Abstract
Mutations in the transcription factor Sox10 or Endothelin Receptor B (Ednrb) result in Waardenburg Syndrome Type IV (WS-IV), which presents with deficiencies of neural crest derived melanocytes (hypopigmentation) and enteric ganglia (hypoganglionosis). As Sox10 and Ednrb are expressed in mouse migratory neural crest cells and melanoblasts, we investigated the possibility that SOX10 and EDNRB function through a hierarchical relationship during melanocyte development. However, our results support a distinct rather than hierarchical relationship. First, SOX10 expression continues in Ednrb null melanoblasts, demonstrating that SOX10 expression is not dependent on EDNRB function. Second, Ednrb expression persists in E10.5 Sox10 null embryos, demonstrating that Ednrb is not dependent on SOX10 for expression in migratory neural crest cells. Third, over-expression of SOX10 in melanoblasts of mice that harbor null or hypomorphic Ednrb alleles does not rescue hypopigmentation, suggesting that SOX10 overexpression can neither complement a lack of EDNRB function nor increase Ednrb expression. Fourth, mice that are double heterozygous for loss-of-function mutations in Sox10 and Ednrb do not demonstrate synergistically increased hypopigmentation compared to mice that are single heterozygotes for either mutation alone, suggesting a lack of direct genetic interaction between these genes. Our results suggest that SOX10 does not directly activate Ednrb transcription in the melanocyte lineage. Given that SOX10 directly activates Ednrb in the enteric nervous system, our results suggest that SOX10 may differentially activate target genes based on the particular cellular context.
INTRODUCTION
A number of human diseases are associated with improper neural crest development (Gammill and Bronner-Fraser, 2003; Le Douarin et al., 2004). Among these, Waardenburg syndrome (WS) is characterized by neurosensory deafness and pigmentation anomalies in the skin, hair and iris (Baxter et al., 2004; Read, 2000; Tachibana et al., 2003). Four types of WS have been classified which share the common features of hereditary auditory-pigmentary abnormalities. Different subtypes are distinguished from one another based on additional symptoms. Individuals with WS type 4 (WS4) present with aganglionosis of the distal colon (referred to as Hirschsprung Disease) in addition to neurosensory deafness and pigment cell loss. WS4 has been associated with mutations in the HMG-box transcription factor Sox10 (Kuhlbrodt et al., 1998; Southard-Smith et al., 1998), the seven transmembrane G protein-coupled receptor endothelin receptor B (Ednrb) (Hosoda et al., 1994), and EDNRB ligand, endothelin 3 (Edn3) (Baynash et al., 1994). This study focuses on the genetic pathways that underlie the pigmentation defects in WS4.
SOX10 is important for both the survival of NC-derived precursor cells and their differentiation to melanocytes and glia (Mollaaghababa and Pavan, 2003). Thus, mice heterozygous for a spontaneous Sox10 mutation (Dominant megacolon, Sox10Dom) or a targeted mutation (Sox10LacZ) present with regional hypopigmentation (head and belly spots) and enteric aganglionosis (Britsch et al., 2001; Southard-Smith et al., 1998). A disruption of melanocyte development can be detected by E10.5 in Sox10Dom homozygous embryos, indicated by the absence of melanoblast markers Dopachrome tautomerase (Dct) and Microphthalmia-associated transcription factor (Mitf) (Potterf et al., 2001; Southard-Smith et al., 1998), both direct targets of SOX10 transcriptional regulation (Bondurand et al., 2000; Britsch et al., 2001; Jiao et al., 2004; Lee et al., 2000; Ludwig et al., 2004; Potterf et al., 2000; Potterf et al., 2001; Verastegui et al., 2000). Consistent with this, Dct expression is significantly reduced in SOX10 haploinsufficent embryos, suggesting that SOX10 target genes are sensitive to its expression levels.
EDNRB is a G-protein coupled receptor that responds to EDN3 and endothelin-1 (EDN1) ligands (McCallion and Chakravarti, 2001). Ednrb exhibits overlapping embryonic expression patterns with Sox10 (Lee et al., 2003; Southard-Smith et al., 1998), and, similar to Sox10, is important for the development of neural crest-derived melanocytes and enteric ganglia (Pla and Larue, 2003). A number of Ednrb alleles have been characterized in mouse (Hosoda et al., 1994; Matsushima et al., 2002; Shin et al., 1997). Ednrbs–l (piebald-lethal) is a null allele carrying a deletion of the Ednrb coding sequence and flanking genomic DNA (Hosoda et al., 1994). Mice homozygous for Ednrbs–l are almost completely white and develop megacolon. Ednrbs (piebald) is a hypomorphic allele, encompassing a wild type coding sequence and with the ability to produce structurally normal mRNA (Hosoda et al., 1994). However, the Ednrb mRNA expression level is reduced to about 28% of the wild type level in Ednrbs/s animals (Hosoda et al., 1994). Reportedly, the piebald mutation corresponds to a retrotransposon insertion within the first intron of Ednrb, introducing a cryptic splice site between the first exon and the insert that leads to formation of abnormal transcripts at the expense of appropriately spliced mRNA (Ohtani S et al., 2004). Unlike the Ednrbs–l allele, mice homozygous for the Ednrbs allele rarely present with megacolon (Hosoda et al., 1994). While Ednrbs–l heterozygotes (Ednrbs–l/+) manifest haploinsufficiency as a white belly spot with variable penetrance, compound heterozygous Ednrbs–l/s mice show a further reduction in Ednrb expression level and demonstrate a vastly hypopigmented ventral surface and extensive dorsal white spotting.
It has been shown that EDNRB is transiently required between E10.5 and E12.5 for proper melanocyte development (Pavan and Tilghman, 1994; Shin et al., 1999), overlapping the time when a requirement for SOX10 in melanocyte development first becomes evident. However, MITF- and DCT-positive melanoblasts are present in Ednrb null embryos, albeit at reduced numbers (Hou et al., 2004b; Lee et al., 2003). Therefore, unlike SOX10, EDNRB is not required for the initial specification of melanoblasts. In the gut, a role for EDNRB in both the generation and migration of NCSCs has been established (Kruger et al., 2003). Recently SOX10 has also been reported to directly bind to and activate Ednrb in the enteric nervous system (Zhu et al., 2004); however, an interaction in melanocyte development was not investigated. Here, we present evidence that while EDNRB is essential for the development of two distinct neural crest derivatives, enteric ganglia and melanocytes, its transcriptional activation by SOX10 may be differentially regulated in these two cell types.
RESULTS
Ednrb and Sox10 have similar expression patterns, but Ednrb expression in migratory neural crest is not dependent on Sox10 function
In situ hybridization was used to examine Ednrb expression in the neural crest of mouse embryos at E10.5 and E11.5 (Figure 1). In E10.5 wild type embryos, Ednrb transcripts can be observed in migratory neural crest along the rostral to caudal axis (Figure 1A). This distribution is similar to that of Sox10 visualized by in situ hybridization (Southard-Smith et al., 1999) and by activity of β-galactosidase expressed from the endogenous Sox10 promoter (Sox10LacZ) in a Sox10 knock-in allele (Britsch et al., 2001) (Figure 1C). In E11.5 wild type embryos, a subset of Ednrb expression is observed in neural crest derivatives which also express Sox10 (Figure 1E and F versus 1G and H). These include a subset of the cranial nerves, the dorsal root ganglia, the sympathetic chain and melanoblasts. Notably, Ednrb+ cells were located rostral and dorsal to the eye, and dorsal to the hindlimbs, locations similar to that observed for Sox10+ melanoblasts (Figure 1G and H, and Figure 3D).
Figure 1.
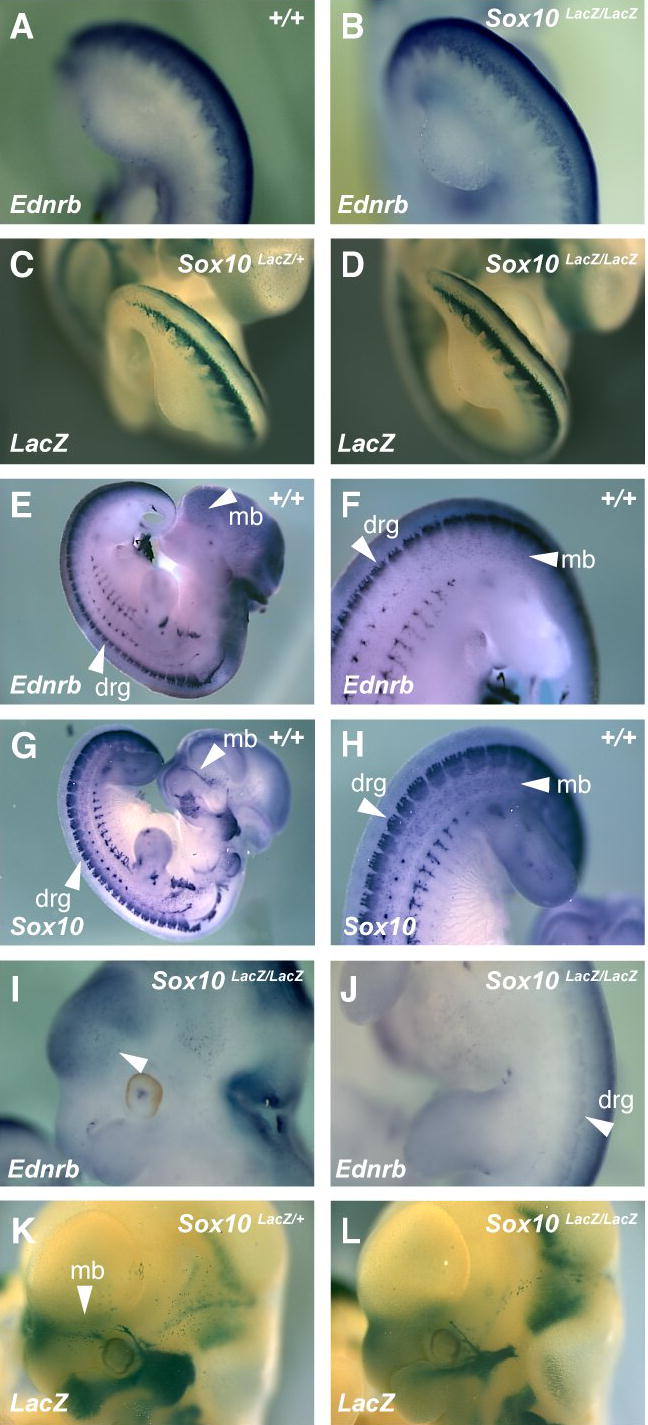
Ednrb and Sox10 have similar patterns of expression in neural crest cells at E10.5 and E11.5. Neural crest gene expression was examined by in situ hybridization for Ednrb (A, B, E, F, I and J), by in situ hybridization for Sox10 (G and H), and by β-galactosidase expression for Sox10 (C,D,K and L). Embryos were analyzed at E10.5 (A – D) and at E11.5 (E – L). Embryos were wild type (A, E–H), Sox10LacZ/+ (C and K), or Sox10LacZ/LacZ (B, D, I, J and L). The expression of Ednrb and Sox10 are similar in migratory neural crest at E10.5 (A versus C) and not significantly reduced in Sox10LacZ/LacZ embryos (A versus B). In E11.5 wild type embryos, similar expression patterns of Ednrb and Sox10 are observed in the dorsal root ganglia (drg) and melanoblasts (mb) (E and F versus G and H). While Ednrb expression is vastly reduced in E11.5 homozygous Sox10LacZ/LacZ embryos, punctate cells are still present dorsal to the eye (white arrow in I) and in the drg (white arrow in J). While Sox10-expressing cells are observed rostral to the eye at 11.5 in Sox10LacZ/+ embryos (K), they are vastly reduced in Sox10LacZ/LacZ embryos (L). Genotypes of embryos are shown in upper right of each panel. For in situ hybridizations, probe used is shown in lower left of panel. Panels marked with LacZ show β-galactosidase stained embryos.
Figure 3.
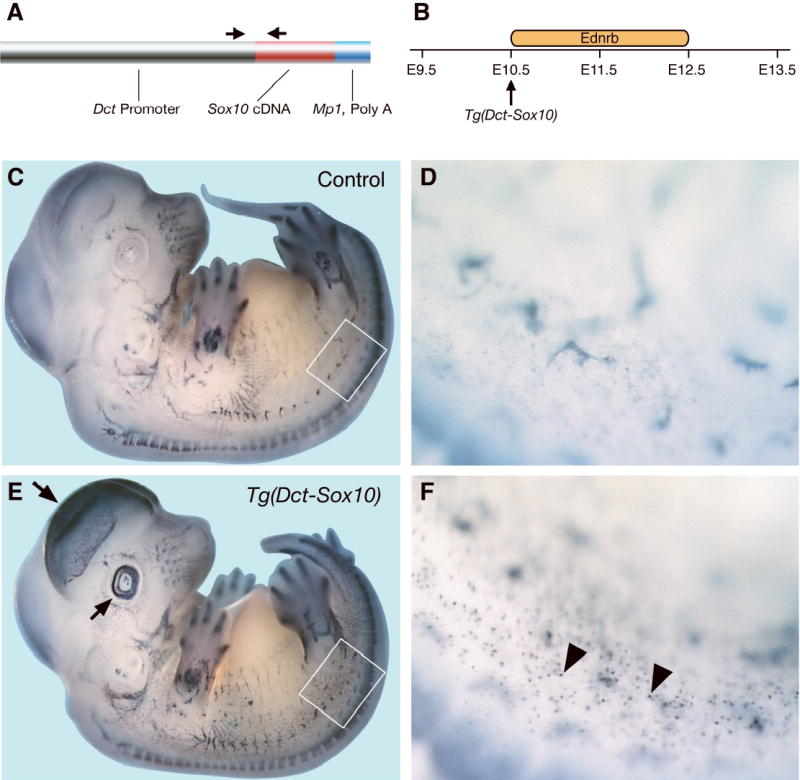
The transgenic mouse line Tg(Dct-Sox10) overexpresses SOX10 in melanoblasts in the time frame critical for EDNRB function. (A) Schematic drawing of Tg(Dct-Sox10) construct. The arrows indicate locations of PCR primers used for genotyping transgenic animals. (B) Time-line for commencement of Sox10 transgene expression during embryogenesis with respect to the time-window that is critical for Ednrb function. (C–F) Whole mount RNA in-situ hybridization for Sox10 expression in non-transgenic and sibling transgenic embryos at the E11.5 stage: (C) Non-transgenic embryo (+/+); (D) Higher magnification of the area in panel C delineated by a white box; (E) Transgenic embryo (Tg(Dct-Sox10)/+); (F) Higher magnification of the area in panel E delineated by a white box. Arrows indicate transgene expression in the eyes and the telencephalon. Arrowheads show Sox10 over-expression in melanoblasts of transgenic embryos compared to non-transgenic siblings (compare panels D and F).
To examine if the expression of Ednrb in neural crest is dependent on Sox10 function, in situ hybridization was used to examine Ednrb transcripts in Sox10 deficient embryos (Figure 1). At E10.5, Sox10-deficient neural crest cells are still present in Sox10 null embryos (Southard-Smith et al., 1999) and can be visualized by the expression of β-galactosidase (Figure 1D). Ednrb transcripts were clearly present in migratory neural crest cells of Sox10 null embryos (Figure 1B) and Sox10 heterozygous embryos (not shown). The observation of Ednrb transcripts in neural crest cells that are null for Sox10 indicates that functional SOX10 is not required for Ednrb expression in migratory neural crest at E10.5. At E11.5, Ednrb expression in Sox10-null embryos is still detectable in neural crest derivatives (such as the dorsal root ganglia), but the in situ hybridization signal is greatly reduced (Figure 1I and J). Assessment of Ednrb expression in Sox10-null melanoblasts is not feasible because two lines of evidence indicate that these cells are missing. First, the melanoblast markers Dct, Si (Pmel17) and Mitf are not observed in locations corresponding to melanoblasts in E11.5 Sox10 null embryos (data not shown and (Potterf et al., 2001)). Secondly, in Sox10LacZ/LacZ embryos, Sox10 null melanoblasts, which would be identified by activity of β-galactosidase, are not observed in these regions (Figure 1L). The reduced Ednrb in situ signal in neural crest derivatives is consistent with the neural crest cells undergoing apoptosis at this time of embryonic development (Kapur, 1999; Sonnenberg-Riethmacher et al., 2001). While a few Ednrb expressing cells can be observed in the ectoderm of the forebrain in Sox10 null embryos (Figure 1I), these cells are not in locations typical for pMel17- or Dct-expressing melanoblasts (Baxter and Pavan, 2003). Moreover, Ednrb+ cells are observed in a similar location in E11.5 Mitf deficient embryos (not shown), suggesting that these forebrain Ednrb+ cells are not presumptive melanoblasts. In summary, our data demonstrate that Ednrb expression is not dependent on Sox10 function in several neural crest derivatives, including migratory neural crest cells at E10.5, a time point at which Ednrb function is essential for melanocyte development (Shin et al., 1999).
Sox10 and Ednrb are co-expressed in neural crest cells, but Sox10 expression is not dependent on EDNRB function
The expression patterns of Sox10 and Ednrb are similar in early neural crest and melanoblast development; however, it has not been determined if the two genes are expressed in the same cells. To determine if Sox10 and Ednrb are co-expressed in neural crest cells, we examined SOX10 expression in neural tube explant cultures isolated from embryos where Ednrb expression is marked by expression of beta-galactosidease from an EdnrbLacZ knock-in allele (Lee et al., 2003). Neural tube explants from EdnrbLacZ/+ embryos were cultured for 48 hours and double-stained for β-galactosidase and SOX10 (Figure 2A). Analysis of these cultures demonstrated co-localization of the two signals in a majority of the cells, indicating the co-expression of Sox10 and Ednrb in neural crest-derived cells.
Figure 2.
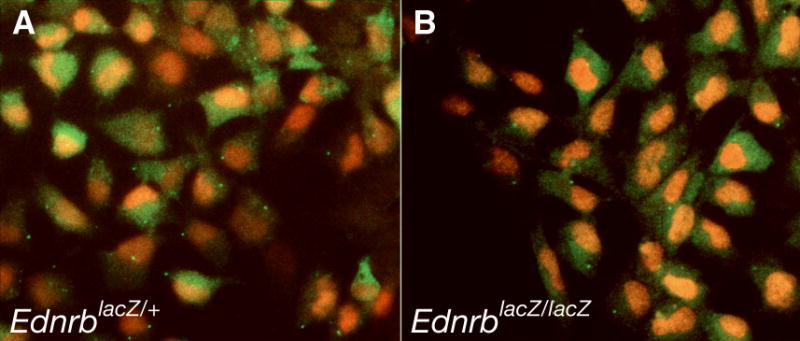
Sox10 and Ednrb are co-expressed in cultured neural crest cells. Double staining of neural tube explant cultures with anti-SOX10 (red) and anti-β-galactosidase (green) antibodies. (A) Heterozygous EdnrbLacZ/+ neural crest progenitors demonstrating co-expression of products from the Sox10 and EdnrbLacZ genes. (B) Homozygous EdnrbLacZ/LacZ neural crest progenitors demonstrating continued expression of SOX10 in the absence of EDNRB.
To assess if SOX10 and EDNRB are co-expressed in melanoblasts, MITF expression was assessed in Sox10 and Ednrb expressing neural crest. Five neural tube explant cultures from wild-type embryos were established and in all cultures 100% of MITF-positive cells were SOX10-positve. Also, four neural tube explant cultures from EdnrbLacZ/+ embryos were established and in all cultures 100% of MITF-positive cells were β-galactosidase-positive. Therefore, combining these results, we can conclude that SOX10 and EDNRB are co-expressed in neural crest derived MITF+ melanoblasts.
SOX10 expression was also assessed in 48-hour neural tube explant cultures derived from EdnrbLacZ/LacZ homozygous embryos to determine if SOX10 expression is dependent on EDNRB function. In these cultures, robust SOX10 expression continues in β-galactosidase-positive neural crest cells that lack EDNRB (Figure 2B). Therefore, SOX10 expression is not dependent on EDNRB function in cultured neural crest-derived cells.
Over-expression of SOX10 from the Dct promoter can correct melanocyte defects associated with mutation in Sox10 but not Ednrb
Transgenic mice were generated that over-express SOX10 during melanoblast development using a minimal Dct promoter (Figure 3A). We generated Dct-Sox10 transgenic mice to achieve SOX10 overexpression in melanoblasts throughout the embryonic time window in which EDNRB function is critical (Shin et al., 1999). Sox10 expression from Tg(Dct-Sox10) mice was determined by embryonic whole mount in situ hybridization, using a probe that recognizes endogenous and Sox10 transgene mRNA. Expression patterns in transgenic embryos were compared with endogenous Sox10 expression in non-transgenic littermates. Consistent with previously characterized Tg(Dct-LacZ) mice, we observe that the Tg(Dct-Sox10) transgene expression has commenced by E10.5 (data not shown). As expected, the transgenic embryos exhibit endogenous Sox10 expression plus ectopic Sox10 expression in the optic vesicle and in the dorsal telencephelon (Figure 3E). In addition, transgenic animals show increased Sox10 expression in the melanoblast population (Compare Figure 3D with Figure 3F). These data show that the Sox10 transgene recapitulates the expression pattern of the minimal Dct promoter. Therefore, in Tg(Dct-Sox10) transgenic mice, Sox10 is over-expressed during the developmental time window that is critical for EDNRB function in melanoblasts (Figure 3B).
A total of 7 founders showed appropriate Dct transgene expression. Four lines were selected for further analysis. Among these, 3 lines demonstrated normal coat pigmentation when maintained in the heterozygous state on a C57/BL6JXFVB/N mixed background (CF1-10, 2272, and 2245). A low percentage of the heterozygous mice from a fourth line (2274) showed a small amount of ventral hypopigmentation (small belly spot).
Heterozygous Tg(Dct-Sox10) mice from the CF1-10 line were crossed with Sox10LacZ/+ animals to assess if over-expression of SOX10 in DCT+ melanoblasts can complement the defects in melanocyte development due to Sox10 haploinsufficiency (reduced Dct expression and belly spot). We first investigated whether expression of the SOX10 transgene in DCT-positive melanoblasts could rescue the transient down-regulation of Dct expression observed in Sox10 mutant embryos (Figure 4). Similar to previous observations for Sox10Dom/+ embryos at E11.5 (Potterf et al., 2001), a severe down-regulation of Dct expression is observed in Sox10LacZ/+ embryos in comparison to their wild type littermates (Figures 4A versus 4C). However, Dct expression is substantially restored in the presence of the SOX10 transgene (compare Figures 4C and 4D), demonstrating that the transgene is functional in early melanoblasts and that overexpression of SOX10 can increase transcription of a direct target gene of SOX10 in the melanocyte lineage.
Figure 4.
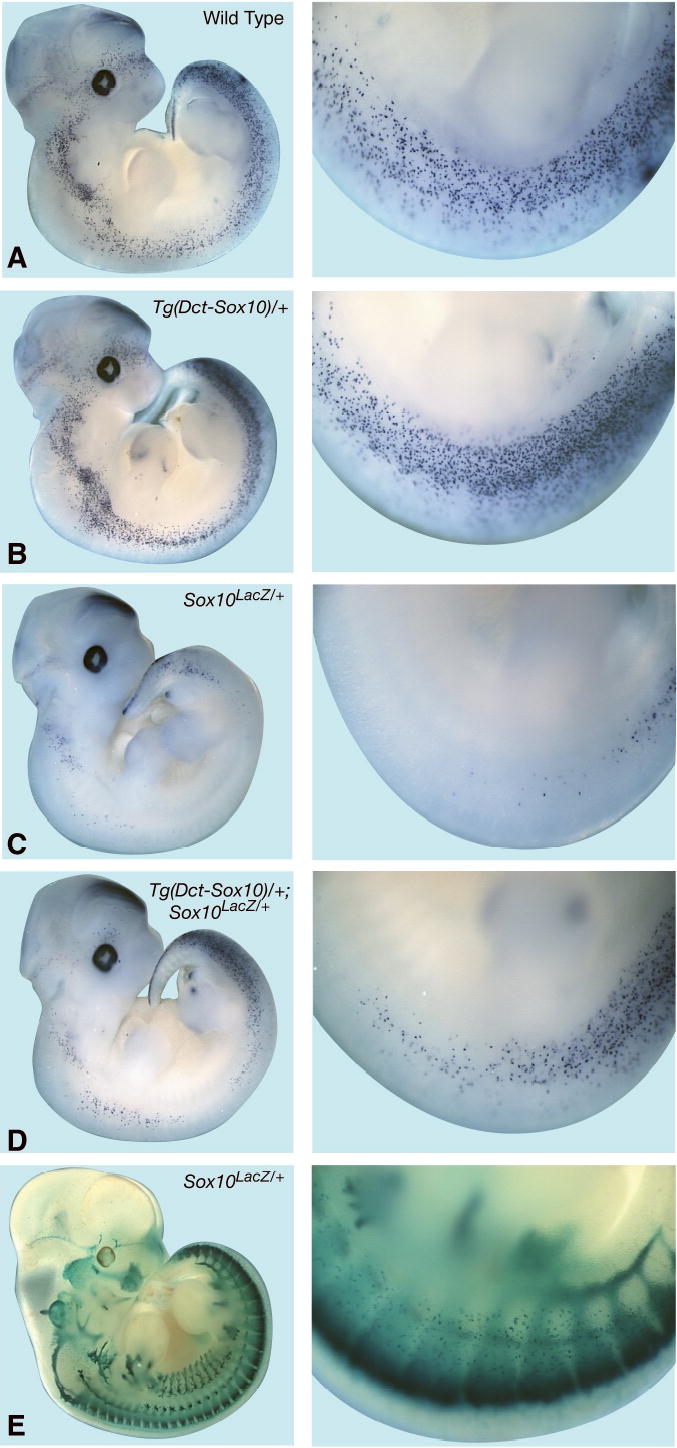
SOX10 transgene rescues Dct expression in Sox10LacZ/+ embryos. Whole mount RNA in-situ for Dct expression is shown for E11.5 littermate embryos of the following genotypes: (A) Wild type (non-trangenic); (B) Tg(Dct-Sox10)/+; (C) Sox10LacZ/+; (D) Tg(Dct-Sox10)/+ ; Sox10LacZ/+. (E) X-gal staining of E11.5 Sox10LacZ/+ embryos to visualize the melanoblast population with reduced Dct expression.
We next assessed if SOX10 overexpression could rescue the belly spot phenotype associated with heterozygosity for the Sox10LacZ mutation. The results in Table 1 demonstrate that the SOX10 transgene can rescue this haploinsufficiency phenotype of the Sox10LacZ/+ mice (p < 0.005). These results, together with the complementation of Dct expression, indicate that the SOX10 transgene is functionally expressed in the melanocyte lineage and during the time in which EDNRB function is critical.
Table 1.
Effects of varying SOX10 and EDNRB dosage on hypopigmentation
| Severity of Hypopigmentation | ||||||
|---|---|---|---|---|---|---|
| Genotype | No. | I | II | III | IV | V |
| Tg(Dct-Sox10)/+CF1-10 | 20 | 100% | 0 | 0 | 0 | 0 |
| Tg(Dct-Sox10)/+2272 | 17 | 100% | 0 | 0 | 0 | 0 |
| Tg(Dct-Sox10)/+2245 | 8 | 100% | 0 | 0 | 0 | 0 |
| Tg(Dct-Sox10)/+2274 | 14 | 86% | 14% | 0 | 0 | 0 |
| Sox10LacZ/+ | 23 | 39% | 61% | 0 | 0 | 0 |
| Sox10LacZ/+; Tg CF1-10 | 23 | 96% | 4% | 0 | 0 | 0 |
| Ednrbs/s | 5 | 0 | 0 | 100% | 0 | 0 |
| Ednrbs/s; Tg CF1-10 | 6 | 0 | 0 | 100% | 0 | 0 |
| Ednrbs/s–l | 9 | 0 | 0 | 0 | 100% | 0 |
| Ednrbs/s–l; Tg CF1-10 | 6 | 0 | 0 | 0 | 100% | 0 |
| Ednrbs–l/s–l | 1 | 0 | 0 | 0 | 0 | 100% |
| Ednrbs–l/s–l; Tg CF1-10 | 2 | 0 | 0 | 0 | 0 | 100% |
| Ednrbs/+ or Ednrbs–l/+ | 16 | 44% | 56% | 0 | 0 | 0 |
| Ednrbs/+; Tg CF1-10 or Ednrbs–l/+;Tg CF1-10 | 10 | 20% | 80% | 0 | 0 | 0 |
| Ednrbs–l/+ | 23 | 78% | 22% | 0 | 0 | 0 |
| Ednrbs–l/+; Sox10Dom/+ | 37 | 16% | 84% | 0 | 0 | 0 |
| Sox10Dom/+ | 14 | 7% | 93% | 0 | 0 | 0 |
No.:Number of animals used for the analysis
I: No hypopigmentation on dorsal or ventral surfaces
II: Ventral hypopigmentation only
III: Hypopigmentation over approximately 25% of the body surface area.
IV: Hypopigmentation over approximately 50 – 70% of the body surface area.
V: Hypopigmentation over approximately 95% of the body surface area.
Next, it was determined if SOX10 over-expression can rescue the hypopigmentation of Ednrb mutant mice. Complementation was tested in mice null for Ednrb (Ednrbs–l/s–l), or haploinsuffcient for Ednrb (Ednrbs–l/+), or carrying a hypomorphic allele (piebald) that has an intact coding sequence but gives reduced levels of wild type mRNA (Table 1; Figure 5). In all three cases, the SOX10 transgene did not rescue the hypopigmentation phenotypes in Ednrb mutant animals.
Figure 5.
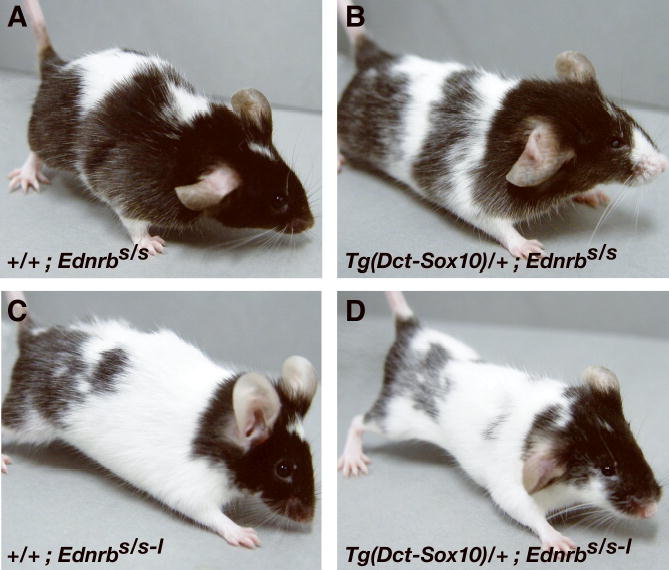
Tg(Dct-Sox10) cannot rescue hypopgmentation in Ednrb mutant mice. Typical examples of the following genotypes are shown: (A) +/+; Ednrbs/s; (B) Tg(Dct-Sox10)/+; Ednrbs/s; (C) +/+; Ednrbs/s–l; (D) Tg(Dct-Sox10)/+; Ednrbs/s-l
Hypopigmentation is not increased in mice that are double heterozygous for Sox10 and Ednrb mutations
We also assessed if reducing both SOX10 and EDNRB expression levels could exacerbate hypopigmention. For this, hypopigmentation of Sox10Dom/+; Ednrbs–l/+ double heterozygous mice was compared with Sox10Dom/+ and Ednrbs–l/+ single heterozygotes. Mice were generated using two independent genetic schemes involving different genetic background contributions to randomize modifier strain variations. Regardless of the cross or background strain used, none of the Sox10Dom/+; Ednrbs–l/+ double heterozygous animals showed increased hypopigmentation when compared to either Sox10Dom/+ or Ednrbs–l/+ single heterozygous siblings (Table 1; Figure 6), showing a lack of synergistic interaction between these genes in the manifestation of hypopigmentation. This is in stark contrast with the observation made in enteric neurons where Ednrb tm1Ywa/+, Sox10Dom/+ double heterozygous animals show dramatic increase in aganglionosis compared to Ednrb tm1Ywa/+ or Sox10Dom/+ single heterozygous siblings (Cantrell et al., 2004). Taken together with the non-complementation experiments described above, these data are consistent with a genetic model where Ednrb expression is not altered by varying SOX10 expression levels in the melanocyte lineage in vivo.
Figure 6.
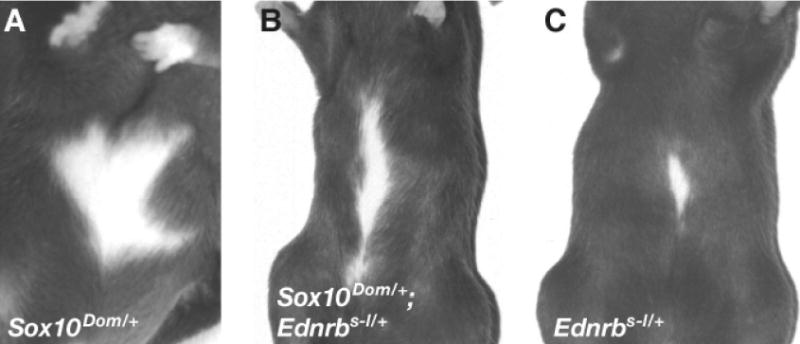
Lack of synergistic hypopigmentation between Sox10 and Ednrb. Ventral photographs of mice: (A) Sox10Dom/+; (B) Sox10Dom/+; Ednrbs–l/+; (C) Ednrbs–l/+
DISCUSSION
Mutations in Ednrb and Sox10 have been associated with pigmentary defects, congenital neurosensory deafness and megacolon in several species. In addition to showing similar loss-of-function phenotypes, Sox10 and Ednrb show similar embryonic expression patterns in mouse migratory neural crest cells in vivo and are co-expressed in individual neural crest cells, including melanoblasts, in vitro (Figure 2). The possibility of Ednrb acting downstream of Sox10 was previously investigated by examining Ednrb expression in Sox10Dom mutant embryos (Southard-Smith et al., 1998). However, the results did not lend themselves to a straightforward interpretation. While there was an apparent lowering of Ednrb expression in the absence of proper SOX10 function, there was also a significant reduction in the number of Ednrb-positive cells, presumably because of the increased apoptosis in the NC-derived population seen in these embryos. Consequently, the apparent lowering of Ednrb expression may have been an indirect consequence of the significant reduction in the number of Ednrb-positive cells. Consistent with a direct hierarchical relationship between these two genes, SOX10 was recently shown to directly bind a defined enhancer element within the Ednrb locus and activate transcription in the neural crest-derived enteric ganglia (Zhu et al., 2004). Transgenic mice harboring an Ednrb BAC containing these SOX10 binding sites show rescued megacolon without any rescue of pigmentation (Zhu et al., 2004), suggesting that either additional SOX10 binding sites are needed for Ednrb expression in melanocytes or that EDNRB and SOX10 act independently in melanocytes. In this study, we tested for a genetic link between Sox10 and Ednrb in neural crest-derived melanocyte development. Our results suggest that unlike the direct regulation of Ednrb by SOX10 in the enteric nervous system these genes may not share a hierarchical relationship in the melanocyte lineage.
Our results do not support Sox10 acting downstream of Ednrb. First, expression analysis demonstrates that SOX10 continues to be robustly expressed in neural crest progenitors that lack Ednrb function. Second, while SOX10 over-expression in the melanocyte lineage can correct the ventral hypopigmentation in Sox10LacZ/+ mice it doesn’t complement the melanocyte deficiency of Ednrb null melanoblasts in vivo. These results are consistent with the finding that SOX10 is required before EDNRB during melanocyte development. Specifically, the expression of MITF and DCT are vastly reduced in Sox10 mutant embryos at E10.5 (Potterf et al., 2001), consistent with SOX10 requirement for direct transcriptional regulation of these two genes (Bondurand et al., 2000; Jiao et al., 2004; Lee et al., 2000; Ludwig et al., 2004; Potterf et al., 2000; Verastegui et al., 2000). However, DCT-expressing melanoblasts are present in Ednrb mutant embryos at E10.5, although they do not expand to fill in the white areas of the coat (Lee et al., 2003; Pavan and Tilghman, 1994). Taken together, these results strongly suggest that Sox10 does not function directly downstream of Ednrb.
Our results also do not support a role for Sox10 acting directly upstream of Ednrb to activate its transcription in migrating neural crest or during melanocyte development. In situ analysis showed similar patterns of Ednrb expression in both wild type and Sox10 null embryos at E10.5. At E11.5, Ednrb signal was greatly reduced but present in multiple cell types, including dorsal root ganglia. These data clearly show that Ednrb expression in migratory neural crest is not dependent on SOX10 function, although we were unable to directly assess expression of Ednrb in Sox10 null melanoblasts. In Sox10 null embryos, the expression of the melanoblast marker Dct is missing from regions where putative melanoblasts should be located. This could be because Dct is dependent on SOX10 for its expression or because the melanoblasts are absent. Consistent with the latter hypothesis, Sox10-LacZ-positive cells are also absent from regions where melanoblasts are expected in E11.5 Sox10 null embryos. The latter hypothesis is also consistent with the increased level of apoptosis observed in Sox10 mutant neural crest cells at this age. It remains a possibility that SOX10 is involved in the maintenance of Ednrb expression in the melanocyte lineage as seen with the regulation of KIT by MITF (Opdecamp et al., 1997).
To further examine if Ednrb expression might be dependent on SOX10 function, we examined if varying SOX10 expression levels could alter hypopigmentation in Ednrb mutant mice. First, the Tg(Dct-Sox10) transgenic mice were used to determine if SOX10 over-expression can rescue hypopigmentation in mutant mice that either carry the Ednrbs allele or are heterozygous for the Ednrbs–l mutation. Ednrbs is a hypomorphic allele that can produce wild type mRNA, albeit at lower levels. If Sox10 acts upstream of Ednrb in the melanocyte lineage, proper spatiotemporal over-expression of SOX10 should in principle induce the Ednrbs allele to produce higher levels of EDNRB and partially rescue hypopigmentation. Or, it could increase the wild type transcript in Ednrbs–l heterozygous mice in which the EDNRB levels are reduced by half, thus rescuing their belly spot phenotype. However, Dct-Sox10 transgenic mice do not rescue hypopigmentation in either Ednrbs–l/+ or in Ednrbs/s mice. We also assayed for possible synergistic effects of lowering SOX10 levels on Ednrb-associated hypopigmentation. Strong in vivo genetic interactions between transcription factors and target genes in melanocyte development have been observed with Sox10 and Mitf (Potterf et al., 2000) and with Mitf and Bcl2 (McGill et al., 2002), providing a very sensitive assay for interacting alleles that affect coat color. In fact, strong in vivo effects were observed on enteric ganglia in Sox10, Ednrb double heterozygous mutant mice (Ednrb tm1Ywa/+, Sox10Dom/+)(Cantrell et al., 2004). Therefore, if Sox10 acted upstream of Ednrb in the melanocyte lineage, one would predict that a significant lowering of both SOX10 and EDNRB levels in Sox10, Ednrb double heterozygous mice would result in noticeably increased hypopigmentation compared to heterozgotes for either gene. However, we did not observe such synergistic interaction between Sox10 and Ednrb.
Our genetic hypothesis that SOX10 and EDNRB function independently in the melanocyte lineage suggests a stark and very interesting difference with the recent finding that SOX10 directly activates Ednrb during the development of enteric neural crest (Zhu et al., 2004) and the report of a strong genetic interaction between these genes in the enteric nervous system (Cantrell et al., 2004). Therefore, SOX10 regulation of a single gene (Ednrb) appears to be dependent on the cellular context. As co-activators are typically involved in SOX gene function, it is possible that the differing milieu of co-factors in enteric and melanocyte derived neural crest cells accounts for the differing transcriptional regulation.
MATERIALS AND METHODS
Mouse Strains
Dominant megacolon (Sox10Dom/+) mice were obtained from the Jackson Laboratory and were maintained on a C57BL6/C3HeB/FeJLe-a/a background. Animals with a LacZ knock-in engineered at the Sox10 locus (Sox10tm1Weg, here referred to as Sox10LacZ/+) were obtained on a mixed genetic background (Britsch et al., 2001) and were crossed to C57BL6/J mice before use in rescue assays. Ednrbs–l/s mice were obtained from the Jackson Laboratory and maintained on SSL/LeJ background. Mice with a LacZ knock-in engineered at the Ednrb locus (Lee et al., 2003) were maintained on a mixed C57BL6/C3H/HeJ genetic background.
Generation of Tg(Dct-Sox10) mice
For the Tg(Dct-Sox10) construct a 0.5 kbp BamHI fragment containing a spliceable intron (MP1) and Poly A sequences was inserted within the unique BamHI restriction site of a pBluescript II SK(+) vector (pBluescript II-MP1). Next, a XhoI/EcoRV fragment of the murine Sox10 cDNA encompassing the full-length open reading frame (Potterf et al., 2000) was directionally cloned into the XhoI and EcoRV sites of the MCS within pBluescript II-MP1, with the 3’ end of the cDNA fragment directly upstream of the MP1 and Poly A sequences (pBluescript II-mSox10-MP1). The Sox10 cDNA fragment used carries 278bp of sequences upstream of the methionine initiation codon and 106bp of sequences downstream of the termination codon, along with a carry-over 45bp fragment from the MCS of pcDNA3.1-mSox10 at the 5’ end. A 3.68kbp minimal Dct promoter fragment (Budd and Jackson, 1995) was obtained by XhoI/NotI double digestion of pMG-1 vector (gift of Dr. Glenn Merlino at the National Cancer Institute) and was subcloned into pBluescript II-mSox10-MP1, directly upstream of the Sox10 cDNA fragment (pDctp-mSox10-Mp1). For this subcloning, the NotI end of the promoter piece and the end of a carry-over SalI site in pBluescript II-mSox10-MP1 were filled in for blunt-end ligation at these sites. For generation of transgenic mice, pDct-mSox10-MP1 was digested with PvuI and NotI and the linear fragment carrying the transgenic cassette was purified and injected into fertilized FVB/N eggs. Candidate founders were tested for presence of the transgene either by Southern or by PCR amplification using the following primers: 5’ primer: agcagtatggctggagcact; 3’ primer: tccagtcgtagccgctgagca, corresponding to sites within the Dct promoter and the Sox10 cDNA respectively (Figure 3). Lines of transgenic mice were maintained by crossing to C57BL/6J mice.
Whole Mount RNA In-situ Hybridizations
Transgene expression was analyzed by whole mount RNA in-situ analysis of E10.5 – E12.5 embryos as described (Loftus et al., 2002). Reverse-transcribed digoxigenin-conjugated probe was made from a PCR-amplified product containing the entire Sox10 ORF region, with the T7 polymerase binding site introduced at the 3’ end. (Forward Primer: ccagggtgtttggtggtgagga; Reverse Primer (contains T7 binding site): gcgggtaatacgactcactatagggcagctcagtcagggcttggcct). The probes for analysis of endogenous Dct and Ednrb expression pattern were prepared as described (Kos et al., 1999; Southard-Smith et al., 1998).
Immunohistochemistry of Neural Tube Explant Cultures
Neural tubes were dissected from E9.5 embryos and explant cultures were prepared as previously described (Hou et al., 2004a). Two-day old explant cultures were fixed for 25 minutes at RT using 4% paraformaldehyde/1 X PBS (pH 7.5), followed by permeabilization for 5 minutes in 0.1% Triton X-100. For double indirect immunolabeling, the explants were incubated with monoclonal anti-β-galactosidase antibody (Promega) and purified anti-SOX10 antibody (Mollaaghababa and Pavan, 2003). The primary antibody signals were revealed using TRITC-coupled goat anti-rabbit (Fab)2 and FITC-coupled goat anti-mouse (Fab)2 (Molecular Probes). MITF expression in explant cultures was as described (Hou et al., 2004b).
Transgenic animal crosses
Sox10LacZ/+ and Tg(Dct-Sox10)/+ heterozygous mice were mated, and the offspring were scored for the presence of the belly spot phenotype. All progeny were genotyped by PCR for both the Sox10LacZ allele and the Dct-Sox10 transgene. For the Sox10LacZ allele, a modification of the published procedure (Britsch et al., 2001) was used; the presence of lacZ sequence was tested using the Sox10-F and Sox10-LacZ primers, while concomitantly Sox10-F and Sox10-R primers were used in a second reaction to verify the presence of the wild type Sox10 allele. For statistical analysis, the Fisher’s exact test was performed to obtain the P value for the colleted data.
For crosses involving Ednrb mutants, Tg(Dct-Sox10)/+ heterozygous mice from the CF1-10 line were mated to Ednrbs/s–l compound heterozygous animals. Recovered Tg(Dct-Sox10)/+ ; Ednrbs/+ double heterozygotes were back-crossed to Ednrbs/s–l animals and the severity of hypopigmentation was scored in their progeny. In a second set, hypopigmentation was scored in progeny from mating of Tg(Dct-Sox10)/+ ; Ednrbs–l/+ mice with Ednrbs–l/s animals. Ednrb genotypes were assigned according to coat pigmentation phenotypes. The severity of spotting was determined by visual examination of dorsal and ventral surfaces of the mice.
Analysis of Synergistic Interactions
Ednrbs–l/s mutants were outcrossed with B6 to expand the line and recover Ednrbs–l/+ individuals. Ednrbs–l/+ and Sox10Dom/+ mice were crossed to generate Ednrbs–l/+, Sox10Dom/+ animals that were backcrossed to Ednrbs–l/+ mice to obtain pups for analysis. Pups were genotyped for both the Ednrbs–l and the Sox10Dom alleles as described (Cantrell et al., 2004). In separate crosses, Ednrbs–l/s–l SSLe females were mated to Sox10Dom/+ males and offspring were genotyped for the Sox10Dom allele and analyzed.
Acknowledgments
We express our appreciation to members of the Pavan laboratory for stimulating discussions and sharing of reagents during the course of this study. We are grateful to Dr. Myung K. Shin of the Fox Chase Cancer Center for providing the EdnrbLacZ mice, to Dr. Michael Wegner at Universitat Erlangen-Nurnberg for providing the Sox10LacZ mice, and to Dr. Heinz Arnheiter of the National Institute of Neurological Disorders and Stroke for a critical reading of the manuscript and for use of their mouse facility for neural tube dissections. We thank Dr. Glenn Merlino of the National Cancer Institute for the pMG1 vector. We are grateful to Ashley Cantrell for mouse husbandry support and phenotypic characterizations for synergy studies. This research was supported in part by the Intramural Research Program of the National Human Genome Research Institute, National Institutes of Health.
References
- Baxter LL, Hou L, Loftus SK, Pavan WJ. Spotlight on spotted mice: a review of white spotting mouse mutants and associated human pigmentation disorders. Pigment Cell Res. 2004;17:215–24. doi: 10.1111/j.1600-0749.2004.00147.x. [DOI] [PubMed] [Google Scholar]
- Baxter LL, Pavan WJ. Pmel17 expression is Mitf-dependent and reveals cranial melanoblast migration during murine development. Gene Expr Patterns. 2003;3:703–7. doi: 10.1016/j.modgep.2003.07.002. [DOI] [PubMed] [Google Scholar]
- Baynash AG, Hosoda K, Giaid A, Richardson JA, Emoto N, Hammer RE, Yanagisawa M. Interaction of endothelin-3 with endothelin-B receptor is essential for development of epidermal melanocytes and enteric neurons. Cell. 1994;79:1277–85. doi: 10.1016/0092-8674(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Bondurand N, Pingault V, Goerich DE, Lemort N, Sock E, Caignec CL, Wegner M, Goossens M. Interaction among SOX10, PAX3 and MITF, three genes altered in Waardenburg syndrome. Hum Mol Genet. 2000;9:1907–17. doi: 10.1093/hmg/9.13.1907. [DOI] [PubMed] [Google Scholar]
- Britsch S, Goerich DE, Riethmacher D, Peirano RI, Rossner M, Nave KA, Birchmeier C, Wegner M. The transcription factor Sox10 is a key regulator of peripheral glial development. Genes Dev. 2001;15:66–78. doi: 10.1101/gad.186601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budd PS, Jackson IJ. Structure of the mouse tyrosinase-related protein-2/dopachrome tautomerase (Tyrp2/Dct) gene and sequence of two novel slaty alleles. Genomics. 1995;29:35–43. doi: 10.1006/geno.1995.1212. [DOI] [PubMed] [Google Scholar]
- Cantrell VA, Owens SE, Chandler RL, Airey DC, Bradley KM, Smith JR, Southard-Smith EM. Interactions between Sox10 and EdnrB modulate penetrance and severity of aganglionosis in the Sox10Dom mouse model of Hirschsprung disease. Hum Mol Genet. 2004;13:2289–301. doi: 10.1093/hmg/ddh243. [DOI] [PubMed] [Google Scholar]
- Gammill LS, Bronner-Fraser M. Neural crest specification: migrating into genomics. Nat Rev Neurosci. 2003;4:795–805. doi: 10.1038/nrn1219. [DOI] [PubMed] [Google Scholar]
- Hosoda K, Hammer RE, Richardson JA, Baynash AG, Cheung JC, Giaid A, Yanagisawa M. Targeted and natural (piebald-lethal) mutations of endothelin-B receptor gene produce megacolon associated with spotted coat color in mice. Cell. 1994;79:1267–76. doi: 10.1016/0092-8674(94)90017-5. [DOI] [PubMed] [Google Scholar]
- Hou L, Loftus SK, Incao A, Chen A, Pavan WJ. Complementation of melanocyte development in SOX10 mutant neural crest using lineage-directed gene transfer. Dev Dyn. 2004a;229:54–62. doi: 10.1002/dvdy.10468. [DOI] [PubMed] [Google Scholar]
- Hou L, Pavan WJ, Shin MK, Arnheiter H. Cell-autonomous and cell non-autonomous signaling through endothelin receptor B during melanocyte development. Development. 2004b;131:3239–47. doi: 10.1242/dev.01193. [DOI] [PubMed] [Google Scholar]
- Jiao Z, Mollaaghababa R, Pavan WJ, Antonellis A, Green ED, Hornyak TJ. Direct interaction of Sox10 with the promoter of murine Dopachrome Tautomerase (Dct) and synergistic activation of Dct expression with Mitf. Pigment Cell Res. 2004;17:352–62. doi: 10.1111/j.1600-0749.2004.00154.x. [DOI] [PubMed] [Google Scholar]
- Kapur RP. Early death of neural crest cells is responsible for total enteric aganglionosis in Sox10(Dom)/Sox10(Dom) mouse embryos. Pediatr Dev Pathol. 1999;2:559–69. doi: 10.1007/s100249900162. [DOI] [PubMed] [Google Scholar]
- Kos L, Aronzon A, Takayama H, Maina F, Ponzetto C, Merlino G, Pavan W. Hepatocyte growth factor/scatter factor-MET signaling in neural crest-derived melanocyte development. Pigment Cell Res. 1999;12:13–21. doi: 10.1111/j.1600-0749.1999.tb00503.x. [DOI] [PubMed] [Google Scholar]
- Kruger GM, Mosher JT, Tsai YH, Yeager KJ, Iwashita T, Gariepy CE, Morrison SJ. Temporally distinct requirements for endothelin receptor B in the generation and migration of gut neural crest stem cells. Neuron. 2003;40:917–29. doi: 10.1016/s0896-6273(03)00727-x. [DOI] [PubMed] [Google Scholar]
- Kuhlbrodt K, Herbarth B, Sock E, Hermans-Borgmeyer I, Wegner M. Sox10, a novel transcriptional modulator in glial cells. J Neurosci. 1998;18:237–50. doi: 10.1523/JNEUROSCI.18-01-00237.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Douarin NM, Creuzet S, Couly G, Dupin E. Neural crest cell plasticity and its limits. Development. 2004;131:4637–50. doi: 10.1242/dev.01350. [DOI] [PubMed] [Google Scholar]
- Lee HO, Levorse JM, Shin MK. The endothelin receptor-B is required for the migration of neural crest-derived melanocyte and enteric neuron precursors. Dev Biol. 2003;259:162–75. doi: 10.1016/s0012-1606(03)00160-x. [DOI] [PubMed] [Google Scholar]
- Lee M, Goodall J, Verastegui C, Ballotti R, Goding CR. Direct regulation of the Microphthalmia promoter by Sox10 links Waardenburg-Shah syndrome (WS4)-associated hypopigmentation and deafness to WS2. J Biol Chem. 2000;275:37978–83. doi: 10.1074/jbc.M003816200. [DOI] [PubMed] [Google Scholar]
- Loftus SK, Larson DM, Baxter LL, Antonellis A, Chen Y, Wu X, Jiang Y, Bittner M, Hammer JA, 3rd, Pavan WJ. Mutation of melanosome protein RAB38 in chocolate mice. Proc Natl Acad Sci U S A. 2002;99:4471–6. doi: 10.1073/pnas.072087599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig A, Rehberg S, Wegner M. Melanocyte-specific expression of dopachrome tautomerase is dependent on synergistic gene activation by the Sox10 and Mitf transcription factors. FEBS Lett. 2004;556:236–44. doi: 10.1016/s0014-5793(03)01446-7. [DOI] [PubMed] [Google Scholar]
- Matsushima Y, Shinkai Y, Kobayashi Y, Sakamoto M, Kunieda T, Tachibana M. A mouse model of Waardenburg syndrome type 4 with a new spontaneous mutation of the endothelin-B receptor gene. Mamm Genome. 2002;13:30–5. doi: 10.1007/s00335-001-3038-2. [DOI] [PubMed] [Google Scholar]
- McCallion AS, Chakravarti A. EDNRB/EDN3 and Hirschsprung disease type II. Pigment Cell Res. 2001;14:161–9. doi: 10.1034/j.1600-0749.2001.140305.x. [DOI] [PubMed] [Google Scholar]
- McGill GG, Horstmann M, Widlund HR, Du J, Motyckova G, Nishimura EK, Lin YL, Ramaswamy S, Avery W, Ding HF, Jordan SA, Jackson IJ, Korsmeyer SJ, Golub TR, Fisher DE. Bcl2 regulation by the melanocyte master regulator Mitf modulates lineage survival and melanoma cell viability. Cell. 2002;109:707–18. doi: 10.1016/s0092-8674(02)00762-6. [DOI] [PubMed] [Google Scholar]
- Mollaaghababa R, Pavan WJ. The importance of having your SOX on: role of SOX10 in the development of neural crest-derived melanocytes and glia. Oncogene. 2003;22:3024–34. doi: 10.1038/sj.onc.1206442. [DOI] [PubMed] [Google Scholar]
- Ohtani, S., Yamada, T., Sakurai, T., Tsuji, T., Yanagisawa, M. and Kunieda, T. (2004) An Insertion of a Retrotransposon in the Ednrb Gene Causes Piebald Coat Color of the s/s Mouse. 18th International Mouse Genome Conference, Poster 77.
- Opdecamp K, Nakayama A, Nguyen MT, Hodgkinson CA, Pavan WJ, Arnheiter H. Melanocyte development in vivo and in neural crest cell cultures: crucial dependence on the Mitf basic-helix-loop-helix-zipper transcription factor. Development. 1997;124:2377–86. doi: 10.1242/dev.124.12.2377. [DOI] [PubMed] [Google Scholar]
- Pavan WJ, Tilghman SM. Piebald lethal (sl) acts early to disrupt the development of neural crest-derived melanocytes. Proc Natl Acad Sci U S A. 1994;91:7159–63. doi: 10.1073/pnas.91.15.7159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pla P, Larue L. Involvement of endothelin receptors in normal and pathological development of neural crest cells. Int J Dev Biol. 2003;47:315–25. [PubMed] [Google Scholar]
- Potterf SB, Furumura M, Dunn KJ, Arnheiter H, Pavan WJ. Transcription factor hierarchy in Waardenburg syndrome: regulation of MITF expression by SOX10 and PAX3. Hum Genet. 2000;107:1–6. doi: 10.1007/s004390000328. [DOI] [PubMed] [Google Scholar]
- Potterf SB, Mollaaghababa R, Hou L, Southard-Smith EM, Hornyak TJ, Arnheiter H, Pavan WJ. Analysis of SOX10 function in neural crest-derived melanocyte development: SOX10-dependent transcriptional control of dopachrome tautomerase. Dev Biol. 2001;237:245–57. doi: 10.1006/dbio.2001.0372. [DOI] [PubMed] [Google Scholar]
- Read AP. Waardenburg syndrome. Adv Otorhinolaryngol. 2000;56:32–8. doi: 10.1159/000059069. [DOI] [PubMed] [Google Scholar]
- Shin MK, Levorse JM, Ingram RS, Tilghman SM. The temporal requirement for endothelin receptor-B signalling during neural crest development. Nature. 1999;402:496–501. doi: 10.1038/990040. [DOI] [PubMed] [Google Scholar]
- Shin MK, Russell LB, Tilghman SM. Molecular characterization of four induced alleles at the Ednrb locus. Proc Natl Acad Sci U S A. 1997;94:13105–10. doi: 10.1073/pnas.94.24.13105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnenberg-Riethmacher E, Miehe M, Stolt CC, Goerich DE, Wegner M, Riethmacher D. Development and degeneration of dorsal root ganglia in the absence of the HMG-domain transcription factor Sox10. Mech Dev. 2001;109:253–65. doi: 10.1016/s0925-4773(01)00547-0. [DOI] [PubMed] [Google Scholar]
- Southard-Smith EM, Angrist M, Ellison JS, Agarwala R, Baxevanis AD, Chakravarti A, Pavan WJ. The Sox10(Dom) mouse: modeling the genetic variation of Waardenburg-Shah (WS4) syndrome. Genome Res. 1999;9:215–25. [PubMed] [Google Scholar]
- Southard-Smith EM, Kos L, Pavan WJ. Sox10 mutation disrupts neural crest development in Dom Hirschsprung mouse model. Nat Genet. 1998;18:60–4. doi: 10.1038/ng0198-60. [DOI] [PubMed] [Google Scholar]
- Tachibana M, Kobayashi Y, Matsushima Y. Mouse models for four types of Waardenburg syndrome. Pigment Cell Res. 2003;16:448–54. doi: 10.1034/j.1600-0749.2003.00066.x. [DOI] [PubMed] [Google Scholar]
- Verastegui C, Bille K, Ortonne JP, Ballotti R. Regulation of the microphthalmia-associated transcription factor gene by the Waardenburg syndrome type 4 gene, SOX10. J Biol Chem. 2000;275:30757–60. doi: 10.1074/jbc.C000445200. [DOI] [PubMed] [Google Scholar]
- Zhu L, Lee HO, Jordan CS, Cantrell VA, Southard-Smith EM, Shin MK. Spatiotemporal regulation of endothelin receptor-B by SOX10 in neural crest-derived enteric neuron precursors. Nat Genet. 2004;36:732–7. doi: 10.1038/ng1371. [DOI] [PubMed] [Google Scholar]


