Alfred Jost’s proposal in the 1940s (1) that testes are crucial for the development of the male phenotype, and that without them the body develops in a female direction, has been driving research in the field of sexual differentiation for more than half a century. Although viewing females as the default outcome may have stunted research on the development of the female phenotype (2), this idea has inspired research on sexual differentiation at every level including that of brains and behavior (see, for example, Refs. 3–5). The more recent discoveries of genes that direct the differentiation of the primordial gonad into the testis (6) require reformulating the theory on the mechanisms of sexual differentiation into ‘Sex chromosomal genes determine the differentiation of the gonads into testes or ovaries; resulting differences in gonadal secretions cause all other differences.’
The Jost doctrine may not be as generally applicable as first thought. Several sex differences, for example in bird plumage, bird song, and the development of pouch vs. scrotum in wallabies, are likely to be caused directly by sex chromosomal genes and not by differences in gonadal hormones (7). Recently, we introduced a model system that can distinguish between direct actions of sex chromosomal complement (XX vs. XY) and gonadal hormones on sexually dimorphic traits (8). Female mice with the familiar XX genotype were crossed with males with an XY−Sry genotype. These mice lacked the Sry gene on the Y chromosome, which normally directs the growth of the testis (6), but developed a male phenotype anyway because they had an Sry transgene inserted on an autosomal chromosome. The offspring included XX and XY− mice of either sex depending on whether they inherited the Sry transgene or not. Comparing XX and XY− mice within sex (defined on basis of gonad) revealed a number of differences that appear to be caused directly by differences in sex chromosomal complement (8, 9).
Finding sex differences that are directly influenced by sex chromosomal complement, however, may only require subtle rewording of the Jost doctrine into ‘Sex chromosomal genes determine the differentiation of the gonads and a select few extragonadal features. Differences in gonadal secretions during development and in adulthood cause the bulk of all remaining sex differences.’ Stated this way, the doctrine may send the subliminal messages that gonadal hormones and sex chromosomal genes are partners in sexual differentiation and that an XY genotype and a male pattern of gonadal hormone release would tend to masculinize sexually dimorphic features. The paper by Palaszynski et al. (10) in this issue of Endocrinology suggests, however, that XY genotype and testosterone may, in some cases, antagonize each other’s action as well.
Palaszynski et al. (10) studied the effects of the autoantigens myelin basic protein and proteolipid protein on the immune response in mice. These proteins have been linked to multiple sclerosis in humans, which is more common in females than in males (11). Likewise, these antigens cause a stronger immune response in female than in male mice (12–14). This sex difference has been attributed to an immunosuppressive effect of circulating testosterone, because gonadectomy decreases this sex difference, mainly by increasing the response in males (14). Palaszynski et al. (10) hypothesized that residual sex differences are either caused by actions of gonadal hormones before gonadectomy or by direct effects of sex chromosomal genes. To test the latter theory, the authors used the same cross of mice as described above. They found that female mice showed a stronger immune response than male mice, irrespective of genotype. Much to their surprise, however, gonadectomized mice showed a stronger immune response in XY− than in XX animals, irrespective of gonadal sex. This shows that, whereas testosterone suppresses the immune response, the XY− complement stimulates it. The authors suggest that this antagonism may be the result of “evolution of a compensatory relationship between the two, serving to decrease immune response differences between females and males in situations in which extreme differences might be maladaptive.” They also suggest that in other mouse strains this compensation may be so effective as to make sex differences in immune response disappear.
This idea is wonderfully counterintuitive. In many fields of biology, we tend to associate physiological and anatomical differences with differences in physiological and behavioral endpoints. Vice versa, if physiological and behavioral endpoints do not differ, we are not inclined to look for differences in the mechanisms underlying these endpoints. Sex differences at one or another level in the mechanisms underlying any physiological or behavioral endpoint, however, may be the norm, not the exception. This is certainly true at the chromosomal level. Every cell in our bodies treats X chromosomes differently, depending on whether we are male or female. In mammals, cells in females show X inactivation, the silencing of most genes on one of the two X chromosomes; cells in male bodies do not (presuming that the corresponding genotypes were XX and XY, respectively) (15). We have no trouble accepting X inactivation as a compensatory mechanism, meant to ensure that X chromosomal genes, many of which serve basic household functions of cells, are expressed at roughly the same rate in males as in females (15). The extra effort needed to inactivate one of the two X chromosomes is, presumably, the price we pay for the evolution of two sexes. Clearly, differential expression of genes on the XX and XY chromosomes is necessary to generate male and female genotype. But for all we know, much of this differential expression is restricted to only a few tissues at rather restricted periods of our life. In mice, for example, expression of the Sry gene is needed for only half a day (embryonic d 11–11.5), in only one cell type, Sertoli cells, to set in motion the cascade of events that leads to the male phenotype (16, 17). In contrast, X inactivation has to take place in all cells of the female body, throughout life, in any tissue, sexually dimorphic or not (15).
Compensatory processes, meant to counter undesired side effects of sexual differentiation, may take place again and again, in developing as well as in adult animals, from the molecular to the macroscopic level. Brains may be a treasure trove for finding such compensatory processes, especially because neural circuits often serve more than one function, some dimorphic, others not. Indeed, one can find sex differences that cause sex differences in some functions while preventing them in others. For example, higher levels of the neuropeptide vasopressin in male brains may cause sex differences in aggressive behavior while preventing them in social recognition memory and parental behavior (18, 19).
These potential compensatory processes force us to rethink how genes and hormones may interact either to differentiate bodies or keep differentiation restricted to essential systems. The cross of mice used by Palaszynski et al. (10) and described in Ref. 8 provides a powerful tool to test this interaction. This is illustrated in Fig. 1, which shows the results of a hypothetical experiment in which a parameter is compared in males and females with a normal XX and XY genotype and in males and females in which sex chromosomal complement is manipulated so that males have XX and females XY chromosomes (XX Sry males and XY− females). The bars on the left (natural state) show three possible scenarios in which the parameter is either larger in males than in females (M > F), larger in females than in males (M < F), or does not differ in males and females with normal sex chromosomes (M = F). The bars to the right show how these parameters could differ in mice with reversed sex chromosomal complement if testosterone and the XY genotype antagonize each other’s action. Under those circumstances, such antagonism may either enhance or eliminate normally occurring sex differences depending on the direction of the antagonism (either testosterone or the XY complement masculinizes the parameter). Likewise, such antagonism may induce differences in traits where they did not exist before because the antagonizing action does not occur in the same animal. Figure 1 does not illustrate all possible permutations. For example, it does not take into account possible interactions of gonadal hormones in females with differential imprinting of the X chromosome or dosage differences of X chromosomal genes that escape X inactivation, both of which may influence sex differences as well (20). With all possible permutations, this becomes a very complex situation indeed. Neglecting the possibility of direct interactions between sex chromosomal genes and gonadal hormones may come with a high price, however, because it may mask potential explanations as to what causes sex differences in human pathology. The paper of Palaszynski et al. (10) demonstrates that interactions between gonadal hormones and sex chromosomal complement certainly deserve to be studied in the field of autoimmune disease. It would be surprising if this phenomenon were restricted to the field of immunology.
Fig. 1.
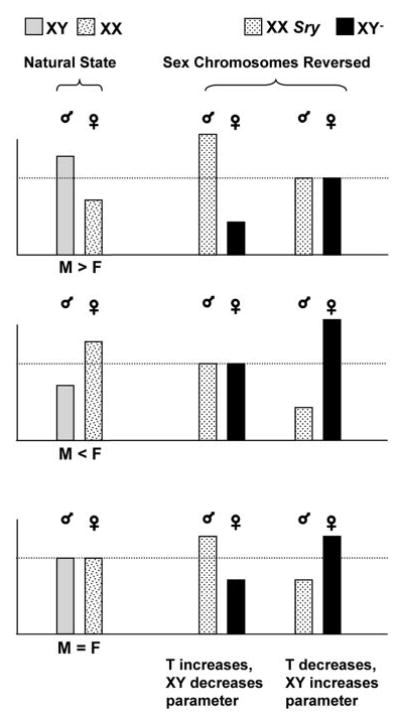
Results of a hypothetical comparison of a trait that differs between males and females (M > F and M < F), or not (M = F), in animals with normal sex chromosomes (XY in males and XX in females; Natural State) or with a reversed sex chromosomal complement (XX in males and XY in females; Sex Chromosomes Reversed). The ♂ and ♀ signs indicate gonadal sex. See text for further explanation.
References
- 1.Jost A. Recherches sur la differenciation sexuelle de l’embryon de lapin. 1. Introduction et embryologie genitale normale. Arch Anat Microsc Morphol Exp. 1947;36:151–200. [Google Scholar]
- 2.Loffler KA, Koopman P. Charting the course of ovarian development in vertebrates. Int J Dev Biol. 2002;46:503–510. [PubMed] [Google Scholar]
- 3.Field PM, Raisman G. A sexual difference in the preoptic area of the rat forebrain. J Physiol. 1971;218:23P–25P. [PubMed] [Google Scholar]
- 4.Gorski RA, Gordon JH, Shryne JE, Southam AM. Evidence for a morphological sex difference within the medial preoptic area of the rat brain. Brain Res. 1978;148:333–346. doi: 10.1016/0006-8993(78)90723-0. [DOI] [PubMed] [Google Scholar]
- 5.Phoenix CH, Goy RW, Gerall AA, Young WC. Organizing action of prenatally administered testosterone propionate on the tissues mediating mating behavior in the female guinea pig. Endocrinology. 1959;65:369–382. doi: 10.1210/endo-65-3-369. [DOI] [PubMed] [Google Scholar]
- 6.Koopman P, Gubbay J, Vivian N, Goodfellow P, Lovell-Badge R. Male development of chromosomally female mice transgenic for Sry. Nature. 1991;351:117–121. doi: 10.1038/351117a0. [DOI] [PubMed] [Google Scholar]
- 7.Arnold AP. Genetically triggered sexual differentiation of brain and behavior. Horm Behav. 1996;30:495–505. doi: 10.1006/hbeh.1996.0053. [DOI] [PubMed] [Google Scholar]
- 8.De Vries GJ, Rissman EF, Simerly RB, Yang LY, Scordalakes EM, Auger CJ, Swain A, Lovell-Badge R, Burgoyne PS, Arnold AP. A model system for study of sex chromosome effects on sexually dimorphic neural and behavioral traits. J Neurosci. 2002;22:9005–9014. doi: 10.1523/JNEUROSCI.22-20-09005.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carruth LL, Reisert I, Arnold AP. Sex chromosome genes directly affect brain sexual differentiation. Nat Neurosci. 2002;5:933–934. doi: 10.1038/nn922. [DOI] [PubMed] [Google Scholar]
- 10.Palaszynski KM, Smith DL, Kamrava S, Burgoyne PS, Arnold AP, Voskuhl RR. A yin-yang effect between sex chromosome complement and sex hormones on the immune response. Endocrinology. 2005;146:3280–3285. doi: 10.1210/en.2005-0284. [DOI] [PubMed] [Google Scholar]
- 11.Whitacre CC, Reingold SC, O’Looney PA. A gender gap in autoimmunity. Science. 1999;283:1277–1278. doi: 10.1126/science.283.5406.1277. [DOI] [PubMed] [Google Scholar]
- 12.Bebo BF, Jr, Schuster JC, Vandenbark AA, Offner H. Gender differences in experimental autoimmune encephalomyelitis develop during the induction of the immune response to encephalitogenic peptides. J Neurosci Res. 1998;52:420–426. doi: 10.1002/(SICI)1097-4547(19980515)52:4<420::AID-JNR5>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 13.Cua DJ, Hinton DR, Stohlman SA. Self-antigen-induced Th2 responses in experimental allergic encephalomyelitis (EAE)-resistant mice. Th2-mediated suppression of autoimmune disease. J Immunol. 1995;155:4052–4059. [PubMed] [Google Scholar]
- 14.Dalal M, Kim S, Voskuhl RR. Testosterone therapy ameliorates experimental autoimmune encephalomyelitis and induces a T helper 2 bias in the autoantigen-specific T lymphocyte response. J Immunol. 1997;159:3–6. [PubMed] [Google Scholar]
- 15.Lyon MF. X-chromosome inactivation. Curr Biol. 1999;9:R235–R237. doi: 10.1016/s0960-9822(99)80151-1. [DOI] [PubMed] [Google Scholar]
- 16.Burgoyne PS, Buehr M, Koopman P, Rossant J, McLaren A. Cell-autonomous action of the testis-determining gene: Sertoli cells are exclusively XY in XX—XY chimaeric mouse testes. Development. 1988;102:443–450. doi: 10.1242/dev.102.2.443. [DOI] [PubMed] [Google Scholar]
- 17.Lovell-Badge R, Hacker A. The molecular genetics of Sry and its role in mammalian sex determination. Philos Trans R Soc Lond B Biol Sci. 1995;350:205–214. doi: 10.1098/rstb.1995.0153. [DOI] [PubMed] [Google Scholar]
- 18.De Vries GJ, Boyle PA. Double duty for sex differences in the brain. Behav Brain Res. 1998;92:205–213. doi: 10.1016/s0166-4328(97)00192-7. [DOI] [PubMed] [Google Scholar]
- 19.De Vries GJ. Minireview: sex differences in adult and developing brains: compensation, compensation, compensation. Endocrinology. 2004;145:1063–1068. doi: 10.1210/en.2003-1504. [DOI] [PubMed] [Google Scholar]
- 20.Arnold AP. Sex chromosomes and brain gender. Nat Rev Neurosci. 2004;5:701–708. doi: 10.1038/nrn1494. [DOI] [PubMed] [Google Scholar]


