg/ml. Detection limit was shown with a straight line. The controls were maintained with 0.1% DMSO.
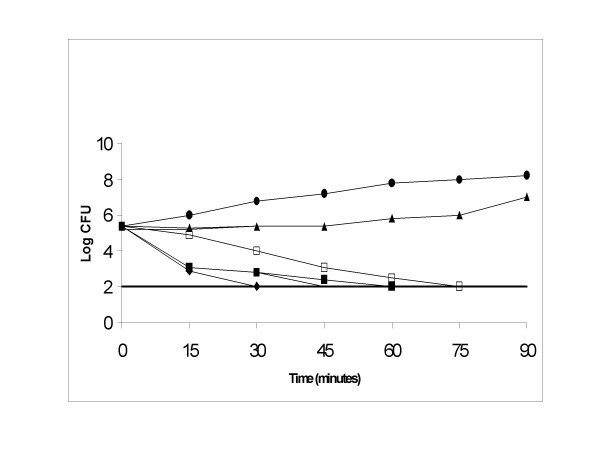
The time course lethal action of the bioactive compounds on E. coli (NCIM-2089) was studied at three different concentrations (1 μg/ml, 2 μg/ml & 0.5 μg/ml). At 1 μg/ml, eugenol inhibited E. coli after 1 hour of incubation whereas cinnamaldehyde inhibited E. coli after 75 minutes of incubation (Figure 2).
Figure 2.
Effects of eugenol and cinnamaldehyde on the growth of E. coli NCIM-2089. E. coli was exposed to eugenol at concentrations of (◆) 2 μg/ml, (■) 1 μg/ml, (▲) 0.5 μg/ml, (●) control, and to cinnamaldehyde at concentrations of (◊)2 μg/ml, (□) 1 μg/ml, (△) 0.5 μg/ml. Detection limit was shown with a straight line. The controls were maintained with 0.1% DMSO.
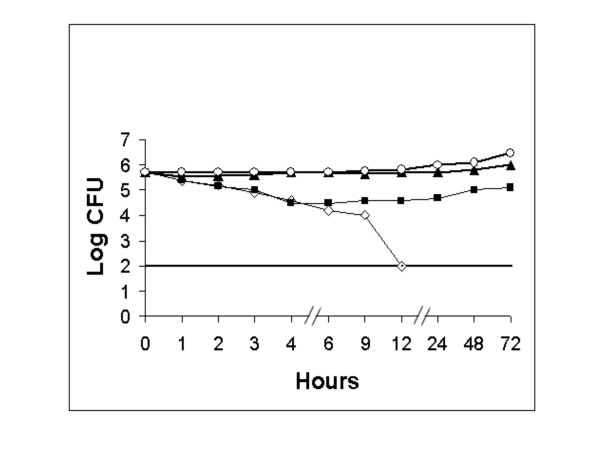
For comparing the efficacy of our bioactive compounds against H. pylori, we studied the effect of a common antibiotic (amoxycillin) at different concentrations such as 1 μg/ml, 0.1 μg/ml and 0.01 μg/ml with an MIC of < 0.016 μg/ml. There was no inhibition of H. pylori seen at the concentration of 0.01 μg/ml. But inhibition was noticed at a concentration of 0.1 μg/ml up to the 4th hour of incubation. However, the bacterium was completely inhibited at a concentration of 1 μg/ml, by the 12th hour of incubation (Figure 3).
Figure 3.
Effect of amoxicillin on the growth of H. pylori (ATCC26695). ATCC26695 was exposed to at concentrations of (◊) 1 μg/ml, (■) 0.1 μg/ml, (▴) 0.01 μg/ml, (○) control. Detection limit was shown with a straight line.
H. pylori morphology
Microscopic observation of slides revealed that at high concentrations of both the bioactive compounds (4 μg/ml) and after 4 hours of incubation, ~70% of the cells assumed coccoid forms with fewer spiral shaped organisms.
Effect of the bioactive compounds on the growth of H. pylori in buffers at acidic pH
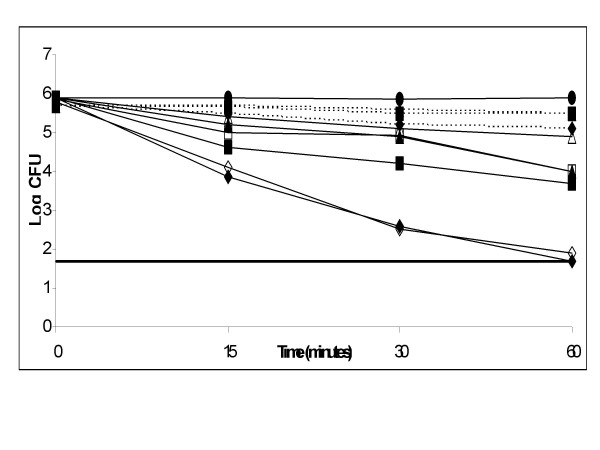
The viability of strain ATCC 26695 at acidic pH was investigated using eugenol and cinnamaldehyde at various concentrations (2 μg/ml, 4 μg/ml and 1 μg/ml). As shown in Figure 4, both the compounds revealed increased activity at acidic pH (pH-4.0) and a dose dependant bactericidal activity was also observed. At a concentration of double the MIC (4 μg/ml), eugenol was found to completely inhibit the bacteria at about 1 hour of incubation. Cinnamaldehyde also revealed bactericidal activity in the similar manner but did not inhibit the organism totally (after one hour of incubation there was a difference of 0.2 Log). For both the compounds, when compared at acidic and neutral pH, we found a Log difference of 1.5 after one hour of incubation at MIC of 2 μg/ml.
Figure 4.
Effect of eugenol and cinnamaldehyde on the growth of H. pylori (ATCC26695) at low pH. ATCC26695 was exposed to eugenol at pH4 (solid lines) and pH7 (dotted lines) at concentrations (◆ or ◊) 4 μg/ml, (■ or □) 2 μg/ml, (ß or △) 1 μg/ml, (●) control. Detection limit was shown as a straight line. The controls were maintained with 0.1% DMSO.
Test for resistance to eugenol and cinnamaldehyde
We investigated whether H. pylori would acquire resistance to the bioactive compounds. Selected H. pylori strains (ATCC26695, MS-27, MS-1, MS-8 and MS-15) did not acquire resistance to both the bioactive compounds at sub inhibitory concentrations (0.25 and 0.5 μg/ml) even after 10 passages.
Discussion
Eradication of H. pylori with monotherapy is difficult and often results in poor clinical outcome [28]. Presently a proton pump inhibitor (PPI) based triple therapy is being used. Though it is having a success rate of 80 to 90% [29], problems like treatment failure and contraindications for some patients are common. Furthermore, the major problem in treating H. pylori infection is rapidly emerging drug resistance during treatment with various antibiotics [30]. Because of the prevalence of antibiotic-resistant H. pylori strains, the search for safe and effective non-antibiotic agents is essential.
In Indian traditional medical system, a number of plants and plant products are known to possess potent medicinal properties, suggesting that plants, plant products and their extracts may be useful for specific medical conditions. Hence in our effort to identify newer compounds, which can inhibit H. pylori growth, we tested eugenol and cinnamaldehyde. These compounds have already been found to inhibit 10 different multi drug resistant pathogenic bacteria such as E. coli, Staphylococcus, Proteus, Klebsiella, Enterobacter, and Pseudomonas isolated from human subjects [20,21]. In our studies, eugenol and cinnamaldehyde completely inhibited all the strains (both sensitive & resistant) at a concentration of 2 μg/ml. This inhibitory concentration is very less when compared against the previously reported MICs {allicins: 6–12 μg/ml, garlic oil: 8–32 μg/ml [11] and ajoenes: 10–25 μg/ml [31]}. Our compounds also proved to be more potent than thiosulfinate {40 μg/ml [19]}, vinyldithiins {<100 μg/ml [31]}, epigallocatechin gallate {50–100 μg/ml [32]} and garlic powder {250 μg/ml [11]}.
In time course viability studies, we found that eugenol and cinnamaldehyde inhibited H. pylori ATCC26695 at 2 μg/ml concentration within 9 and 12 hours of incubation respectively. Highly potent effects of some essential oils of lemon grass on H. pylori have been previously described [26]. However, our observations may be more accurate with the fact that we used active principles and not the extracts or oils as used by Ohno et al., [26]. These essential oils may contain unknown substances that might provide synergistic, inhibitory effects. Eugenol in our studies was found to be a potent antimicrobial than the essential oils of Ohno and colleagues [26] whereas the effect of cinnamaldehyde was approximately equivalent to the oils used by them. Further, our compounds were more potent than the common antibiotic amoxycillin, which inhibited H. pylori ATCC26695 at 12th hour (similar to cinnamaldehyde) whereas eugenol inhibited microbial growth at the 9th hour of incubation itself, at its minimum inhibitory concentration. Amoxycillin was found to completely inhibit bacteria at a higher concentration (1 μg/ml), than it's minimum inhibitory concentration (0.016 μg/ml) (Figure 3). As H. pylori resides in the stomach, where pH levels are lower than 3.0, it is important to check the activity and stability of a compound at acidic pH. In our study, H. pylori isolates survived at pH 4.0, despite some reports showing H. pylori survival at pH-3.0 [25], and bactericidal effects of the bioactive compounds were found to be stronger even at low pH. Several agents that have bactericidal effects against H. pylori are dependent on pH (for example, ecabet sodium is active at acidic pH [25] and tea cathechin is active at alkaline pH [33]).
Furthermore, we found that the organism did not develop any resistance to the test compounds even after 10 successive passages grown at sub-inhibitory concentrations (0.25 and 0.5 μg/ml) [21], whereas H. pylori strains acquired resistance to amoxycillin and clarithromycin after 10 sequential passages as reported previously [26,34]. In addition, these bioactive compounds had equivalent bactericidal activities against both the susceptible and resistant H. pylori strains.
Eugenol and cinnamaldehyde form a part of regular diet in tropical countries since ages. Many reports suggest these compounds are nontoxic at doses being consumed [34], and they are known for their antimicrobial and antioxidant properties [21,36]. Moreover, to heal peptic ulcer, or to prevent the oxidative stress, prescribing antioxidants is quite a normal practice these days with the medical practitioners. As both these compounds are natural antioxidant [37], their benefits could be multiple. Two animal studies carried out in the past suggest that an extract of cinnamon bark taken orally may help prevent stomach ulcer [36] and one study described eugenol to be both anti-inflammatory and antiulcerogenic [38]. It was used as early as 1950 for treating ulcer disease [39]. Given all the advantages we observed for the two bioactive compounds tested, our data should not be construed as certification of the compounds in clinical application until their stability, specificity and overall efficacy is established in different laboratories and against different bacterial strains. Further studies are therefore clearly needed in the context of reproducibility of these findings under different laboratory conditions.
Conclusion
The data obtained in this study show that eugenol and cinnamaldehyde at 2 μg/ml produce significant decrease of viability of the organism H. pylori, irrespective of the strain. Activity of these compounds at low pH levels may help them achieve their efficacy in an environment such as human stomach. We trust these findings are encouraging in view of growing treatment failures and antibiotic resistance in the area of H. pylori management. Additional in vivo studies and clinical trials would be needed to justify and further evaluate the potential of these compounds as anti H. pylori agents.
Acknowledgments
Acknowledgements
SMA, HP, LVR and CMH would like to thank University Grants Commission, New Delhi for providing financial support to carryout this investigation. NA and LAS would like to thank the ISOGEM http://www.isogem.org for the support.
Contributor Information
Shaik Mahaboob Ali, Email: alimicro@rediffmail.com.
Aleem A Khan, Email: aleem_a_khan@rediffmail.com.
Irshad Ahmed, Email: sirfirshadahmed@gmail.com.
M Musaddiq, Email: sirfirshadahmed@gmail.com.
Khaja S Ahmed, Email: khajashakeel@gmail.com.
H Polasa, Email: hpolasa@yahoo.co.in.
L Venkateswar Rao, Email: vrlinga@yahoo.com.
Chittoor M Habibullah, Email: cmhabib@rediffmail.com.
Leonardo A Sechi, Email: sechila@uniss.it.
Niyaz Ahmed, Email: niyaz@cdfd.org.in.
References
- Warren JR, Marshall BJ. Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet. pp. 1273–1275. 1983 Jun 4. [PubMed]
- Dixon MF. Helicobacter pylori and peptic ulceration: histopathological aspects. J Gastroenterol Hepatol. 1991;6:125–130. doi: 10.1111/j.1440-1746.1991.tb01451.x. [DOI] [PubMed] [Google Scholar]
- Parsonnet J, Friedman GD, Daniel MS, Vandersteen DP, Chang Y, Vogelman JH, Orentreich N, Sibley RK. Helicobacter pylori infection and the risk of gastric carcinoma. N Engl J Med. 1991;325:1127–1131. doi: 10.1056/NEJM199110173251603. [DOI] [PubMed] [Google Scholar]
- EUROGAST study group An International Association between Helicobacter pylori infection and Gastric Cancer. Lancet. 1993;341:1359–1362. doi: 10.1016/0140-6736(93)90938-D. [DOI] [PubMed] [Google Scholar]
- Marshall BJ, Goodwin CS, Warren JR, Murray R, Blincow ED, Blackbourn SJ, Phillips M, Waters TE, Sanderson CR. Prospective double blind trail of duodenal ulcer relapse after eradication of Campylobacter pylori. Lancet. 1988;2:1437–1442. doi: 10.1016/S0140-6736(88)90929-4. [DOI] [PubMed] [Google Scholar]
- Rauws EA, Langenberg W, Houthoff HJ, Zanen HC, Tytgat GN. Campylobacter pyloridis -associated chronic active antral gastritis. A prospective study of its prevalence and the effects of antibacterial and antiulcer treatment. Gastroenterol. 1988;94:33–40. [PubMed] [Google Scholar]
- Lacy BE, Rosemore J. Helicobacter pylori :ulcers and more: the beginning of an era. J Nutr. 2001;131:2789S–2793S. doi: 10.1093/jn/131.10.2789S. [DOI] [PubMed] [Google Scholar]
- Buiatti E, Munoz N, Vivas J, Cano E, Peraza S. Difficulty in eradicating Helicobacter pylori in a population at high risk for gastric cancer in Venezuela. Cancer Causes Control. 1994;5:249–254. doi: 10.1007/BF01830244. [DOI] [PubMed] [Google Scholar]
- Bell GD, Powell KU, Burridge SM, Bowden AN, Ramesh B, Bolten G, Purser K, Harrison G, Brown C, Gant PW, Jones PH, Trowell JE. Helicobacter pylori eradication: efficacy and side effect profile of a combination of omeprazole, amoxycillin and metronidazole compared with four alternative regimens. Q J Med J Assoc Phys. 1993;86:743–750. [PubMed] [Google Scholar]
- Broutet N, Tchamgoue S, Pereira E, Lamouliatte H, Salamon R, Megraud F. Risk factors for failure of Helicobacter pylori therapy-results of an individual data analysis of 2751 patients. Aliment Pharmacol Ther. 2003;17:99–109. doi: 10.1046/j.1365-2036.2003.01396.x. [DOI] [PubMed] [Google Scholar]
- O'Gara EA, Hill DJ, Maslin DJ. Activities of garlic oil, garlic powder, and their diallyl constituents against Helicobacter pylori. Appl Environ Microbiol. 2000;66:2269–2273. doi: 10.1128/AEM.66.5.2269-2273.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman A, Choudhary I, Farooq A, Ahmed A, Iqbal Z, Demirci B, Demirci F, Can Baser KH. Anti fungal activities and essential oil constituents of some spices from Pakistan. 3rd International Electronic Conference on Synthetic Organic Chemistry [ECSOC-3] 1999. http://pages.unibas.ch/mdpi/ecsoc-3/posters.htm
- Arora DS, Kaur J. Antimicrobial activity of spices. Int J Antimicrob agents. 1999;12:257–262. doi: 10.1016/S0924-8579(99)00074-6. [DOI] [PubMed] [Google Scholar]
- Pacheco P, Sierra J, Schmeda-Hirschmann G, Potter CW, Jones BM, Moshref M. Antiviral activities of Chilean medicinal plant extracts. Phytother Res. 1993;7:415–418. [Google Scholar]
- Buiatti E, Palli D, Decarli A, Amadori D, Avellini C, Bianchi S, Biserni R, Cipriani F, Cocco P, Giacosa A, Marubini E, Puntoni R, Vindigni C, Fraumeni J, Blot W. A Case-Control study of Gastric Cancer and Diet in Italy. Int J Cancer. 1989;44:611–616. doi: 10.1002/ijc.2910440409. [DOI] [PubMed] [Google Scholar]
- You WC, Blott WJ, Chang YS, Ershow AG, Yang ZT, An Q, Henderson B, Xu GW, Fraumeni JF, Jr, Wang TG. Diet and High Risk of Stomach Cancer in Shandong, China. Cancer Res. 1988;48:3518–3523. [PubMed] [Google Scholar]
- Tabak M, Armon R, Neeman I. Cinnamon extracts inhibitory effect on Helicobacter pylori. J Ethnopharmacol. 1999;67:269–277. doi: 10.1016/S0378-8741(99)00054-9. [DOI] [PubMed] [Google Scholar]
- Tabak M, Armon R, Potasman I, Neeman I. In vitro inhibition of Helicobacter pylori by extracts of thyme. J Appl Bacteriol. 1996;80:667–672. doi: 10.1111/j.1365-2672.1996.tb03272.x. [DOI] [PubMed] [Google Scholar]
- Sivam GP, Lampe JW, Ulness B, Swanzy SR, Potter JD. Helicobacter pylori – In vitro susceptibility to garlic (Allium sativum) extract. Nutr Cancer. 1997;27:118–121. doi: 10.1080/01635589709514512. [DOI] [PubMed] [Google Scholar]
- Suresh P, Ingle VK, Vijaya Lakshmi V. Antibacterial activity of Eugenol in comparison with other antibiotics. J Fd Sci Technol. 1992;29:254–256. [Google Scholar]
- Shashidar NS. Ph D thesis. Dept. of Microbiology, Osmania University, Hyderabad; 2002. Studies on bioactive natural compounds for their Antimicrobial and antioxidant properties. [Google Scholar]
- Bilgrami KS, Sinha KK, Sinha AK. Inhibition of aflatoxin production and growth of Aspergillus flavus by eugenol & onion & garlic extracts. Indian J Med Res. 1992;96:171–175. [PubMed] [Google Scholar]
- McNulty C, the PHLS Helicobacter Working Group. Owen R, Tompkins D, Hawtin P, Mc Coll K, Price A, Smith G, Teare L. Helicobacter pylori susceptibility testing by disc diffusion. J Antimicrob Chemother. 2002;49:601–609. doi: 10.1093/jac/49.4.601. [DOI] [PubMed] [Google Scholar]
- Anita POG, Marcelo LR, Yune HBB, Lea V, Maira de CBM, Sergio M, Pedrazzoli J., Jr Analysis of antimicrobial susceptibility and virulence factors in Helicobacter pylori clinical isolates. BMC Gastroenterol. http://www.biomedcentral.com/1471-230X/3/20 [DOI] [PMC free article] [PubMed]
- Shibata K, Ito Y, Hongo A, Yasoshima A, Endo T, Ohashi M. Bactrericidal activity of a new antiulcer agent, ecabet sodium, against Helicobacter pylori under acidic conditions. Antimicrob Agents Chemother. 1995;39:1295–1299. doi: 10.1128/aac.39.6.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno T, Kita M, Yamaoka Y, Imamura S, Yamamoto T, Mitsufuji S, Kodama T, Kashima K, Imanishi J. Antimicrobial activity of essential oils against Helicobacter pylori. Helicobacter. 2003;8:207–215. doi: 10.1046/j.1523-5378.2003.00146.x. [DOI] [PubMed] [Google Scholar]
- Glupczynski Y, Megraud F, Anderson LP, Lopez-Brea M. Antibiotic susceptibility of Helicobacter pylori in Europe in 1998: results of the third multicentre study. Gut. 1999;45:A3. [Google Scholar]
- Glupczynski Y, Burette A. Drug therapy for Helicobacter pylori infection: Problems and pitfalls. Am J Gastroenterol. 1990;85:1545–1551. [PubMed] [Google Scholar]
- Peitz U, Menegatti M, Vaira D, Malfertheiner P. The European meeting on Helicobacter pylori : therapeutic news from Lisbon. Gut. 1998;43:S66–69. doi: 10.1136/gut.43.2008.s66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alarcon T, Domingo D, Lopez-Brea M. Antibiotic resistance problems with Helicobacter pylori. Int J Antimicrob Agents. 1999;12:19–26. doi: 10.1016/S0924-8579(99)00051-5. [DOI] [PubMed] [Google Scholar]
- Ohta R, Yamada N, Kaneko H, Ishikawa K, Fukuda H, Fujino T, Suzuki A. In Vitro Inhibition of the growth of Helicobacter pylori by Oil-Macerated Garlic Constituents. Antimicrob Agents Chemother. 1999;43:1811–1812. doi: 10.1128/aac.43.7.1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee YK, Koo MW. Anti – Helicobacter pylori activity of Chinese tea: in vitro study. Alimient Pharmacol Ther. 2000;14:635–638. doi: 10.1046/j.1365-2036.2000.00747.x. [DOI] [PubMed] [Google Scholar]
- Mabe K, Yamada M, Oguni I, Takahashi T. In vitro and in vivo activities of tea cathechins against Helicobacter pylori. Antimicrob Agents Chemother. 1999;43:1788–1791. doi: 10.1128/aac.43.7.1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLoney CR, Schiller NL. Characterization of an In Vitro-Selected Amoxicillin-Resistant Strain of Helicobacter pylori. Antimicrob Agents Chemother. 2000;44:3368–3373. doi: 10.1128/AAC.44.12.3368-3373.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAO . Technical Report Series no 20. FAO/WHO Expert Committee on Food Additives; 1982. Evaluation of certain food additives and contaminants. [Google Scholar]
- Blumenthal M, ed . The Complete Commission E Monographs, Therapeutic Guide Herbal Medicines. Boston, Mass: Integrative MedicineCommunications; 1998. p. 110. [Google Scholar]
- Juliani HR, Simon JE, Ramboatiana MMR, Behra O, Garvey A, Raskin I. Malagasy aromatic plants: Essential oils, antioxidant and antimicrobial activities. XXVI International Horticultural Congress: The Future for Medicinal and Aromatic Plants http://www.actahort.org/members/showpdf?booknrarnr=629_9
- Capasso R, Pinto L, Vuotto ML, Di Carlo G. Preventive effect of eugenol on PAF and ethanol-induced gastric mucosal damage. Fitoterapia. 2000;71:S131–137. doi: 10.1016/S0367-326X(00)00188-X. [DOI] [PubMed] [Google Scholar]
- Bandes J, Samuels NA, Hollander F, Goldfischer RL, Winkelstein A. The clinical response of patients with peptic ulcer to a topical mucigogue (eugenol) Gastroenterol. 1951;18:391–399. [PubMed] [Google Scholar]