Abstract
Background
Oncolytic herpes viruses are attenuated, replication-competent viruses that selectively infect, replicate within, and lyse cancer cells and are highly efficacious in the treatment of a wide variety experimental cancers. The current study seeks to define the pharmacologic interactions between chemotherapeutic drugs and the oncolytic herpes viral strain NV1066 in the treatment of pancreatic cancer cell lines.
Methods
The human pancreatic cancer cell lines Hs 700T, PANC-1, and MIA PaCa-2 were treated in vitro with NV1066 at multiplicities of infection (MOI; ratio of the number of viral particles per tumor cell) ranging from 0.01 to 1.0 with or without 5-fluorouracil or gemcitabine. Synergistic efficacy was determined by the isobologram and combination-index methods of Chou and Talalay. Viral replication was measured using a standard plaque assay.
Results
Six days after combination therapy, 76% of Hs 700T cells were killed compared to 43% with NV1066 infection alone (MOI 0.1) or 0% with 5-FU alone (2 uM) (p<0.01). Isobologram and combination-index analyses confirmed a strongly synergistic pharmacologic interaction between the agents at all viral and drug combinations tested (LD5 – LD95) in the three cell lines. Dose reductions up to 6- and 78-fold may be achieved with combination therapy for NV1066 and 5-FU, respectively, without compromising cell kill. 5-FU increased viral replication up to 19-fold compared to cells treated with virus alone. Similar results were observed by combining gemcitabine and NV1066.
Conclusions
We have demonstrated that 5-FU and gemcitabine potentiate oncolytic herpes viral replication and cytotoxicity across a range of clinically achievable doses in the treatment of human pancreatic cancer cell lines. The potential clinical implications of this synergistic interaction include improvements in efficacy, treatment-associated toxicity, tolerability of therapeutic regimens, and quality of life. These data provide the cellular basis for the clinical investigation of combined oncolytic herpes virus therapy and chemotherapy in the treatment of pancreatic cancer.
Keywords: herpes simplex virus, NV1066, synergism, viral oncolysis
Introduction
Pancreatic cancer is an aggressive malignancy which is currently the fourth leading cause of cancer-related deaths for both men and women in the United States.1,2 In 2005, adenocarcinoma of the exocrine pancreas will account for an estimated 31,860 new cases and 31,270 deaths.1 Despite the recent advances in anticancer drugs, surgical resection remains the only potentially curative option for patients with pancreatic cancer. Only 10-15% of patients, however, are resectable at the time of diagnosis.3 Moreover, survival after presumed curative resection remains poor with median survivals ranging from 17 to 21 months.3-8
For patients with distant disease or locally-advanced disease precluding resection, the prognosis is worse with median survivals ranging from 3 to 10 months.2 A multitude of therapeutic regimens using chemotherapeutic agents and radiation have been investigated. While 5-fluorouracil (5-FU)- and gemcitabine-based regimens have demonstrated the greatest antitumor effect, only negligible improvements in survival have been realized. It is clear that novel therapeutic options are needed for the treatment of pancreatic cancer.
Oncolytic herpes viruses are attenuated, replication-competent herpes simplex type-1 viruses (HSV) that selectively infect, replicate within, and lyse cancer cells. These viruses have been shown to be highly efficacious in the treatment of a wide variety of human and animal cancers.9-18 Recent studies in our laboratory and others suggest that the efficacy of oncolytic HSV is enhanced when administered in combination with ionizing radiation or chemotherapeutic agents.12,19-22 This observation is not universal, however, depending on both the chemotherapeutic agent or viral strain used and the individual cancer. Combined modality therapeutic regimens are attractive as they aim to exploit a synergistic interaction between two agents to maximize efficacy and reduce treatment-associated toxicity and the development of drug resistance. As we envision combining oncolytic HSV with current chemotherapeutic regimens for the initiation of human clinical trials, this study sought to investigate whether 5-fluorouracil or gemcitabine potentiates efficacy of oncolytic HSV in the treatment of pancreatic cancer.
Materials and Methods
Cells
The human pancreatic cancer cell lines Hs 700T, PANC-1, and MIA PaCa-2 were obtained from the American Type Culture Collection (Rockville, MD) and grown in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with high glucose, 1.5 g/L sodium bicarbonate, 100 U/ml penicillin, 100 mg/ml streptomycin, and 10% fetal calf serum. Vero cells (American Type Culture Collection, Rockville, MD) were grown in minimum essential medium supplemented with 100 U/ml penicillin, 100 mg/ml streptomycin, and 10% fetal calf serum. Cells were maintained in a 5% CO2 humidified incubator at 37°C.
Virus
NV1066 is an attenuated, replication-competent oncolytic herpes simplex-1 virus whose construction has been previously described.23,24 Briefly, NV1066 is derived from the wild-type HSV-1 virus (F strain) and is rendered safe via deletions in the viral replicative and virulence genes ICP0, ICP4, and γ134.5. NV1066 is a derivative of the oncolytic HSV strain NV1020 which has already been tested in human phase I clinical trials demonstrating a favorable safety profile.25 Viral stocks were propagated on Vero cells and titered by standard plaque assay.17
Cytotoxicity assay
Cytotoxicity assays were performed by plating 2 × 104 cells into 24-well flat-bottom assay plates (Becton Dickinson, Franklin Lakes, NJ) in 1 ml of media. After overnight incubation at 37°C, cells were treated with either media alone (control), 1 - 4 μM 5-fluorouracil (5-FU) (American Pharmaceutical Partners, Inc., Schaumburg, IL), 0.5 - 2 nM gemcitabine (Eli Lilly and Company, Indianapolis, IN), or NV1066 at multiplicities of infection (MOI, the ratio of viral plaque forming units [PFU] to tumor cell) ranging from 0.01 - 1.0. Combination therapy experiments were performed by first exposing cells to either 5-FU or gemcitabine for six hours. After exposure, the media containing the chemotherapeutic agent was removed, cells were washed with phosphate buffered saline (PBS), and fresh media was added. Cells were then infected with NV1066 diluted in 100 μL media and incubated at 37°C. Daily after infection, media was removed and cells were lysed with 1.35% Triton-X solution to release intracellular lactate dehydrogenase (LDH). LDH was then quantified with the Cytotox 96 nonradioactive cytotoxicity assay (Promega, Madison, WI) that measures conversion of a tetrazolium salt into a red formazan product. Absorbance was measured at 450 nm with a microplate reader (EL321e, Bio-Tek Instruments, Winooski, VT). Results are expressed as the surviving percentage of cells as determined by the measured absorbance of each sample relative to control, untreated cells. All samples were tested and experiments were replicated, in triplicate.
Quantitative analysis of synergy
The isobologram and combination-index methods, derived from the median-effect principle of Chou and Talalay, were used to define the pharmacologic interaction between the chemotherapeutic drugs and NV1066.26 Details of these equations and of the software used to perform the computerized analyses have been described previously.26-30 Briefly, by taking into account the potency of the individual drugs, the potency of the combination of the drugs, and the shapes of their dose-effect curves, these methods enable the precise analysis of the pharmacologic interaction of two-drug combinations by comparing observed drug effects with predicted additive drug effects.
Data obtained from the cytotoxicity experiments were used to perform these analyses. The isobologram method is a graphical representation of the pharmacologic interaction and is formed by selecting a desired fractional cell kill (Fa) and plotting the individual drug and viral doses required to generate that Fa on their respective x- and y-axes. A straight line is then drawn to connect the points. The observed dose combination of the two agents which achieved that particular Fa is then plotted on the isobologram. Combination data points that fall on the line represent an additive drug-drug interaction, whereas data points that fall below or above the line represent synergism or antagonism, respectively.
The combination-index (CI) method is a mathematical and quantitative representation of a two-drug pharmacologic interaction. Using data from the cytotoxicity experiments and computerized software, CI values are generated over a range of Fa levels from 0.05 – 0.95 (5% - 95% cell kill). CI of 1 indicates an additive effect between two agents, whereas a CI < 1 or CI > 1 indicates synergism or antagonism, respectively. More clinically pertinent, data generated from the combination-index method can be used to quantify the dose-reduction index (DRI) for the combination of two drugs. DRI represents the fold-decrease of each individual agent attainable if the two drugs are used in combination as opposed to alone to achieve a particular Fa.
Viral replication assay
Standard plaque assays were performed to quantify viral proliferation within pancreatic cancer cells. Cells (2 × 104) were plated in 12-well flat-bottom assay plates in 2 ml of media. After overnight incubation at 37°C, cells were treated with NV1066 alone (MOI 0.01) or in combination with 5-FU (1 - 5 μM) or gemcitabine (1 - 5 nM). Supernatants and cells were collected from culture wells six days following treatment. Samples were subjected to three freeze-thaw lysis cycles to release intracellular viral particles. Serial dilutions of supernatants and cell lysates were cultured on confluent layers of Vero cells and viral titers were determined by counting viral plaques 72 hours later. All samples were tested, and experiments were replicated, in triplicate.
Real-time reverse transcriptase polymerase chain reaction (RT-PCR) quantitation of GADD34 expression
Cells (3 × 105) were plated in 6-well plates with 2 ml of media, incubated overnight, and treated with 5-fluorouracil (10 uM) or vehicle alone (control). Six hours following exposure, media was removed, cells were washed with PBS, and fresh media was added. Cells were harvested 12, 24, 36, and 60 hours after exposure and total RNA was isolated using an RNeasy Mini Kit (Qiagen Inc., Valencia, CA). SYBR green–based real-time quantitative reverse transcriptase polymerase chain reaction (RT-PCR) was performed using an ABI Prism 7900HT Sequence Detection System (Applied Biosystems, Foster City, CA). The following primers were used: GADD34 forward (5′-GGAGGAAGAGAATCAAGCCA-3′); GADD34 reverse (5′-TGGGGTCGGAGCCTGAAGAT-3′); 18S forward (5′-GTAACCCGTTGAACCCCATT-3′); and 18S reverse (5′-CCATCCAATCGGTAGTAGCG-3′). Co-amplification of the 18S ribosomal RNA housekeeping gene was used to normalize the amount of total RNA present. Thermal cycling conditions for amplification of GADD34 were as follows: 95°C for 9 minutes and 30 seconds; 40 cycles of 94°C for 30 seconds, 55.6°C for 30 seconds, 72°C for 30 seconds, and 78ºC for 30 seconds.
Statistical Analysis
All data are expressed as mean ± standard error of the mean. Two-tailed Student’s t-test was used to determine significance between treatment groups.
Results
Cytotoxicity of NV1066 in the treatment of Hs 700T human pancreatic cancer cells
To examine the oncolytic efficacy of NV1066, dose-dependent cytotoxicity experiments were performed. NV1066 demonstrates dose-dependent cytotoxicity against the Hs 700T human pancreatic cancer cell line (Figure 1). Cell kill progressively increases for the duration of the experiment at all doses tested. Seven days following infection at an MOI of 1.0, 90% (± 0.3%) of cells were killed (p <0.01). At 10- and 100-fold lower MOIs of 0.1 and 0.01, 84% (± 1%) and 63% (± 3%) of cells were killed 7 days following infection, respectively (p <0.01).
Figure 1. NV1066 demonstrates dose-dependent cytotoxicity against the Hs 700T human pancreatic cancer cell line.
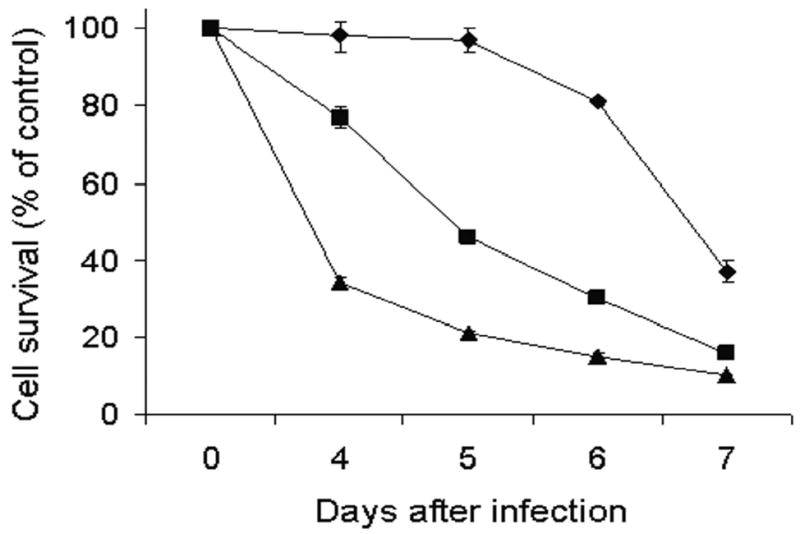
Monolayer cell cultures of Hs 700T were infected with NV1066 at multiplicities of infection (MOI) of 0.01 (diamond), 0.1 (square), and 1.0 (triangle). Lactate dehydrogenase cytotoxicity assays were used to measure cell kill for seven days following infection (last four days are shown). Mean cell survival is presented as a percentage compared to uninfected cells (± standard error of the mean). MOI represents the ratio of the number of viral particles to the number of tumor cells.
Cytotoxicity of combination NV1066 and 5-fluorouracil or gemcitabine
To examine the cytotoxic effect of combining chemotherapeutic agents and NV1066, Hs 700T cells were treated with either 5-FU or gemcitabine alone, NV1066 alone, or a combination of a 5-FU or gemcitabine and NV1066. Treatment of Hs 700T cells with 2 μM 5-FU alone or NV1066 at an MOI of 0.1 alone resulted in 0% (± 1%) and 43% (± 3%) cell kill, respectively, six days following treatment (Figure 2a). The expected cytotoxicity of combining treatments assuming an additive pharmacologic interaction was calculated and plotted (Figure 2a, dotted line). Observed cytotoxicity with combination 2 μM 5-FU and NV1066 at an MOI of 0.1 demonstrated 76% (± 4%) cell kill six days after treatment and is significantly greater than predicted cell kill (p <0.01).
Figure 2. Combination chemotherapy and oncolytic viral therapy demonstrates enhanced efficacy in the treatment of a human pancreatic cancer cell line compared with single-agent therapy.
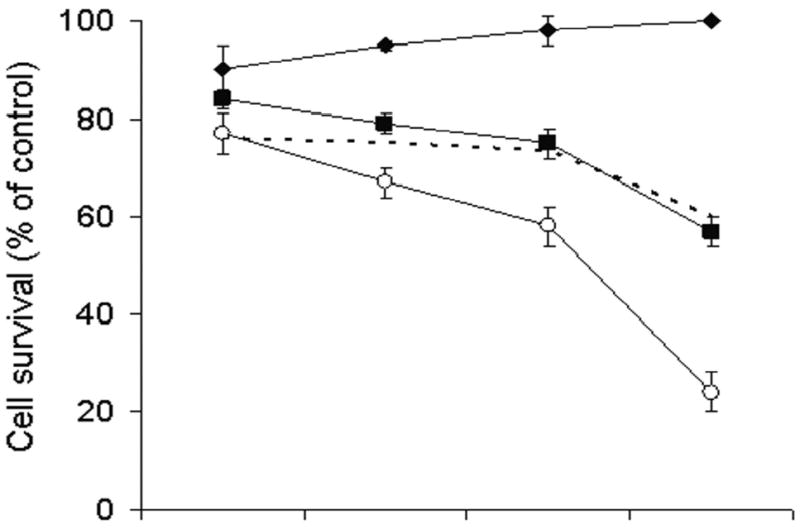
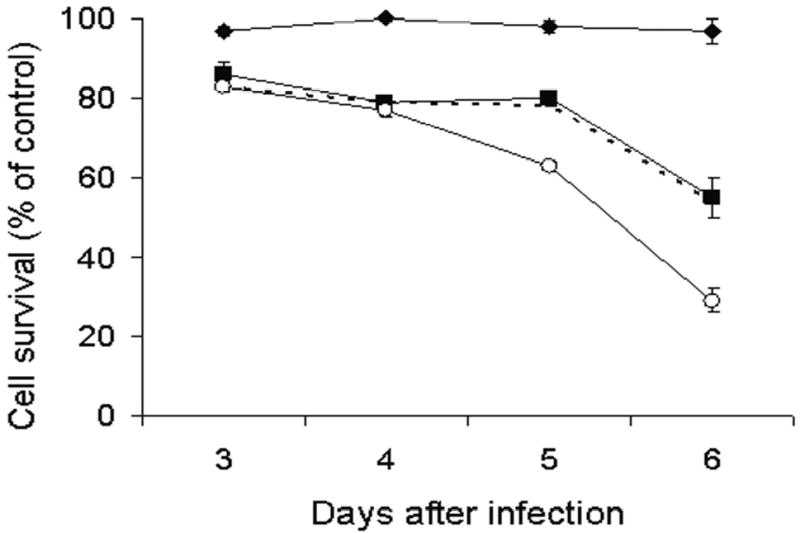
Monolayer cell cultures of Hs 700T cells were treated with 2 uM 5-fluorouracil (panel A, diamond), 1 nM gemcitabine ( panel B, diamond), NV1066 at an MOI of 0.1 (square), or a combination of the chemotherapeutic agent with NV1066 (open circle). Lactate dehydrogenase cytotoxicity assays were used to measure cell kill on days 3, 4, 5, and 6. Expected additive cell kill was calculated and is plotted (dotted line). Mean cell survival is presented as a percentage compared to uninfected cells (± standard error of the mean). MOI represents the ratio of the number of viral particles to the number of tumor cells.
Similar results were obtained combining gemcitabine and NV1066. Six days following treatment of Hs 700T cells with 1 nM gemcitabine alone or NV1066 at an MOI of 0.1 alone, in 3% (± 3%) and 45% (± 5%) of cells were killed, respectively (Figure 2b). Observed cytotoxicity with combination gemcitabine and NV1066 at the same doses demonstrated 71% (± 3%) cell kill and is significantly greater than predicted cell kill (p <0.01). Synergism was observed over a range of clinically achievable doses of both 5-FU (Figure 3a-c) and gemcitabine (Figure 3d-f). Synergism was also observed in the treatment of PANC-1 and MIA-PaCa-2 human pancreatic cancer cell lines (data not shown).
Figure 3. Combination chemotherapy and oncolytic viral therapy demonstrates enhanced efficacy in the treatment of a human pancreatic cancer cell line compared with single-agent therapy over a range of chemotherapeutic and viral doses.
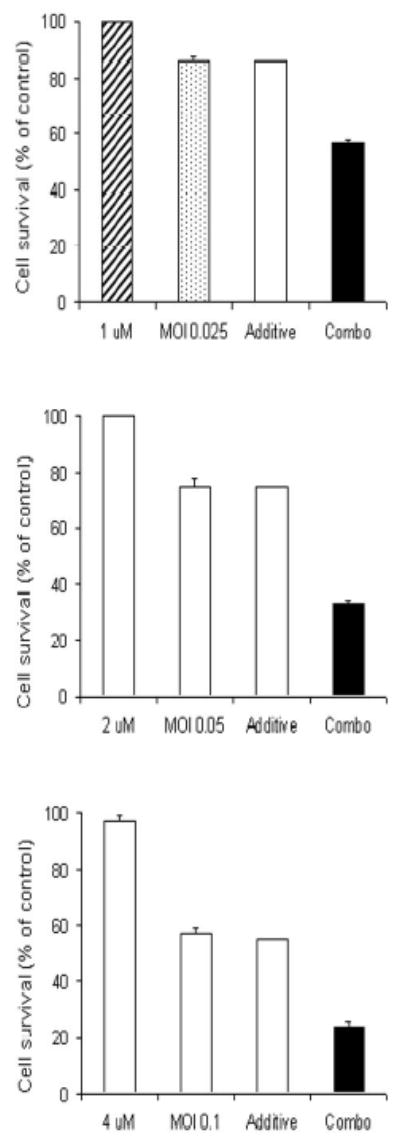
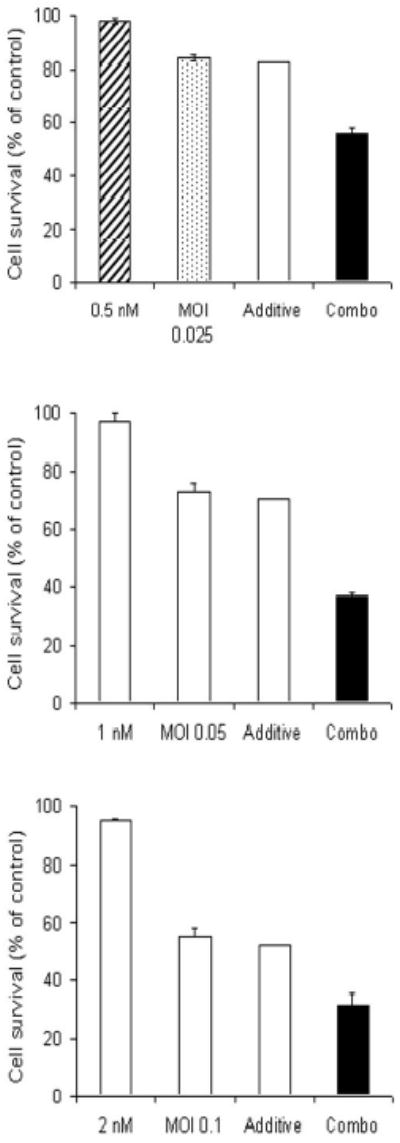
Hs 700T cells were treated with a range of doses of 5-fluorouracil alone (panels A-C, hatched bar), gemcitabine alone (panels D-F, hatched bar), or NV1066 alone (dotted bar), or a combination of the agents (solid bar). Expected additive cell kill was calculated and is plotted (clear bar). Lactate dehydrogenase cytotoxicity assays were used to measure cell kill 6 days following treatment. Mean cell survival is presented as a percentage compared to uninfected cells (± standard error of the mean). MOI represents the ratio of the number of viral particles to the number of tumor cells.
Quantitative analysis of synergy
The isobologram and combination-index methods developed by Chou and Talalay were used to confirm and quantify the synergism observed between the chemotherapeutic drugs and NV1066. Isobolograms were constructed for Fa values ranging from 0.5-.95 (5% - 95% cell kill). Experimental combination therapy data points plot well below the expected additive line at each Fa value for both combinations indicating strong synergism across a broad range of doses and agents. Representative isobolograms are shown in Figure 4.
Figure 4. Isobolograms demonstrate synergism between NV1066 and 5-FU (panels A-C) and NV1066 and gemcitabine (panels D-F).
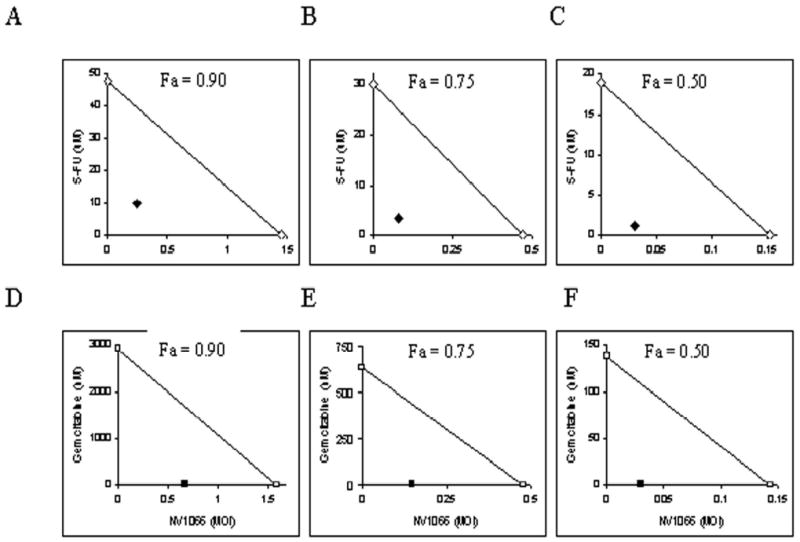
The individual doses of NV1066 and 5-FU observed to achieve 90% cell kill (Fa = 0.90, panel A), 75% cell kill (Fa = 0.75, panel B), and 50% cell kill (Fa = 0.50, panel C) are plotted on the x- and y-axes, respectively (open diamonds). The individual doses of NV1066 and gemcitabine needed to achieve 90% cell kill (Fa = 0.90, panel D), 75% cell kill (Fa = 0.75, panel E), and 50% cell kill (Fa = 0.50, panel F) are plotted on the x- and y-axes, respectively (open squares). The solid line connecting these points represents drug combination doses required to achieve the desired cell kill (Fa) if the pharmacologic interaction between the two agents was additive. Observed drug combination doses are plotted (NV1066 and 5-FU = closed diamonds; NV1066 and gemcitabine = closed squares) and fall well below the additive line, indicating strong synergism for both combinations over a range of doses and cytotoxic effect levels. Fa, fraction cell kill. 5-FU, 5-fluorouracil.
CI values for the interaction between 5-FU and NV1066 (0.25 – 0.47) and gemcitabine and NV1066 (0.10 – 0.53) are <1 over the entire range of Fa values tested (0.05 – 0.95) indicating strong synergism (Table I). Using these data, the dose-reduction index (DRI) was then calculated for each Fa value. For combination 5-FU and NV1066, compared to either drug alone, up to 78-fold and 6-fold dose reductions can be achieved, respectively, without compromising cell kill (Table 2). For combination gemcitabine and NV1066, up to 207-fold and 10-fold dose reductions can be achieved, respectively, without compromising cell kill (Table 3). These mathematical methods therefore demonstrate a strong synergistic interaction between each chemotherapeutic drug and NV1066 over a wide range of therapeutic doses and cytotoxic effect levels.
Table 1.
Combination index (CI) values determined for pharmacologic interaction between NV1066 and 5-fluorouracil and NV1066 and gemcitabine over a broad range of cytotoxic effect levels.
| Fractional Cell Kill (Fa) | CI value (NV1066 and 5-FU) | CI value (NV1066 and gemcitabine) |
|---|---|---|
| 0.05 | 0.25 | 0.10 |
| 0.10 | 0.24 | 0.13 |
| 0.20 | 0.24 | 0.16 |
| 0.30 | 0.25 | 0.18 |
| 0.40 | 0.25 | 0.21 |
| 0.50 | 0.26 | 0.23 |
| 0.60 | 0.27 | 0.26 |
| 0.70 | 0.28 | 0.29 |
| 0.80 | 0.31 | 0.34 |
| 0.90 | 0.38 | 0.43 |
| 0.95 | 0.47 | 0.53 |
Footnote to Table 1. Interpretation of combination-index (CI) values in quantifying two-drug pharmacologic interactions: CI 0.90 – 1.10 = nearly additive; CI 0.85 – 0.90 = slight synergism; CI 0.70 – 0.85 = moderate synergism; CI 0.30 – 0.70 = synergism; CI 0.10 – 0.30 = strong synergism; CI < 0.10 = very strong synergism. Fa = Fractional cell kill.
Table 2. Doses of 5-fluorouracil (5-FU) and NV1066 needed to kill various fractions (Fa) of Hs 700T cells and dose reductions (-fold) achievable when agents are used in combination.
Dose reduction index is the achievable dose reduction (-fold) for each single-agent when used in combination to attain the same cell kill. Fa, fractional cell kill.
| Fractional Cell Kill (Fa) | 5-FU alone (uM) | NV1066 alone (MOI) | 5-FU dose reduction index | NV1066 dose reduction index |
|---|---|---|---|---|
| 0.05 | 5.3 | 0.007 | 78 | 4 |
| 0.1 | 7.6 | 0.016 | 52 | 5 |
| 0.15 | 9.2 | 0.026 | 41 | 5 |
| 0.2 | 10.6 | 0.037 | 34 | 5 |
| 0.25 | 12 | 0.05 | 29 | 5 |
| 0.3 | 13.3 | 0.064 | 25 | 5 |
| 0.35 | 14.6 | 0.081 | 22 | 5 |
| 0.4 | 16 | 0.101 | 20 | 5 |
| 0.45 | 17.4 | 0.125 | 18 | 5 |
| 0.5 | 19 | 0.153 | 16 | 5 |
| 0.55 | 20.6 | 0.188 | 14 | 5 |
| 0.6 | 22.5 | 0.232 | 13 | 5 |
| 0.65 | 24.6 | 0.289 | 11 | 5 |
| 0.7 | 27 | 0.367 | 10 | 5 |
| 0.75 | 30 | 0.472 | 9 | 6 |
| 0.8 | 33.9 | 0.634 | 8 | 6 |
| 0.85 | 39.2 | 0.905 | 6 | 6 |
| 0.9 | 47.6 | 1.454 | 5 | 6 |
| 0.95 | 65.1 | 3.128 | 3 | 6 |
Table 3. Doses of gemcitabine and NV1066 needed to kill various fractions (Fa) of Hs 700T cells and dose reductions (-fold) achievable when agents are used in combination.
Dose reduction index is the achievable dose reduction (-fold) for each single-agent when used in combination to attain the same cell kill. Fa, fractional cell kill.
| Fractional Cell Kill (Fa) | Gemcitabine alone (nM) | NV1066 alone (MOI) | Gemcitabine dose reduction index | NV1066 dose reduction index |
|---|---|---|---|---|
| 0.05 | 2.3 | 0.006 | 208 | 10 |
| 0.1 | 6.5 | 0.013 | 209 | 8 |
| 0.15 | 12.3 | 0.021 | 209 | 7 |
| 0.2 | 20 | 0.031 | 210 | 7 |
| 0.25 | 29.9 | 0.043 | 211 | 6 |
| 0.3 | 42.4 | 0.057 | 211 | 6 |
| 0.35 | 58.2 | 0.073 | 211 | 5 |
| 0.4 | 78.3 | 0.092 | 212 | 5 |
| 0.45 | 104.1 | 0.115 | 212 | 5 |
| 0.5 | 137.6 | 0.143 | 212 | 4 |
| 0.55 | 181.8 | 0.178 | 213 | 4 |
| 0.6 | 241.7 | 0.223 | 213 | 4 |
| 0.65 | 325.3 | 0.282 | 213 | 4 |
| 0.7 | 446.7 | 0.363 | 214 | 4 |
| 0.75 | 633.5 | 0.478 | 214 | 3 |
| 0.8 | 944.9 | 0.655 | 215 | 3 |
| 0.85 | 1533.3 | 0.959 | 215 | 3 |
| 0.9 | 2916.6 | 1.59 | 216 | 2 |
| 0.95 | 8239.5 | 3.61 | 217 | 2 |
Viral replication assay
Viral progeny production in Hs 700T cells was quantified in the absence or presence of 5-FU or gemcitabine using standard plaque assays (Figure 5). Six days following infection of Hs 700T cells with NV1066 alone at an MOI of 0.01 (200 PFU), 5.6 × 104 PFU were produced. Addition of 5-FU or gemcitabine resulted in a dose-dependent increase in viral progeny production. Combination treatment with 1 uM, 2.5 uM, or 5 uM 5-FU resulted in the production of 1.5 × 105 PFU, 1.1 × 106 PFU, and 8 × 105 PFU, respectively, six days following infection (Figure 5a). This represents a 750- to 5 500-fold amplification of the initial infecting viral dose (200 PFU) and a 3- to 19-fold increase in viral titer production compared with cells treated with virus alone (p <0.05). Combination therapy with gemcitabine resulted in a 2 800- to 8 000-fold amplification of the initial infecting viral dose representing a 10- to 30-fold increase in viral progeny compared with infection with NV1066 alone (p <0.01) (Figure 5b).
Figure 5. Combination chemotherapy and oncolytic viral therapy demonstrates enhanced viral replication in the treatment of a human pancreatic cancer cell line.
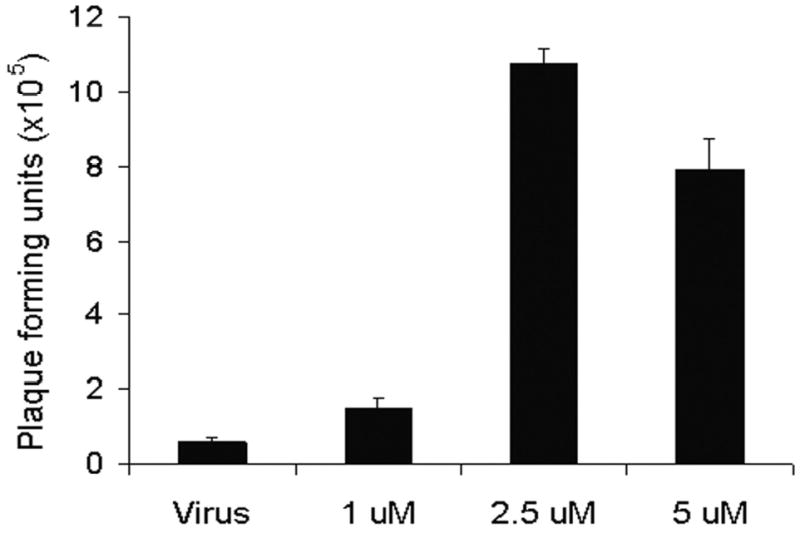
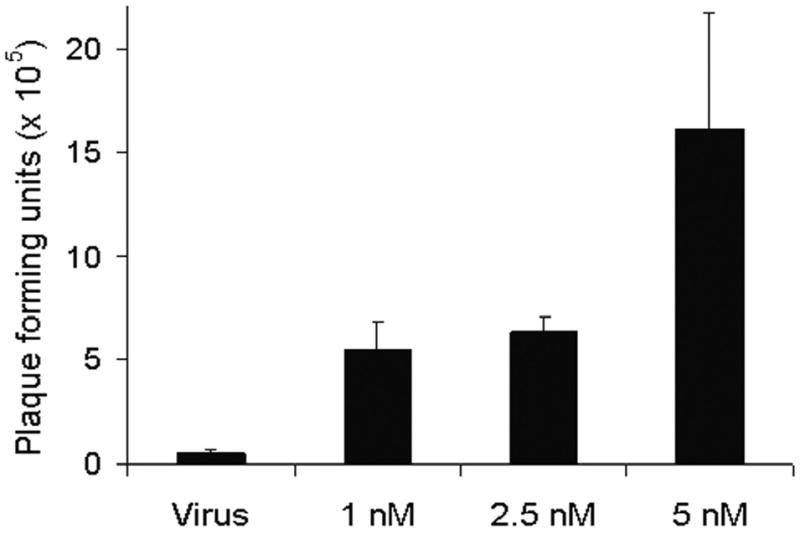
Monolayer cell cultures of Hs 700T cells were treated with NV1066 at an MOI of 0.01 (200 plaque forming units, PFU) either alone or in combination with a range of doses of 5-fluorouracil (1 – 5 uM) (panel A) or gemcitabine (1 – 5 nM) (panel B). Viral progeny were quantified 6 days following infection using a standard plaque assay. Mean PFU for triplicate samples are plotted (± standard error of the mean). MOI represents the ratio of the number of viral particles to the number of tumor cells.
Real-time reverse transcriptase polymerase chain reaction (RT-PCR) quantitation of GADD34 expression
Real-time RT-PCR was used to assess genetic expression of the cellular stress response gene GADD34 following treatment of pancreatic cell lines with 5-FU. Six-hour exposure of Hs 700T cells to 5-FU resulted in a 2.3-fold upregulation of GADD34 mRNA expression 24 hours following treatment (p < 0.01) (Figure 6).
Discussion
Novel therapies are desperately needed for the treatment of pancreatic cancer. Current chemoradiation-based therapeutic regimens are largely limited in both efficacy and toxicity. Oncolytic herpes viruses are attenuated, replication-competent viruses that selectively infect, replicate within, and lyse cancer cells and are highly efficacious in the treatment of a wide variety of experimental therapies. We have previously shown that oncolytic herpes viruses are effective as a single-agent in the treatment of an experimental model of pancreatic cancer.14 We now use the isobologram and CI methods of Chou and Talalay to define the pharmacologic interactions between 5-FU, gemcitabine and the oncolytic HSV strain NV1066 and demonstrate a synergistic enhancement in cytotoxicity in the treatment of a human pancreatic cancer cell line.26 Synergism was observed across a range of clinically achievable doses of two of the most effective and widely used chemotherapeutic agents in the treatment of this disease. Furthermore, synergism was observed in the treatment of all three cell lines tested although representative data from one cell line is shown.
A fundamental advantage of oncolytic viral therapy compared to standard cancer treatment modalities, is the in vivo amplification of the administered viral dose. Following completion of the viral life cycle in a cancer cell, cellular lysis results in the release of many new infectious viral particles which can then infect additional viable cancer cells. We demonstrate that the production of viral progeny is significantly enhanced in the presence of either 5-FU or gemcitabine. Our data also suggest that this potentiation of viral replication is responsible for the synergism observed. The differential improvement in cell kill in the combination therapy arms of the experiments does not appear to be an initial cytotoxic effect. Rather, it becomes evident five to six days following treatment after several viral life cycles have been completed and after the differential increase in viral progeny production is evident by plaque assay. This amplification is limited only to the extent that viable cancer cells are present to support viral replication.
Several molecular mechanisms have been proposed to explain the potentiation of viral replication by chemotherapy and radiation therapy.11,12,19-22,31,32 These mechanisms describe viral exploitation of the host cellular stress response following exposure to chemotherapeutic agents or ionizing radiation. Previous work in our laboratory has demonstrated that up-regulation of host cellular ribonucleotide reductase (RR) following exposure of cancer cells to ionization radiation enhances viral replication and cell kill in the treatment of a colorectal cancer cell line.21 RR reduces ribonucleotides to deoxyribonucleotides and is responsible for the production of the substrates of DNA synthesis. These studies were performed using the second-generation oncolytic herpes simplex type-1 viral strain G207, which has an insertional inactivation of the large subunit of the viral ribonucleotide reductase gene and is therefore dependent on host cell RR for viral replication. Up-regulation of host cell RR following DNA damage has therefore been proposed to complement the viral genomic deletion enhancing viral replication.
In comparison to G207, NV1066 is not deficient for viral RR and is therefore not dependent on host cell RR for viral replication. More recent work from our laboratory has shown that upregulation of the host cellular stress response gene GADD34 (Growth Arrest and DNA Damage Protein 34) mediates a synergistic cytotoxic effect following exposure of gastric cancer cells to mitomycin C.22 GADD34 is homologous to the viral replicative gene γ134.5—both copies of which are deleted in NV1066 for attenuation. Upregulation of GADD34 following exposure to the chemotherapeutic agent therefore complements this viral genomic deletion and enhances viral replication. We similarly demonstrate upregulation of GADD34 following exposure of Hs 700T cells to 5-FU.
The clinical implications of this synergism are evident and are not limited to enhanced efficacy. The dose-reduction index, the most relevant clinical parameter derived from the Chou and Talalay analysis, reveals the potential for significant dose reductions without compromising cell kill. Dose reductions minimize treatment-associated toxicity thereby improving the tolerability of therapeutic regimens and quality of life.
The use of these agents alone or in combination with systemic chemotherapy can be conceivably employed in several clinical settings. The retroperitoneal resection margin is the site of local failure in up to 50% of cases following pancreaticoduodenectomy.2 Oncolytic HSV could be administered intraoperatively into the tumor bed following resection for clearance of microscopic residual disease and sterilization of this difficult margin. Locally-advanced primary disease precluding resection could be approached with percutaneous or endoscopically-administered virus in combination with neoadjuvant systemic chemotherapy in an attempt to down-stage disease and permit subsequent curative resection. Additionally, common sites of recurrent metastatic disease include the peritoneal cavity and liver which could be treated with regional intraperitoneal or intrahepatic arterial perfusion, respectively.
The toxicity of these agents has also been extensively investigated both in animal models and humans. Oncolytic herpes viruses are highly specific for infection of cancer cells sparing normal cells. The safety of these oncolytic viruses have been tested in preclinical toxicology studies in Aotus monkeys that are extremely sensitive to wild-type herpes viral infections. These monkeys demonstrated no toxicity when administered attenuated virus33,34. Viral dissemination following administration has been extensively investigated in our laboratory utilizing both quantitative PCR detection of the viral gene ICP0 and radiolabeled herpes virus. These studies repetitively demonstrate no viral proliferation in non-cancerous tissues. Finally, the safety of use of several oncolytic HSV strains has been demonstrated in humans with the recent completion of several phase I clinical trials.25
Conclusions
We have demonstrated that 5-FU and gemcitabine potentiate oncolytic herpes viral replication and cytotoxicity across a range of clinically achievable doses in the treatment of human pancreatic cancer cell lines. The clinical implications of this synergistic interaction are paramount and include improvements in efficacy, treatment-associated toxicity, tolerability of therapeutic regimens, and quality of life. This data also corroborates prior studies suggesting a synergistic interaction between chemotherapy and oncolytic viral therapy and supports the commencement of clinical trials incorporating oncolytic herpes viruses into investigative therapeutic regimens in the treatment of pancreatic cancer.
Footnotes
Meeting presentation: Presented at the Forty-Sixth Annual Meeting of The Society for Surgery of the Alimentary Tract, Chicago, Illinois, May 14-18, 2005
Grants: Supported in part by training grant T 32 CA09501 (D.P.E and K.H.) AACR-AstraZeneca Cancer Research and Prevention fellowship (P.S.A), grants RO1 CA 75416 and RO1 CA/DK80982 (Y.F.) from the National Institutes of Health, grant BC024118 from the US Army (Y.F.), grant IMG0402501 from the Susan G. Komen Foundation (Y.F.) and grant 032047 from Flight Attendant Medical Research Institute (Y.F.)
References
- 1.Jemal A, Murray T, Ward E, et al. Cancer statistics, 2005. Ca: a Cancer Journal for Clinicians. 2005;55:10–30. doi: 10.3322/canjclin.55.1.10. [DOI] [PubMed] [Google Scholar]
- 2.Wolff R, Abbruzzese J, Evans D. Neoplasms of the Exocrine Pancreas. In: Kufe D, Pollock R, Weichselbaum R et al, eds. Holland Frei Cancer Medicine 6th ed. Canada: BC Decker; 2003.
- 3.Picozzi VJ, Traverso LW. The Virginia Mason approach to localized pancreatic cancer. [Review] [28 refs] Surgical Oncology Clinics of North America. 2004;13:663–674. doi: 10.1016/j.soc.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 4.Geer RJ, Brennan MF. Prognostic indicators for survival after resection of pancreatic adenocarcinoma. Am J Surg. 1993;165:68–73. doi: 10.1016/s0002-9610(05)80406-4. [DOI] [PubMed] [Google Scholar]
- 5.Brennan MF. Adjuvant therapy following resection for pancreatic adenocarcinoma. [Review] [20 refs] Surgical Oncology Clinics of North America. 2004;13:555–566. doi: 10.1016/j.soc.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 6.Breslin TM, Hess KR, Harbison DB, et al. Neoadjuvant chemoradiotherapy for adenocarcinoma of the pancreas: treatment variables and survival duration. Annals of Surgical Oncology. 2001;8:123–132. doi: 10.1007/s10434-001-0123-4. [DOI] [PubMed] [Google Scholar]
- 7.Sohn TA, Yeo CJ, Cameron JL, et al. Resected adenocarcinoma of the pancreas-616 patients: results, outcomes, and prognostic indicators.[see comment] Journal of Gastrointestinal Surgery. 2000;4:567–579. doi: 10.1016/s1091-255x(00)80105-5. [DOI] [PubMed] [Google Scholar]
- 8.Nitecki SS, Sarr MG, Colby TV, Van Heerden JA. Long-term survival after resection for ductal adenocarcinoma of the pancreas. Is it really improving? Annals Of Surgery. 1995;221:59–66. doi: 10.1097/00000658-199501000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Andreansky S, Soroceanu L, Flotte ER, et al. Evaluation of genetically engineered herpes simplex viruses as oncolytic agents for human malignant brain tumors. Cancer Research. 1997;57:1502–1509. [PubMed] [Google Scholar]
- 10.Chambers R, Gillespie GY, Soroceanu L, et al. Comparison of genetically engineered herpes simplex viruses for the treatment of brain tumors in a scid mouse model of human malignant glioma. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:1411–1415. doi: 10.1073/pnas.92.5.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chahlavi A, Todo T, Martuza RL, Rabkin SD. Replication-competent herpes simplex virus vector G207 and cisplatin combination therapy for head and neck squamous cell carcinoma. Neoplasia. 1999;1:162–169. doi: 10.1038/sj.neo.7900016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Advani SJ, Chung SM, Yan SY, et al. Replication-competent, nonneuroinvasive genetically engineered herpes virus is highly effective in the treatment of therapy-resistant experimental human tumors. Cancer Res. 1999;59:2055–2058. [PubMed] [Google Scholar]
- 13.Mineta T, Rabkin SD, Yazaki T, Hunter WD, Martuza RL. Attenuated multi-mutated herpes simplex virus-1 for the treatment of malignant gliomas. Nat Med. 1995;1:938–943. doi: 10.1038/nm0995-938. [DOI] [PubMed] [Google Scholar]
- 14.McAuliffe PF, Jarnagin WR, Johnson P, Delman KA, Federoff H, Fong Y. Effective treatment of pancreatic tumors with two multimutated herpes simplex oncolytic viruses. Journal of Gastrointestinal Surgery. 2000;4:580–588. doi: 10.1016/s1091-255x(00)80106-7. [DOI] [PubMed] [Google Scholar]
- 15.Wong RJ, Kim SH, Joe JK, Shah JP, Johnson PA, Fong Y. Effective treatment of head and neck squamous cell carcinoma by an oncolytic herpes simplex virus. Journal of the American College of Surgeons. 2001;193:12–21. doi: 10.1016/s1072-7515(01)00866-3. [DOI] [PubMed] [Google Scholar]
- 16.Bennett JJ, Kooby DA, Delman K, et al. Antitumor efficacy of regional oncolytic viral therapy for peritoneally disseminated cancer. Journal of Molecular Medicine. 2000;78:166–174. doi: 10.1007/s001090000092. [DOI] [PubMed] [Google Scholar]
- 17.Kooby DA, Carew JF, Halterman MW, et al. Oncolytic viral therapy for human colorectal cancer and liver metastases using a multi-mutated herpes simplex virus type-1 (G207) FASEB Journal. 1999;13:1325–1334. doi: 10.1096/fasebj.13.11.1325. [DOI] [PubMed] [Google Scholar]
- 18.Yazaki T, Manz HJ, Rabkin SD, Martuza RL. Treatment of human malignant meningiomas by G207, a replication-competent multimutated herpes simplex virus 1. Cancer Research. 1995;55:4752–4756. [PubMed] [Google Scholar]
- 19.Advani SJ, Chmura SJ, Weichselbaum RR. Radiogenetic therapy: on the interaction of viral therapy and ionizing radiation for improving local control of tumors. Semin Oncol. 1997;24:633–638. [PubMed] [Google Scholar]
- 20.Advani SJ, Sibley GS, Song PY, et al. Enhancement of replication of genetically engineered herpes simplex viruses by ionizing radiation: a new paradigm for destruction of therapeutically intractable tumors. Gene Ther. 1998;5:160–165. doi: 10.1038/sj.gt.3300546. [DOI] [PubMed] [Google Scholar]
- 21.Stanziale SF, Petrowsky H, Joe JK, et al. Ionizing radiation potentiates the antitumor efficacy of oncolytic herpes simplex virus G207 by upregulating ribonucleotide reductase. Surgery. 2002;132:353–359. doi: 10.1067/msy.2002.125715. [DOI] [PubMed] [Google Scholar]
- 22.Bennett JJ, Adusumilli P, Petrowsky H, et al. Up-regulation of GADD34 mediates the synergistic anticancer activity of mitomycin C and a gamma134.5 deleted oncolytic herpes virus (G207) FASEB J. 2004;18:1001–1003. doi: 10.1096/fj.02-1080fje. [DOI] [PubMed] [Google Scholar]
- 23.Longnecker R, Chatterjee S, Whitley RJ, Roizman B. Identification of a herpes simplex virus 1 glycoprotein gene within a gene cluster dispensable for growth in cell culture. Proceedings of the National Academy of Sciences of the United States of America. 1987;84:4303–4307. doi: 10.1073/pnas.84.12.4303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wong RJ, Joe JK, Kim SH, Shah JP, Horsburgh B, Fong Y. Oncolytic herpesvirus effectively treats murine squamous cell carcinoma and spreads by natural lymphatics to treat sites of lymphatic metastases. Human Gene Therapy. 2002;13:1213–1223. doi: 10.1089/104303402320138998. [DOI] [PubMed] [Google Scholar]
- 25.Fong Y, Kemeny N, Jarnagin W, et al. Phase 1 study of a replication-competent herpes simplex oncolytic virus for treatment of hepatic colorectal metastases. Proc Am Soc Clin Oncol. 2002;21:8a. [Google Scholar]
- 26.Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Advances in Enzyme Regulation. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 27.Chou TC. The median-effect principle and the combination index for quantitation of synergism and antagonism. In: Chou TCR, ed. Synergism and antagonism in chemotherapy. New York: Academic Press; 1991:61–102.
- 28.Chou JCTC. Dose-effect analysis with microcomputers: quantitation of ED50, LD50, synergism, antagonism, low-dose risk, receptor-ligand binding and enzyme kinetics. Manual and Software for IBM-PC. 1987.
- 29.Chou J. Quantitation of synergism and antagonism of two or more drugs by computerized analysis. In: Chou TCR, ed. New York: Academic Press; 1991:223–44.
- 30.Chou TC, Motzer RJ, Tong Y, Bosl GJ. Computerized quantitation of synergism and antagonism of taxol, topotecan, and cisplatin against human teratocarcinoma cell growth: a rational approach to clinical protocol design.[see comment] Journal of the National Cancer Institute. 1994;86:1517–1524. doi: 10.1093/jnci/86.20.1517. [DOI] [PubMed] [Google Scholar]
- 31.Toyoizumi T, Mick R, Abbas AE, Kang EH, Kaiser LR, Molnar-Kimber KL. Combined therapy with chemotherapeutic agents and herpes simplex virus type 1 ICP34.5 mutant (HSV-1716) in human non-small cell lung cancer. Hum Gene Ther. 1999;10:3013–3029. doi: 10.1089/10430349950016410. [DOI] [PubMed] [Google Scholar]
- 32.Bradley JD, Kataoka Y, Advani S, et al. Ionizing radiation improves survival in mice bearing intracranial high- grade gliomas injected with genetically modified herpes simplex virus. Clin Cancer Res. 1999;5:1517–1522. [PubMed] [Google Scholar]
- 33.Todo T, Feigenbaum F, Rabkin SD, et al. Viral shedding and biodistribution of G207, a multimutated, conditionally replicating herpes simplex virus type 1, after intracerebral inoculation in aotus. Mol Ther. 2000;2:588–595. doi: 10.1006/mthe.2000.0200. [DOI] [PubMed] [Google Scholar]
- 34.Varghese S, Newsome JT, Rabkin SD, et al. Preclinical safety evaluation of G207, a replication-competent herpes simplex virus type 1, inoculated intraprostatically in mice and nonhuman primates. Hum Gene Ther. 2001;12:999–1010. doi: 10.1089/104303401750195944. [DOI] [PubMed] [Google Scholar]


