Abstract
Purpose
To determine if α1-adrenergic receptors utilize the nitric oxide (NO)/cGMP pathway to stimulate protein secretion from rat lacrimal gland.
Methods
Identification and cellular location of endothelial nitric oxide synthase (eNOS) and neuronal nitric oxide synthase (nNOS) were determined by western blot and immunofluorescence techniques, respectively. Rat lacrimal gland acini were isolated by collagenase digestion, and protein secretion stimulated by phenylephrine, an α1-adrenergic agonist, was measured using a fluorescent assay system. Acini were preincubated with inhibitors for 20 min prior to addition of phenylephrine (10−4 M). NO and cGMP were measured in response to phenylephrine stimulation. Activation of p42/p44 MAPK was determined using western blot analysis using an antibody against phosphorylated (active) p42/p44 MAPK.
Results
eNOS and nNOS were both present in lacrimal gland. eNOS appeared to be localized with caveoli while nNOS was present in nerves surrounding acini. Inhibition of eNOS with L-NAME (10−6 M) completely inhibited phenylephrine-stimulated protein secretion whereas the inactive isomer D-NAME and inhibition of nNOS with S-methyl-L-thiocitrulline did not. Phenylephrine increased NO production in a time- and concentration-dependent manner that was abolished by the α1D-adrenergic receptor inhibitor BMY-7378. Inhibition of guanylate cyclase with ODQ also inhibited phenylephrine-induced protein secretion while phenylephrine caused a 2.2 fold increase in cGMP. In addition, preincubation with L-NAME and ODQ inhibited phenylephrine-stimulated p42/p44 MAPK activation.
Conclusions
α1D-Adrenergic agonists stimulate eNOS to produce NO leading to production of cGMP by guanylate cyclase to transduce their extracellular signal through the cell to stimulate protein secretion in rat lacrimal gland.
Nitric oxide (NO) is a small, diffusible gaseous molecule that has been identified as a mediator in a variety of cellular functions including secretion, inflammation, and blood flow 1,2,3. NO is synthesized from arginine and molecular oxygen resulting in NO and L-citrulline by nitric oxide synthase (NOS). Nitric oxide synthase is a family of enzymes consisting of three known isoforms, endothelial nitric oxide synthase (eNOS or NOS-3), neuronal nitric oxide synthase (nNOS or NOS-1), and inducible nitric oxide synthase (iNOS or NOS-2). eNOS was first identified in endothelial cells as the enzyme which produced endothelial-derived relaxing factor, later identified as NO 4. It has since been shown to be present in a variety of tissues. nNOS was originally identified in neuronal cells, but has also been shown to be present in many different tissues. eNOS and nNOS are constitutively expressed hence they are referred to as constitutive enzymes. These two isoforms are activated by intracellular calcium and calmodulin. iNOS is not present in resting cells and is induced in a variety of cells by cytokines, infection, or lipopolysaccaride. This isoform is calcium independent and constitutively active 5. Induction of iNOS results in a large, rapid increase in NO that can have detrimental effects to surrounding tissue. In contrast, activation of eNOS and nNOS results in a smaller, slower increase in NO that interacts with a variety of signaling pathways.
The effects of NO can be classified as either cGMP-dependent or cGMP-independent. NO interacts with many different types of proteins however interactions with heme-containing proteins such as hemoglobin, cytochrome P450, ryanodine receptors, or guanylate cyclase (GC) are well-documented 4,6. Ryanodine receptors are sarcoplasmic reticulum calcium-release channels whereas GCs convert GTP to cGMP. The family of GCs is divided into soluble GCs and particulate GCs 7. NO is a potent activator of soluble GCs but not of particulate GC 7. Signal transduction by cGMP is dependent upon its synthesis by GCs, its targeting, and its degradation by cGMP-dependent phosphodiesterases (PDEs). Once produced, cGMP interacts with protein kinase G (PKG) to phosphorylate downstream proteins, many which have yet to be identified that activate a variety of cellular functions.
The lacrimal gland is an exocrine gland responsible for producing the majority of the aqueous portion of the tear film 8. Acinar cells are the major cell type present in the lacrimal gland. In addition, myoepithelial and ductal epithelial cells are also present. The function of the lacrimal gland is to secrete water, electrolytes and protein, which maintain, nourish, and protect the cells of the cornea and conjunctiva. If lacrimal gland secretion is altered in either amount or composition, a spectrum of diseases called dry eye syndromes results. Therefore secretion from the lacrimal gland is tightly regulated. As a result, parasympathetic and sympathetic nerves extensively innervate the lacrimal gland. Stimulation of the afferent sensory nerves in the cornea triggers tear secretion through the efferent parasympathetic and sympathetic nerves that innervate the lacrimal gland 8. Receptors for these neurotransmitters are located on the basolateral side of the acinar cells8. Activation of these receptors initiates signal transduction pathways that culminate in secretion of proteins, electrolytes, and water across the apical membranes into the lumen and onto the cornea and conjunctiva.
We have previously shown that cholinergic agonists released from parasympathetic nerves and α1-adrenergic agonists released from sympathetic nerves are potent stimuli of protein secretion from the lacrimal gland. The signal transduction pathways used by cholinergic agonists have been well characterized 8. These agonists bind to the M3 muscarinic receptor to activate Gαq/11, which in turn activates phospholipase C (PLC). PLC causes the production of inositol trisphosphate, which causes the release of intracellular Ca2+. Diacylglycerol is also produced by PLC, which activates protein kinase C (PKC)α,-δ, and -ɛ9. In contrast, the pathways utilized by α1-adrenergic agonists have remained elusive. We have also shown that α1-adrenergic receptors mediate both protein secretion and activation of p42/p44 MAPK 10. In spite of this information, the G protein(s) involved in transduction of this signal are unknown, though Meneray et al have shown that inhibition Gαq is responsible for approximately 32% of the α1-adrenergic agonist-induced protein secretion 11. The phospholipase involved has also not been identified though it is known that neither phospholipase C nor D are involved 12,13. It is known that phenylephrine (an α1-adrenergic agonist in the lacrimal gland) activates PKC-ɛ, which stimulates protein secretion and PKCα and –δ to inhibit secretion. We have also shown that α1-adrenergic agonists transactivate the EGF receptor, recruiting Shc and Grb2 leading to activation of p42/p44 MAPK. Activation of p42/p44 MAPK inhibits protein secretion and may act to attenuate overall protein secretion from the lacrimal gland or to terminate stimulated secretion.
It is known that the lacrimal gland contains many of the proteins necessary for the production of NO and cGMP. nNOS has been shown to be present in the lacrimal gland surrounding ducts, blood vessels, and acinar cells 14,15. Exogenous addition of NO, through the use of NO donors, has been shown to increase total protein secretion, which is inhibited by guanylate cyclase inhibitors in cultured lacrimal gland acinar cells 3,16. In addition, NO donors which increase cGMP induced a rise in intracellular [Ca2+] ([Ca2+]i) through the release of Ca2+ from intracellular stores 17. Jorgensen et al demonstrated that α1-adrenergic agonist-induced increase in [Ca ]i was inhibited by inhibitors of GC and cGMP-dependent protein kinase Ia 18. Although it is not known if activation of muscarinic receptors increases NO, it is known that when total IgGs and purified autoantibodies against M3 muscarinic receptors from patients suffering from the autoimmune disease Sjogren’s syndrome were added to freshly prepared rat lacrimal gland slices, NOS activity was increased. This activity was mediated via the M3 muscarinic receptor as the M3 muscarinic inhibitor, 4-DAMP, inhibited NOS activity 19. Thus evidence supporting a role for α1-adrenergic, β-adrenergic, or muscarinic agonists in stimulating lacrimal gland protein secretion via the NO/cGMP pathway is lacking.
In the present study, we confirm the presence of eNOS and nNOS in the lacrimal gland and demonstrate that the α1-adrenergic agonist phenylephrine, using α1D-adrenergic receptors, activates eNOS, but not nNOS, to increase NO production. NO, in turn, activates GC to increase cGMP levels. α1-Adrenergic agonist activation of the NO/cGMP pathway leads to an increase in protein secretion and p42/p44 MAPK induction from freshly isolated rat lacrimal gland acinar cells.
MATERIALS AND METHODS
Materials
Rabbit polyclonal antibodies directed against eNOS, nNOS, and caveolin-1 were purchased from Transduction Labs (San Jose, CA). Monoclonal antibody against α-smooth muscle actin was purchased from Diagonstic Biosystems (Pleasanton, CA). MAP 2 antibody was from Sigma-Aldrich (St. Louis, MO). Antibodies to phosphorylated p42/p44 and total p42 MAPK were purchases from Santa Cruz Biotechnology (Santa Cruz, CA). L-NAME, D-NAME, S-methyl-L-thiocitrulline, L-thiocitrulline and ODQ were from Calbiochem (San Diego, CA). NO and cGMP assay kits were from Biomol Research Laboratories (Plymouth Meeting, PA). Amplex red was acquired from Molecular Probes (Eugene, OR). All other reagents were obtained from Sigma (St. Louis, MO).
Animals
Male Sprague-Dawley rats weighing between 125 – 150g were obtained from Taconic Farms (Germantown, NY). They were maintained in constant temperature rooms with fixed light/dark intervals of 12 hours and were fed ad libitum. The rats were anesthetized for 1 minute in CO2, decapitated, and both exorbital lacrimal glands were removed. All experiments were in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and were approved by the Schepens Eye Research Institute Animal Care and Use Committee.
Preparation of Rat Lacrimal Gland Acini
Acini were prepared using a modification of a method developed by Herzog et al 20. In brief, lacrimal glands were trimmed, fragmented, and incubated with collagenase CLSIII (100 U/ml) in Krebs-Ringer Bicarbonate (KRB) buffer (119 mM NaCl, 4.8 mM KCl, 1 mM CaCl2, 1.2 mM MgSO4, 1.2 mM KH2PO4, and 25 mM NaHCO3) supplemented with 10 mM HEPES, 5.5 mM glucose (KRB-HEPES), and 0.5% BSA, pH 7.4. Lobules were subjected to gentle pipetting through tips of decreasing diameter, filtered through nylon mesh (150 μm pore size), and centrifuged briefly. The pellet was washed twice by centrifugation (50 x g, 2minutes) through a 4% BSA solution made in KRB-HEPES buffer. The dispersed acini were allowed to recover for 60 min in fresh KRB-HEPES buffer containing 0.5% BSA.
Western Blot Analysis
Lacrimal glands were homogenized in RIPA buffer (9.1 mM dibasic sodium phosphate, 1.7 mM monobasic sodium phosphate 150 mM NaCl pH 7.4, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS). Proteins in the supernatant were separated by SDS-PAGE on an 8% gel. Separated proteins were transferred onto nitrocellulose membranes, which were blocked overnight at 4°C in 5% non-fat dried milk in buffer containing 10 mM Tris-HCl, pH 8.0, 150 mM NaCl, and 0.05% Tween-20 (TBST), and then incubated with the primary antibody (1:200 for eNOS and 1:100 for nNOS) for 2 hours at room temperature. After washing the membranes three times in TBST, the membranes were incubated with HRP-conjugated secondary antibody. Immunoreactive bands were detected by the enhanced chemiluminescence method.
Immunohistochemistry
The lacrimal glands were fixed by immersion in 4% formaldehyde diluted in phosphate-buffered saline (PBS, 145 mM NaCl, 7.3 mM Na2HPO4, and 2.7 mM NaH2PO4, pH 7.2) for 4 hours at 4°C. After cryopreservation overnight at 4°C in 30% sucrose dissolved in PBS, the tissue was frozen in optimal cutting embedding compound. Cryostat sections (6 μm) were placed on gelatin-coated slides and air-dried for 2 hours. The sections were air-dried and rinsed for 5 minutes in TBS (10 mM Tris-HCl, pH 8.0 and 150 mM NaCl). Non-specific sites were blocked in PBS containing 10% normal goat serum, 1% bovine serum albumin (BSA) and 0.2% Triton X-100 for 45 minutes. After rinsing for 5 minutes in TBS, the sections were then incubated with the primary antibody for 2 hours at room temperature in a humidified chamber. Antibodies to eNOS, nNOS, α-smooth muscle actin, or caveolin-1 were used at a concentration of 1:50 diluted in TBS while the antibody to microtubule-associated protein 2 (MAP 2) was used at 1:500 diluted in TBS. The secondary antibodies conjugated to either FITC (1:150) or Cy3 (1:300), diluted in TBS with 1% BSA, were applied for 1 hour at room temperature. Coverslips were mounted with a medium consisting of glycerol and paraphenylenediamine. For negative controls, the primary antibody was omitted. The sections were viewed with a Nikon Eclipse E80i microscope and pictures were taken with a SPOT Diagnostic Instruments Inc. camera.
Measurement of peroxidase secretion
Lacrimal gland acini were incubated in 0.5% BSA-KRB-HEPES buffer in duplicate for 20 minutes at 37°C in the absence or presence the α1-adrenergic agonist phenylephrine (10−4 M). Inhibitors were added 20 min prior to addition of phenylephrine. After a brief centrifugation, supernatant was collected, and peroxidase activity was measured in duplicate in both the supernatant and the pellet fraction using Amplex Red. Oxidation of this reagent by peroxidase in the presence of hydrogen peroxide produces a highly fluorescent molecule, resorufin. The amount of fluorescence was quantified by a fluorescence microplate reader (model FL600; Bio-Tek, Winooski, VT) with an excitation wavelength of 530 nm and an emission wavelength of 590 nm. Peroxidase secretion, an index of lacrimal gland protein secretion, was expressed as the percent peroxidase secreted into the medium (supernatant) compared to total peroxidase in the cells (pellet) plus the medium.
Measurement of NO
Lacrimal gland acini were incubated with phenylephrine (10−4 M) for 0–30 seconds or with increasing concentrations of phenylephrine (10−7 – 10−3 M) for 20 seconds. The supernatant was collected and filtered through 10,000 MW cut off filters to remove proteins. The amount of NO was determined using a nitric oxide assay kit by reducing nitrate to nitrite with nitrate reductase. Nitrite was determined using the Greiss reaction and the color reaction was read at 540 nm according to the manufacturer’s protocol.
Measurement of cGMP
Lacrimal gland acini were preincubated for 10 minutes with the phosphodiesterase inhibitor 3-isobutylmethylxanthine prior to stimulation with phenylephrine (10−4 M) for 0 – 30 minutes. The reaction was stopped by brief centrifugation and supernatant removed and acini were lysed in 0.1 N HCl. The cells were sonicated briefly and centrifuged for 5 minutes at 5,000 x g at room temperature. The amount of cGMP in the supernatant was measured using a cGMP assay kit according to the manufacturer’s instructions.
Measurement of MAPK activity
The activation of p42/44 MAPK was examined using western blot techniques. Acini were preincubated with the inhibitors L-NAME, its inactive isomer D-NAME, and ODQ for 20 minutes prior to addition of phenylephrine (10−4 M) for 5 minutes. To terminate incubation, ice-cold KRB buffer was added and centrifuged briefly. The pellet was homogenized in RIPA buffer (10 mM Tris-HCl, pH7.4, 150 mM NaCl, 1% sodium deoxycholate, 1% Triton X-100, 0.1% SDS, 1 mM EDTA, 100 μg/ml phenylmethylsulfonyl fluoride, 30 μl/ml aprotinin, 1 mM Na3VO3), sonicated, and centrifuged at 20,000 x g for 30 minutes. As described for Western blot analysis, the proteins in the supernatant were separated by SDS-PAGE, transferred onto nitrocellulose membrane, and probed with either total p42 MAPK (1:1000) or phosphorylated p42/44 MAPK (1:100) followed by HRP-conjugated secondary antibody. Immunoreactive bands were digitally scanned and analysed using ImageJ (National Institutes of Health). The amount of phosphorylated p42/p44 MAPK in each sample was standardized to the amount of total p42 MAPK.
Data presentation and statistical analysis
Data are expressed as fold increase above basal value, which was standardized to 1.0. Results are expressed as mean ± SEM. Data were analysed by Student’s t-test. p<0.05 was considered statistically significant.
RESULTS
Presence of eNOS and nNOS in the Lacrimal Gland
To confirm the presence of nNOS and determine if eNOS is present in rat lacrimal gland, either the whole lacrimal gland or acini were homogenated in RIPA buffer and proteins separated by SDS-PAGE as described in methods. Western blot analysis was then performed using antibodies to the eNOS and nNOS isoforms. Homogenates from liver, aorta, and brain were used as positive controls. In lacrimal gland, eNOS was present at the same molecular weight as that in the brain and liver. Interestingly, the major band present in lacrimal gland homogenate was a slightly higher molecular weight than the major band present in the lacrimal gland acini. eNOS expressed in the aorta was a lower molecular weight than either the liver, brain, or lacrimal gland (Figure 1).
Figure 1. Western Blot Analysis of eNOS and nNOS in rat lacrimal gland.
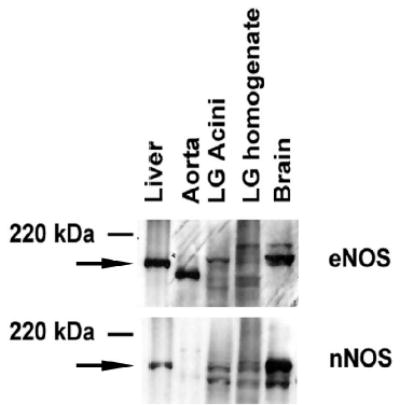
Rat liver, cultured rat aorta cells, rat lacrimal gland acini, whole rat lacrimal gland and rat brain were homogenized and proteins separated by SDS-PAGE and transferred to nitrocellulose. Blots were probed with antibodies against eNOS (upper blot) and nNOS (lower blot). Blots shown are representative of three independent experiments.
nNOS was also present in both the lacrimal gland homogenate and acini. The major band in both lacrimal gland homogenate and acini were at the same molecular weight as the nNOS present in liver and brain (Figure 1). An additional, lower molecular weight band was also observed in the brain, lacrimal gland acini, and homogenate and may represent a different phosphorylation state 21.
These data indicate that the lacrimal gland expresses the eNOS and nNOS isoforms of NOS.
Localization of eNOS and nNOS in the Lacrimal Gland
To determine the localization of eNOS, lacrimal gland sections were incubated with an antibody directed against eNOS and immunofluorescence was performed. eNOS was present in a cobblestone pattern with immunoreactivity present in the basal membranes of acinar cells (Figure 2A). As eNOS has been shown to be present in caveolae 22,23, lacrimal gland sections were also labeled with an antibody against caveolin-1 to determine if eNOS expressed in the lacrimal gland was also present in the caveolin. As shown in Figure 2B, caveolin-1 was located on the basal membranes of acinar cells in a pattern similar to that seen with eNOS. When sections were double-labeled with both eNOS and caveolin-1, there was significant co-localization of both proteins in the basal membranes (Figure 2C).
Figure 2. Localization of eNOS in rat lacrimal gland.
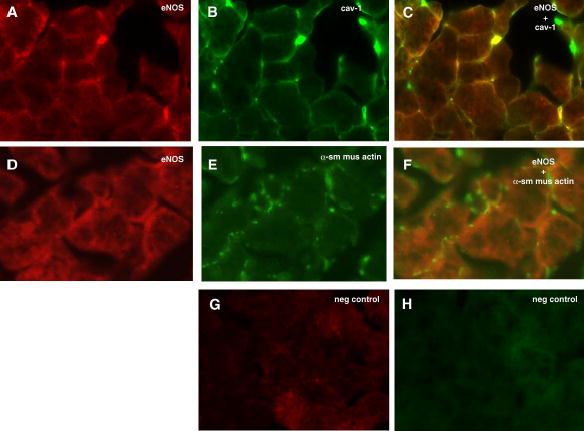
Rat lacrimal gland sections were double labeled with antibodies against eNOS (A) and caveolin-1 (B). Co-localization of eNOS and caveolin-1 is shown in yellow in C. Sections were also double labeled with eNOS (D) and α-smooth muscle actin (E) to label myoepithelial cells. Overlay of D and E is shown in F. Negative control for Cy3 is shown in G while negative control for FITC is shown in H. Magnification 400 x. Pictures are representative of three independent experiments.
As NO is a diffusible molecule and myoepithelial cells are present in the lacrimal gland and are believed to play a role in secretion, we determined if eNOS was also present in these cells. Myoepithelial cells contain an abundance of α-smooth muscle actin, which can be used to identify these cells 24. As shown in Figure 2D, myoepithelial cells were abundant throughout the gland and present surrounding acinar cells. When double labeled with eNOS antibody, there was no apparent co-localization indicating that myoepithelial cells do not contain eNOS (Figure 2F). Negative controls were devoid of staining (Figures 2G and H).
The localization of nNOS was also determined using an antibody against nNOS and performing immunofluorescence experiments. nNOS surrounded a population of acini near the basal membrane (Figure 3A). nNOS has been localized to nerve filaments in many different tissues 25,26,27 including the lacrimal gland 14,15 6,28 therefore lacrimal gland sections were also double-labeled with microtubule associated protein 2 (MAP 2). MAP 2 is highly expressed in mammalian nerves 29. Localization of MAP 2 was observed in nerve filaments (Figure 3B). When the staining of nNOS and MAP 2 were overlayed, significant co-localization of nNOS and MAP 2 was observed (Figure 3C).
Figure 3. Localization of nNOS in rat lacrimal gland.
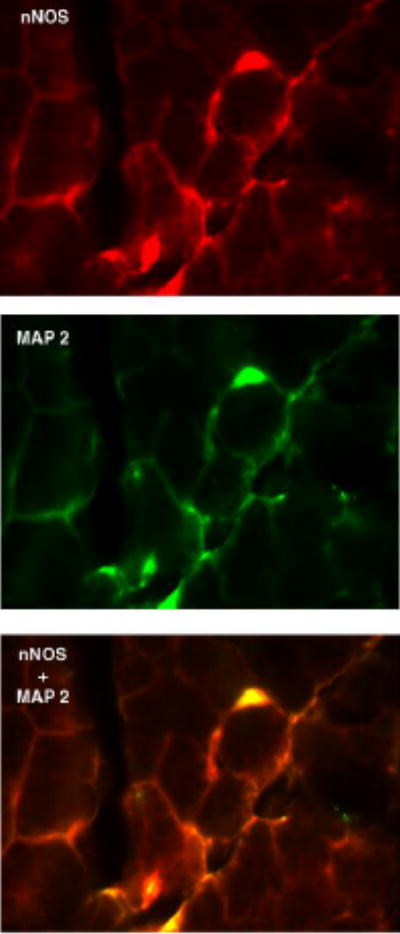
Rat lacrimal gland sections were double labeled with antibodies against nNOS (A) and MAP 2 (B). Co-localization of nNOS and MAP 2 is shown in yellow in C. Magnification 400 x. Pictures are representative of three independent experiments.
These data indicate that eNOS is present the in the caveolae and nNOS is present in the nerve fibers of the rat lacrimal gland.
Effect of Inhibition of eNOS and nNOS on α1-Adrenergic Agonist-induced Protein Secretion
As the α1-adrenergic agonist phenylephrine is a potent stimulator of protein secretion, we determined the effects of inhibitors of eNOS and nNOS on phenylephrine-stimulated lacrimal gland secretion. Acini were preincubated with either the eNOS inhibitor L-NAME, its inactive isomer D-NAME or the nNOS specific inhibitors S-methyl-L-thiocitrulline or L-thiocitrulline for 20 minutes. Phenylephrine (10−4 M) was then added for an additional 20 minutes. As shown in Figure 4A, phenylephrine increased peroxidase secretion by 2.9 ± 0.7 fold above control. L-NAME, which has an IC50 of 0.5 x 10−6 M for eNOS 30 and is reportedly 2000 fold more selective for eNOS than nNOS in an allergic rat model 31, completely inhibited phenylephrine-induced protein secretion at 10−6 M. The inactive isomer, D-NAME had no effect on phenylephrine-induced protein secretion (Figure 4A). In addition, neither L-thiocitruline nor S-methyl-L-thiocitrulline, which is more selective for nNOS than eNOS 1,32, had any effect on phenylephrine-stimulated protein secretion (Figure 4B).
Figure 4. Effect of inhibition of eNOS and nNOS on α1-adrenergic agonist-stimulated protein secretion.
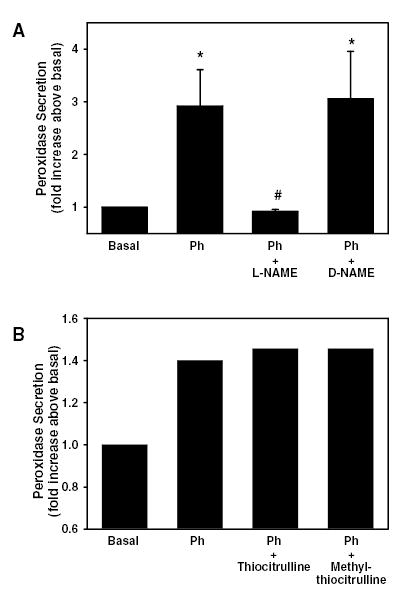
A. Lacrimal gland acini were preincubated with the eNOS inhibitor L-NAME or its inactive isomer D-NAME at the indicated concentration for 20 minutes prior to stimulation with the α1-adrenergic agonist phenylephrine (10−4 M) for 20 minutes. Peroxidase secretion was then measured. Values are mean ± SEM from 4–6 independent experiments. * indicates statistically significant difference from basal. # indicates statistically significant difference from phenylephrine alone. B. Acini were preincubated with the nNOS inhibitors thiocitrulline or methyl-thiocitrulline at the indicated concentration for 20 minutes prior to stimulation with the α1-adrenergic agonist phenylephrine (10−4 M) for 20 minutes. Peroxidase secretion was then measured. Values are mean from 2 independent experiments.
As a control, acini were also incubated with the cholinergic agonist carbachol (Cch), which has been shown to stimulate peroxidase secretion through the Ca2+/PKC pathway 8. Cch (10−4 M) increased secretion 1.2 ± 0.03 fold above basal (data not shown). Cch-stimulated secretion was unchanged after preincubation with L-NAME or D-NAME (10−6 M, data not shown).
These data imply that phenylephrine stimulates eNOS, but not nNOS, to activate peroxidase secretion.
Effect of α1-Adrenergic Agonists on NO Production
Since an inhibitor of NOS, inhibited phenylephrine-induced protein secretion, we determined if phenylephrine increased NO production. Acini were incubated with phenylephrine (10−4 M) for 0–30 seconds and the amount of NO was measured in the supernatant as described above. Phenylephrine statistically significantly increased the production of NO in a time dependent manner 2.1 ± 0.6 and 2.8 ± 0.6 fold above basal after 20 seconds and 30 seconds, respectively (Figure 5A).
Figure 5. Effect of α1-adrenergic agonist-stimulated on nitric oxide production.
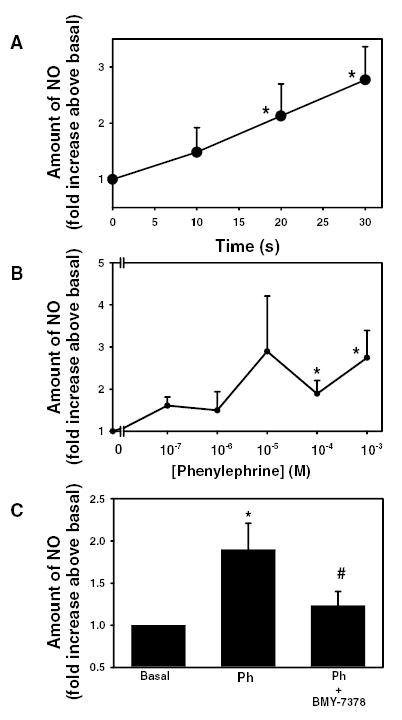
Rat lacrimal gland acini were stimulated with phenylephrine (10−4 M) for varying times (A) or with varying concentrations of phenylephrine for 20 seconds (B) and the amount of NO was measured. (C) Acini were also preincubated with BMY-7378 (10−8 M) for 20 minutes prior to addition of phenylephrine (10−4 M) for 20 seconds. Values are mean ± SEM from 3–9 independent experiments. * indicates statistically significant difference from basal. # indicates statistically significant difference from phenylephrine alone.
Phenylephrine also increased NO in a concentration dependent manner. Acini were incubated for 20 seconds with phenylephrine concentrations ranging from 10−7–10−3 M. Phenylephrine 10−4 and 10−3 M statistically significantly increased NO 1.9 ± 0.3 and 2.7 ± 0.6 fold above basal, respectively (Figure 5B).
To determine if phenylephrine-induced increase in NO is mediated through the α1D-adrenergic receptor, acini were preincubated with α1D-adrenergic receptor antagonist BMY-7378 (10−8 M) for 20 minutes prior to addition of phenylephrine (10−4 M) for 20 seconds. BMY-7378 completely inhibited phenylephrine-induced increase in NO (Figure 5C).
These data indicate that phenylephrine, acting through the α1D-adrenergic receptor, increases NO production in a time- and concentration-dependent manner.
Effect of NO Donors and cGMP Analogs on Lacrimal Gland Protein Secretion
As a positive control, lacrimal gland acini were incubated with the NO donor, S-nitroso-N-acetyl penicillamine (SNAP). SNAP (1.7 x 10−4 M) was added for 20 min and peroxidase secretion was measured. SNAP statistically significantly increased by 1.4 ± 0.2 fold above basal (Figure 6). This is compared to phenylephrine-stimulated protein secretion which increased peroxidase secretion by 1.6 ± 0.2 fold above basal.
Figure 6. Effect of NO donors and cGMP on lacrimal gland protein secretion.
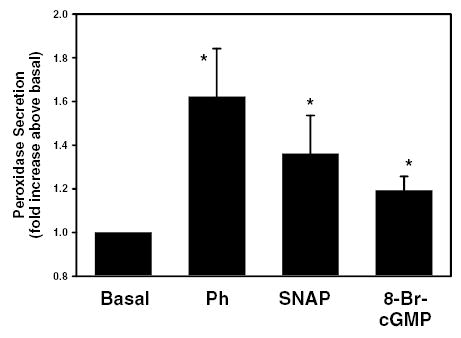
Rat lacrimal gland acini were incubated with either the nitric oxide donor S-nitroso-N-acetyl penicillamine (SNAP, 1.7 x 10−4 M), the α1-adrenergic agonist phenylephrine (10−4 M), or 8-bromocGMP (10−3 M) for 20 min and protein secretion was measured. Values are mean ± SEM from 6–9 independent experiments. * indicates statistically significant difference from basal.
One possible consequence of an increase in the production of NO production is to stimulate the activity of guanylate cyclase to produce cGMP. To determine if an increase in intracellular concentrations of cGMP had an effect on peroxidase secretion, acini were incubated with the cell permeable analog of cGMP, 8-bromo-cGMP and peroxidase secretion was measured. 8-Br-cGMP, (10−3 M) statistically significantly increased peroxidase to 1.2 ± 0.1 fold above basal (Figure 6).
These data indicate that exogenous addition of NO or cGMP is sufficient to increase peroxidase secretion in lacrimal gland acini.
Effect of Guanylate Cyclase Inhibitors on α1-Adrenergic Agonist-stimulated Protein Secretion
Since phenylephrine stimulation increases NO production through activation of NOS to stimulate protein secretion and addition of exogenous cGMP also increases protein secretion, we next investigated the effects of inhibition of guanylate cyclase, the enzyme which synthesize cGMP from GTP, on phenylephrine-stimulated peroxidase secretion. Lacrimal gland acini were preincubated for 20 minutes with oxadiazoloquinoxalin (ODQ, 10−10 – 10−7 M) prior to incubation with phenylephrine (10−4 M) for 20 minutes. Phenylephrine statistically significantly increased peroxidase secretion 1.7 ± 0.1 fold above basal (Figure 7). ODQ statistically significantly inhibited phenylephrine-induced peroxidase secretion by 64.3 ± 23.5%, 43.7 ± 24.1, and 90.7 ± 7.9% to 1.1 ± 0.2, 1.3 ± 0.1, and 1.0 ± 0.1 at 10−9, 10−8, and 10−7 M, respectively (Figure 7). As a control, acini were also stimulated with Cch (10−4 M) for 20 minutes. Cch stimulated secretion 1.2 ± 0.1 fold above basal (data not shown). Similar to L-NAME and D-NAME, ODQ (10−9 M) did not have any effect on Cch-stimulated protein secretion (data not shown).
Figure 7. Effect of inhibition of guanylate cyclase on α1-adrenergic agonist-stimulated protein secretion.
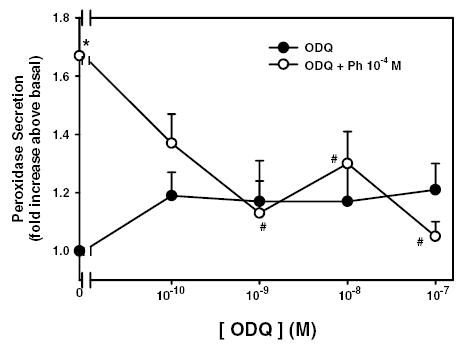
Lacrimal gland acini were preincubated with the guanylate cyclase inhibitor ODQ from 10−10–10−7 M for 20 minutes either alone (closed circles) or prior to addition of the α1-adrenergic agonist phenylephrine (10−4 M) for 20 minutes (open circles). Values are mean ± SEM from 3 independent experiments. * indicates statistically significant difference from basal. # indicates statistically significant difference from phenylephrine alone.
These data indicate that guanylate cyclase is activated by α1-adrenergic agonists to stimulate protein secretion.
Effect of Phenylephrine on cGMP Concentration
Since phenylephrine generates NO and inhibition of guanylate cyclase leads to decreased peroxidase secretion, we next determined if phenylephrine increases the cGMP concentration within the acinar cells. Acini were incubated with phenylephrine (10−4 M) for 0 – 30 minutes and the amount of cGMP was determined via enzyme immunoassay as described in the Methods. As shown in Figure 7, phenylephrine statistically significantly increased the amount of cGMP 2.2 fold above control from 0.05 ± 0.02 to 0.11 ± 0.01 pmol/ml cGMP after 5 minutes (Figure 8). The amount of cGMP declined to 0.10 ± 0.04 after 10 minutes and returned to basal value 15 minutes after addition of phenylephrine (Figure 8).
Figure 8. Effect of α1-adrenergic agonists on cGMP concentration.
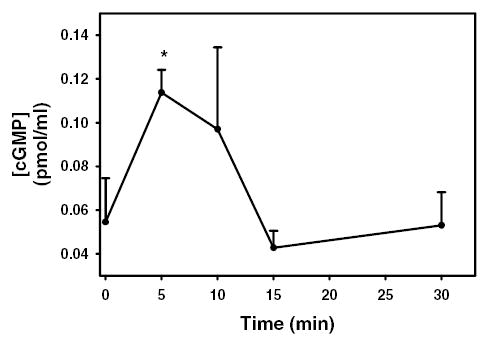
Lacrimal gland acini were incubated with the α1-adrenergic agonist phenylephrine (10−4 M) for 0–30 minutes. cGMP concentration was then measured. Values are mean ± SEM from 3 independent experiments. * indicates statistically significant difference from basal.
These data indicate that phenylephrine increases cGMP concentration in lacrimal gland acini.
Effect of Inhibition of NO and Guanylate Cyclase on α1-Adrenergic agonist-stimulated MAPK Activation
We previously showed that phenylephrine transactivates the EGFR to activate p42/p44 MAPK 10. Activation of p42/p44 MAPK negatively modulates peroxidase secretion 10. To determine if generation of NO and cGMP play a role in the transactivation of the EGFR, acini were incubated with either the NOS inhibitor L-NAME (10−6 M), its inactive isomer D-NAME (10−6 M) or the guanylate cyclase inhibitor ODQ (10−9 M) for 20 minutes prior to stimulation with phenylephrine (10−4 M) for 5 minutes. Western blot analysis was performed using antibodies against phosphorylated (active) and total p42/p44 MAPK. Phenylephrine statistically significantly increased phosphorylation of p42/p44 MAPK 1.2 ± 0.1 fold above basal (data not shown). The amount of phosphorylated p42/p44 MAPK stimulated with phenylephrine, in the presence of L-NAME was statistically significantly reduced to 19.5 ± 16.9% of the response seen with phenylephrine alone (Figure 9). The inactive isomer D-NAME decreased phosphorylated p42/p44 MAPK though the response was not statistically significant. Neither L-NAME nor D-NAME alone had an effect on basal p42/p44 MAPK activity (data not shown).
Figure 9. Effect of inhibition of eNOS and guanylate cyclase on α1-adrenergic agonist-stimulated p42/p44 MAPK.
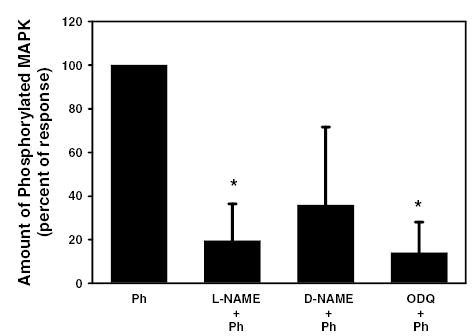
Lacrimal gland acini were incubated for 20 minutes with the NOS inhibitor L-NAME (10−6 M), the inactive isomer D-NAME (10−6 M), or the guanylate cyclase inhibitor ODQ (10−9 M) prior to stimulate with the α1-adrenergic agonist phenylephrine (10−4 M) for 5 minutes. Activated p42/p44 MAPK was determined via western blotting techniques using an antibody directed against phosphorylated (activated) p42/p44 MAPK. Values are mean ± SEM from 3 independent experiments. * indicates statistically significant difference from phenylephrine.
Similar to L-NAME, the activation of p42/p44 MAPK in the presence of phenylephrine and ODQ was statistically significantly reduced to 14 ± 14% of the response seen with phenylephrine alone (Figure 9). ODQ alone did not have any effect on basal p42/p44 MAPK activity (data not shown).
These data indicate that generation of NO and cGMP are necessary for phenylephrine-induced activation of p42/p44 MAPK.
DISCUSSION
In this study we have shown that α1-adrenergic agonists, acting through the α1D-adrenergic receptors, stimulate NOS, probably eNOS, to generate NO leading to production of cGMP. Activation of this pathway by α1-adrenergic agonists leads to increased protein secretion and activation of p42/p44 MAPK from freshly isolated rat lacrimal gland acini.
We have identified the presence and localization of eNOS and confirmed the presence and location of nNOS in the lacrimal gland. Many studies have shown that eNOS is localized to caveolae 22,23,33, and in vitro studies have shown that interactions between caveolin-1 and eNOS lead to an inhibition of eNOS activity 34. Caveolae are microdomains of the plasma membrane in which numerous signaling molecules are targeted 34. In endothelial cells, eNOS translocates from the caveoli to the cytoplasm upon stimulation and is presumably activated 34. While activation of eNOS upon stimulation has not been shown in epithelial cells, it is known that many agonists stimulate eNOS activation. In the lacrimal gland, similar to other tissues, eNOS colocalizes with caveolin-1 in unstimulated cells. Thus it is possible that addition of α1-adrenergic agonists could translocate eNOS from the caveolae to the cytoplasm.
Previous studies have demonstrated the presence and localization of nNOS in the mouse lacrimal gland with nNOS immunoreactivity appearing in nerve fibers and surrounding a few acini 14. In the present study, nNOS immunoreactivity was abundantly present surrounding numerous acini of the rat lacrimal gland. In addition, nNOS was present in nerves as this immunoreactivity co-localized with MAP 2, a protein abundant in mammalian nerves. It is possible that either a species difference exists such that the rat lacrimal gland contains more nNOS surrounding acini than the mouse. It is also possible that different antibodies used in each study could account for the differences between the previous and present study.
We did not investigate the role of iNOS in protein secretion. iNOS is known as an inducible enzyme and its expression is stimulated by inflammation and bacterial products 5. It is unlikely that iNOS would be involved in α1-adrenergic agonist-stimulated protein secretion, as this stimulation is a very fast process occurring in seconds whereas induction of iNOS would be a longer-term process. Beauregard et al showed maximum expression of iNOS occurred four hours after addition of interleukin-1β to cultured rabbit lacrimal gland acinar cells 16.
In the lacrimal gland, L-NAME was the only NOS inhibitor to inhibit α1-adrenergic-induced protein secretion. The specificity of NOS inhibitors, including L-NAME, is controversial, as some studies have indicated that L-NAME is more selective for eNOS than nNOS or iNOS. Others have determined that L-NAME is more selective for the constitutive NOS isoforms (eNOS and nNOS) than the inducible form (iNOS)35. In rat aorta L-NAME reverses the vasodilation effects caused by acetylcholine with an IC50 of 0.5 μM 36. In the lacrimal gland, inhibition of NOS with L-NAME completely inhibited α1-adrenergic agonist-stimulated protein secretion while the inhibitors L-thiocitruline nor S-methyl-L-thiocitrulline, which have greater selectivity for nNOS than eNOS 1, had no effect on this secretion. These data imply that eNOS is primarily responsible for the increased generation of NO seen with α1-adrenergic agonists leading to protein secretion. eNOS has been shown to be activated by α2-adrenergic receptors to inhibit NaCl absorption in thick ascending limb of the loop of Henle 37 and to increase mucus secretion in estradiol-stimulated cervical epithelial cells 38.
The effects of the NOS inhibitor L-NAME and guanylate cyclase inhibitor ODQ are specific to α1-adrenergic receptors, as neither inhibitor had any effect on cholinergic agonist-stimulated secretion. Bacman et al previously showed that Cch increases activity of NOS and stimulates the production of cGMP in rat lacrimal gland 39. These authors, however, did not link the increase in cGMP to a specific cellular function. In the present study, we measured the effects of NOS and guanylate cyclase inhibitors on a cellular function, namely protein secretion. Thus it is possible that cholinergic agonists do increase NOS activity and stimulate the production of cGMP, but these molecules do not play a role in cholinergic agonist-stimulated protein secretion.
Previous studies of the lacrimal gland have shown that generation of NO leads to increased protein secretion as demonstrated by Beauregard et al 3. In that study, these authors measured the effects of NO generators on total protein release in cultured rabbit lacrimal gland acinar cells. Measurement of total protein secretion can be problematic as this method measures not only proteins secreted in a regulated manner such as peroxidase, but also constitutively released proteins and proteins that can be released by ectodomain shedding such as occurs with growth factors such as epidermal growth factor. Almost 4% of cell surface proteins can be released by ectodomain shedding. In the present study, we show that NO production can occur via agonist-induced receptor activation leading to regulated protein secretion in freshly isolated acinar cells. Receptor-linked generation of NO also occurs in other exocrine glands such as the pancreas where cholecystokinin and carbachol stimulate NO production and amylase release and the submandibular glands where Cch-stimulated amylase secretion was inhibited by ODQ 2,40.
NO and cGMP production have also been linked to release of calcium from rat lacrimal gland 17,18. Jorgensen et al reported that inhibitors of guanylate cyclase and cGMP-dependent protein kinase Ia inhibited phenylephrine induced-Ca2+ release 18. In addition, NO donors also stimulated release of Ca2+ and NO meditated the release of Ca2+ mediated by β-adrenergic but not α1-adrenergic, agonists increase the production of NO 17,41. Addition of cGMP analogs did not increase [Ca2+]i themselves nor did they have any effect on cyclic ADP-ribose-induced Ca2+ release nor did α1-adrenergic agonists increase NO production17. In these studies, NO was measured using the fluorescent molecule DAF-2. However, it is not possible to use the ratio method with DAF-2 similar to what is used with fura2 41. Thus, its fluorescence is dependent upon the amount of dye entering the cells, the extent of ester hydrolysis within the cell altering the non-fluorescent DAF-2 DA to the fluorescent DAF-2, and the number of cells in each preparation. These factors change with each cell preparation making this method unreliable. In the present study, α1-adrenergic agonists caused a significant increase in NO as determined by reducing nitrate to nitrite and measuring the amount of nitrite using the Greiss reaction, a well-established reaction. As previous studies showed that α1-adrenergic agonist-induced protein secretion is dependent on an increase in [Ca2+]i 12, it is possible that the synthesis of cGMP by NOS leads to an activation of guanylate cyclase followed by an increase in [Ca2+]i which ultimately leads to protein secretion. We did not examine the role of NO in the release of Ca2+ in this study.
In addition to protein secretion, NO/cGMP production was also linked to another lacrimal gland function, namely activation of p42/p44 MAPK. The NOS inhibitor L-NAME and the guanylate cyclase inhibitor ODQ both inhibited α1-adrenergic agonist-induced activation of p42/p44 MAPK.
We previously showed that PKCɛ, but not PKCα or –δ, played a significant role in α1-adrenergic agonist stimulated protein secretion 42. Inhibition of PKCɛ blocks phenylephrine-induced protein secretion by approximately 80%. As inhibition of eNOS also substantially inhibits phenylephrine-induced protein secretion, it is likely that there is an interaction between PKCɛ and eNOS. Interestingly, PKCɛ has been shown to bind to and phosphorylate both Akt and eNOS in murine heart leading to an increase in activation of both molecules 43. While we did not examine the interactions between PKCɛ and eNOS, it is possible that a similar interaction also occurs in the lacrimal gland.
In summary, we have shown that α1-adrenergic agonists, acting through the α1D-adrenergic receptors, stimulate NOS, probably eNOS, to generate NO leading to production of cGMP. Activation of this pathway by α1-adrenergic agonists leads to increased protein secretion and induction of p42/p44 MAPK from freshly isolated rat lacrimal gland acini.
Acknowledgments
The authors would like to thank Dr. Diane Darland for providing the cultured aorta cell homogenate and Dr. Driss Zoukhri for many helpful discussions and reading of the manuscript.
Footnotes
Commercial Relationship: None
References
- 1.Furfine ES, Harmon MF, Paith JE, Knowles RG, Salter M, Kiff RJ, Duffy C, Hazelwood R, Oplinger JA, Garvey EP. Potent and selective inhibition of human nitric oxide synthases. Selective inhibition of neuronal nitric oxide synthase by S-methyl-L-thiocitrulline and S-ethyl-L-thiocitrulline. J Biol Chem. 1994;269(43):26677–26683. [PubMed] [Google Scholar]
- 2.DiMagno MJ, Hao Y, Tsunoda Y, Williams JA, Owyang C. Secretagogue-stimulated pancreatic secretion is differentially regulated by constitutive NOS isoforms in mice. Am J Physiol Gastrointest Liver Physiol. 2004;286(3):G428–436. doi: 10.1152/ajpgi.00368.2003. [DOI] [PubMed] [Google Scholar]
- 3.Beauregard C, Brandt PC, Chiou GC. Nitric oxide and cyclic GMP-mediated protein secretion from cultured lacrimal gland acinar cells. J Ocul Pharmacol Ther. 2002;18(5):429–443. doi: 10.1089/10807680260362713. [DOI] [PubMed] [Google Scholar]
- 4.Krumenacker JS, Hanafy KA, Murad F. Regulation of nitric oxide and soluble guanylyl cyclase. Brain Res Bull. 2004;62(6):505–515. doi: 10.1016/S0361-9230(03)00102-3. [DOI] [PubMed] [Google Scholar]
- 5.Aktan F. iNOS-mediated nitric oxide production and its regulation. Life Sci. 2004;75(6):639–653. doi: 10.1016/j.lfs.2003.10.042. [DOI] [PubMed] [Google Scholar]
- 6.Stamler JS, Meissner G. Physiology of nitric oxide in skeletal muscle. Physiol Rev. 2001;81(1):209–237. doi: 10.1152/physrev.2001.81.1.209. [DOI] [PubMed] [Google Scholar]
- 7.Lucas KA, Pitari GM, Kazerounian S, Ruiz-Stewart I, Park J, Schulz S, Chepenik KP, Waldman SA. Guanylyl cyclases and signaling by cyclic GMP. Pharmacol Rev. 2000;52(3):375–414. [PubMed] [Google Scholar]
- 8.Hodges RR, Dartt DA. Regulatory pathways in lacrimal gland epithelium. Int Rev Cytol. 2003;231:129–196. doi: 10.1016/s0074-7696(03)31004-6. [DOI] [PubMed] [Google Scholar]
- 9.Zoukhri D, Hodges RR, Sergheraert C, Dartt DA. Protein kinase C isoforms differentially control lacrimal gland functions. Adv Exp Med Biol. 1998;438:181–186. doi: 10.1007/978-1-4615-5359-5_26. [DOI] [PubMed] [Google Scholar]
- 10.Ota I, Zoukhri D, Hodges RR, Rios JD, Tepavcevic V, Raddassi I, Chen LL, Dartt DA. Alpha 1-adrenergic and cholinergic agonists activate MAPK by separate mechanisms to inhibit secretion in lacrimal gland. Am J Physiol Cell Physiol. 2003;284(1):C168–178. doi: 10.1152/ajpcell.00151.2002. [DOI] [PubMed] [Google Scholar]
- 11.Meneray MA, Fields TY. Adrenergic stimulation of lacrimal protein secretion is mediated by G(q/11)alpha and G(s)alpha. Curr Eye Res. 2000;21(2):602–607. [PubMed] [Google Scholar]
- 12.Hodges RR, Dicker DM, Rose PE, Dartt DA. Alpha 1-adrenergic and cholinergic agonists use separate signal transduction pathways in lacrimal gland. Am J Physiol. 1992;262(6 Pt 1):G1087–1096. doi: 10.1152/ajpgi.1992.262.6.G1087. [DOI] [PubMed] [Google Scholar]
- 13.Zoukhri D, Dartt DA. Cholinergic activation of phospholipase D in lacrimal gland acini is independent of protein kinase C and calcium. Am J Physiol. 1995;268(3 Pt 1):C713–720. doi: 10.1152/ajpcell.1995.268.3.C713. [DOI] [PubMed] [Google Scholar]
- 14.Ding C, Walcott B, Keyser KT. Neuronal nitric oxide synthase and the autonomic innervation of the mouse lacrimal gland. Invest Ophthalmol Vis Sci. 2001;42(12):2789–2794. [PubMed] [Google Scholar]
- 15.Cheng SB, Kuchiiwa S, Kuchiiwa T, Nonaka S, Nakagawa S. Presence of neuronal nitric oxide synthase in autonomic and sensory ganglion neurons innervating the lacrimal glands of the cat: an immunofluorescent and retrograde tracer double-labeling study. J Chem Neuroanat. 2001;22(3):147–155. doi: 10.1016/s0891-0618(01)00125-9. [DOI] [PubMed] [Google Scholar]
- 16.Beauregard C, Brandt PC, Chiou GC. Induction of nitric oxide synthase and overproduction of nitric oxide by interleukin-1beta in cultured lacrimal gland acinar cells. Exp Eye Res. 2003;77(1):109–114. doi: 10.1016/s0014-4835(03)00058-7. [DOI] [PubMed] [Google Scholar]
- 17.Looms DK, Tritsaris K, Dissing S. Nitric oxide-induced signalling in rat lacrimal acinar cells. Acta Physiol Scand. 2002;174(2):109–115. doi: 10.1046/j.1365-201X.2002.00935.x. [DOI] [PubMed] [Google Scholar]
- 18.Jorgensen TD, Dissing S, Gromada J. Cyclic GMP potentiates phenylephrine but not cyclic ADP-ribose-evoked calcium release from rat lacrimal acinar cells. FEBS Lett. 1996;391(1–2):117–120. doi: 10.1016/0014-5793(96)00715-6. [DOI] [PubMed] [Google Scholar]
- 19.Bacman S, Berra A, Sterin-Borda L, Borda E. Muscarinic acetylcholine receptor antibodies as a new marker of dry eye Sjogren syndrome. Invest Ophthalmol Vis Sci. 2001;42(2):321–327. [PubMed] [Google Scholar]
- 20.Herzog V, Sies H, Miller F. Exocytosis in secretory cells of rat lacrimal gland. Peroxidase release from lobules and isolated cells upon cholinergic stimulation. J Cell Biol. 1976;70(3):692–706. doi: 10.1083/jcb.70.3.692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hayashi Y, Nishio M, Naito Y, Yokokura H, Nimura Y, Hidaka H, Watanabe Y. Regulation of neuronal nitric-oxide synthase by calmodulin kinases. J Biol Chem. 1999;274(29):20597–20602. doi: 10.1074/jbc.274.29.20597. [DOI] [PubMed] [Google Scholar]
- 22.Solomonson LP, Flam BR, Pendleton LC, Goodwin BL, Eichler DC. The caveolar nitric oxide synthase/arginine regeneration system for NO production in endothelial cells. J Exp Biol. 2003;206(Pt 12):2083–2087. doi: 10.1242/jeb.00361. [DOI] [PubMed] [Google Scholar]
- 23.Sullivan JC, Pollock JS. NOS 3 subcellular localization in the regulation of nitric oxide production. Acta Physiol Scand. 2003;179(2):115–122. doi: 10.1046/j.1365-201X.2003.01181.x. [DOI] [PubMed] [Google Scholar]
- 24.Hodges RR, Zoukhri D, Sergheraert C, Zieske JD, Dartt DA. Identification of vasoactive intestinal peptide receptor subtypes in the lacrimal gland and their signal-transducing components. Invest Ophthalmol Vis Sci. 1997;38(3):610–619. [PubMed] [Google Scholar]
- 25.Yago MD, Manas M, Ember Z, Singh J. Nitric oxide and the pancreas: morphological base and role in the control of the exocrine pancreatic secretion. Mol Cell Biochem. 2001;219(1–2):107–120. doi: 10.1023/a:1010834611480. [DOI] [PubMed] [Google Scholar]
- 26.McCuskey RS. Anatomy of efferent hepatic nerves. Anat Rec. 2004;280A(1):821–826. doi: 10.1002/ar.a.20087. [DOI] [PubMed] [Google Scholar]
- 27.Wang YF, Mao YK, Fox-Threlkeld JE, McDonald TJ, Daniel EE. Colocalization of inhibitory mediators, NO, VIP and galanin, in canine enteric nerves. Peptides. 1998;19(1):99–112. doi: 10.1016/s0196-9781(97)00262-3. [DOI] [PubMed] [Google Scholar]
- 28.Ziolo MT, Bers DM. The real estate of NOS signaling: location, location, location. Circ Res. 2003;92(12):1279–1281. doi: 10.1161/01.RES.0000080783.34092.AF. [DOI] [PubMed] [Google Scholar]
- 29.Sanchez C, Diaz-Nido J, Avila J. Phosphorylation of microtubule-associated protein 2 (MAP2) and its relevance for the regulation of the neuronal cytoskeleton function. Prog Neurobiol. 2000;61(2):133–168. doi: 10.1016/s0301-0082(99)00046-5. [DOI] [PubMed] [Google Scholar]
- 30.Valdes V, Mosqueira M, Rey S, Del Rio R, Iturriaga R. Inhibitory effects of NO on carotid body: contribution of neural and endothelial nitric oxide synthase isoforms. Am J Physiol Lung Cell Mol Physiol. 2003;284(1):L57–68. doi: 10.1152/ajplung.00494.2001. [DOI] [PubMed] [Google Scholar]
- 31.Tulic MK, Wale JL, Holt PG, Sly PD. Differential effects of nitric oxide synthase inhibitors in an in vivo allergic rat model. Eur Respir J. 2000;15(5):870–877. doi: 10.1034/j.1399-3003.2000.15e10.x. [DOI] [PubMed] [Google Scholar]
- 32.Narayanan K, Spack L, McMillan K, Kilbourn R, Hayward M, Masters B, Griffith O. S-alkyl-L-thiocitrullines. Potent stereoselective inhibitors of nitric oxide synthase with strong pressor activity in vivo. . J Biol Chem. 1995;270(19):11103–11110. doi: 10.1074/jbc.270.19.11103. [DOI] [PubMed] [Google Scholar]
- 33.Goligorsky MS, Li H, Brodsky S, Chen J. Relationships between caveolae and eNOS: everything in proximity and the proximity of everything. Am J Physiol Renal Physiol. 2002;283(1):F1–10. doi: 10.1152/ajprenal.00377.2001. [DOI] [PubMed] [Google Scholar]
- 34.Ortiz PA, Garvin JL. Trafficking and activation of eNOS in epithelial cells. Acta Physiol Scand. 2003;179(2):107–114. doi: 10.1046/j.1365-201X.2003.01207.x. [DOI] [PubMed] [Google Scholar]
- 35.Boer R, Ulrich WR, Klein T, Mirau B, Haas S, Baur I. The inhibitory potency and selectivity of arginine substrate site nitric-oxide synthase inhibitors is solely determined by their affinity toward the different isoenzymes. Mol Pharmacol. 2000;58(5):1026–1034. [PubMed] [Google Scholar]
- 36.Moore PK, al-Swayeh OA, Chong NW, Evans RA, Gibson A. L-NG-nitro arginine (L-NOARG), a novel, L-arginine-reversible inhibitor of endothelium-dependent vasodilatation in vitro. Br J Pharmacol. 1990;99(2):408–412. doi: 10.1111/j.1476-5381.1990.tb14717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Plato CF, Garvin JL. alpha 2-Adrenergic-mediated tubular NO production inhibits thick ascending limb chloride absorption. Am J Physiol Renal Physiol. 2001;281(4):F679–686. doi: 10.1152/ajprenal.2001.281.4.F679. [DOI] [PubMed] [Google Scholar]
- 38.Gorodeski GI. Role of Nitric Oxide and Cyclic Guanosine 3′,5′-Monophosphate in the Estrogen Regulation of Cervical Epithelial Permeability. Endocrinology. 2000;141(5):1658–1666. doi: 10.1210/endo.141.5.7473. [DOI] [PubMed] [Google Scholar]
- 39.Bacman SR, Berra A, Sterin-Borda L, Borda ES. Human primary Sjogren’s syndrome autoantibodies as mediators of nitric oxide release coupled to lacrimal gland muscarinic acetylcholine receptors. Curr Eye Res. 1998;17(12):1135–1142. doi: 10.1076/ceyr.17.12.1135.5124. [DOI] [PubMed] [Google Scholar]
- 40.Nashida T, Imai A, Shimomura H. Stimulation of guanylate cyclase in rat parotid membranes by phosphate. Odontology. 2004;92(1):9–13. doi: 10.1007/s10266-004-0033-6. [DOI] [PubMed] [Google Scholar]
- 41.Looms D, Tritsaris K, Pedersen AM, Nauntofte B, Dissing S. Nitric oxide signalling in salivary glands. J Oral Pathol Med. 2002;31(10):569–584. doi: 10.1034/j.1600-0714.2002.00047.x. [DOI] [PubMed] [Google Scholar]
- 42.Zoukhri D, Hodges RR, Sergheraert C, Toker A, Dartt DA. Lacrimal gland PKC isoforms are differentially involved in agonist-induced protein secretion. Am J Physiol. 1997;272(1 Pt 1):C263–269. doi: 10.1152/ajpcell.1997.272.1.C263. [DOI] [PubMed] [Google Scholar]
- 43.Zhang J, Baines C, Zong N, Cardwell E, Wang G, Vondriska T and Ping P. Functional proteomic analysis of a three tier PKCe-Akt-eNOS signaling module in cardiac protection. Am J Physiol Heart Circ Physiol. 2004;In press. [DOI] [PubMed]


