Abstract
Background
Replication-competent oncolytic herpes simplex viruses (HSV) with deletion of the γ134.5 gene preferentially replicate in and kill malignant cells. γ134.5 codes for ICP 34.5, a protein that enhances viral replication, and is homologous to Growth Arrest and DNA damage Protein 34 (GADD34), a radiation-inducible DNA repair gene. We hypothesized that radiation therapy may potentiate efficacy of oncolytic viral therapy by up-regulating GADD 34 and promoting viral replication.
Methods
A549 and H1299 lung cancer cell lines were infected with NV1066, an oncolytic HSV at multiplicities of infection (MOI; number of viral particles per tumor cell) of 0.1 to 0.5 in vitro with radiation (2 to 10 Gy) or without radiation. Viral replication was determined by plaque assay, cell-to cell spread by flow cytometry, cell kill by lactate dehydrogenase assay and GADD 34 induction by real time RT-PCR and western blot method. Evidence of synergistic cytotoxicity dependence with GADD34 induction is further confirmed by siRNA inhibition of GADD34 expression.
Results
Using both the isobologram method and combination-index method of Chou-Talalay, significant synergism was demonstrated between radiation therapy and NV1066 both in vitro and in vivo. As a result of such synergism, a dose-reduction for each agent (2 to 6000-fold) can be accomplished over a wide range of therapeutic-effect levels without sacrificing tumor cell kill. This effect is correlated with increased GADD34 expression and inhibited by transfection of siRNA directed against GADD34.
Conclusion
These data provide the cellular basis for the clinical investigation of combined use of radiation therapy with oncolytic HSV therapy in the treatment of lung cancer to achieve synergistic efficacy while minimizing dosage and toxicity.
Keywords: Ionizing radiation, Gene therapy, Viruses, Non small cell lung cancer
Abbreviations: 7-AAD 7-amino-actinomycin D, DRI Dose-reduction index, EGFP Enhanced green fluorescent protein, Fa Fraction affected, GADD34 Growth arrest and DNA damage repair gene 34, HSV Herpes simplex virus, ICP 34.5 Infectious cell protein 34.5, LDH Lactate dehydrogenase, MOI Multiplicity of infection, NSCLC Non-small cell lung cancer, PFU Plaque forming unit, RT Radiation therapy, siRNA Small inhibitory RNA
Introduction
Lung cancer is the leading cause of cancer deaths in the Untied States (1). An estimated 173,770 new patients were expected to be diagnosed with lung cancer in the year 2004, one-third with advanced disease that is unresectable (1). All patients with unresectable lung cancer die of this malignancy in spite of current therapies. Therefore, ongoing investigation is directed at finding effective novel therapies for this common cancer. Currently, radiation therapy (RT) plays an important role in the treatment of inoperable non-small cell lung cancer (NSCLC) in achieving local control and in the relief of symptoms of metastatic disease (2). However, higher doses of radiation needed to achieve local control (3;4) are associated with significant toxicity(5). Thus, therapies are also sought that may synergize with radiation therapy to increase the tumor response and to decrease toxicities.
Herpes simplex virus (HSV) mediated oncolysis and gene therapy have emerged as promising treatment modalities against cancer(6–11). Oncolysis results from the replicative life cycle of the virus, which lyses infected tumor cells and releases viral progeny for further infection and killing of neighboring cancer cells. NV1066 is one such multi-mutated replication-competent oncolytic HSV-1 virus, which carries a gene for the marker protein enhanced green fluorescent protein (EGFP). We have previously demonstrated the efficacy of NV1066 and its predecessor NV1020 in the treatment of lung cancer both in vitro and in vivo (12;13). NV 1066 has deletions from the internal repeat sequences, which results in deletion of one copy of each of the genes for ICP0, ICP34.5, and ICP4(14;15). These deletions attenuate the virus and help to ensure that it preferentially replicates within cancer cells. One of these genes, HSV-1 γ134.5, which codes for ICP 34.5, has significant homology at its carboxy- terminus to a radiation-inducible gene, GADD34 (Growth Arrest and DNA Damage repair gene)(16–19). Therefore we hypothesized that radiation-induced up-regulation of GADD34 in tumor cells would functionally complement the γ134.5 deletions in NV1066 and increase viral tumoricidal activity.
Methods
Cell culture
The human NSCLC cell lines A549 (p53+), H1299 (p53−) and Vero cells (from the African green monkey kidney) were obtained from the American Type Culture Collection (ATCC, Rockville, MD). All the cells were maintained in appropriate media as recommended by ATCC and were incubated in a humidified incubator supplied with 5% carbon dioxide.
Viruses
NV1066 is a replication-competent attenuated HSV-1 oncolytic virus with deletion of a single copy of the viral gene γ134.5. G207 and NV3616 are replication-competent attenuated HSV-1 mutant viruses that have been characterized previously(19–21). G207 is attenuated by deletion of a 1-kilobase coding sequence from both γ134.5 loci and a deletion of ICP6, a gene coding for the enzyme ribonucleotide reductase. R3616 contains the same mutation in the γ134.5 loci, but the ICP 6 gene is intact, preserving ribonucleotide reductase function. Viral stocks were propagated on Vero cells, harvested by freeze-thaw lysis and sonication, and titered by standard plaque assay.
In Vitro cytotoxicity assay
A549 and H1299 cells were plated in 24 well flat-bottom plates (Becton Dickinson, Franklin lake, NJ) in 1 ml of media. Cells were treated with media alone (control wells), radiation alone (137Cs source irradiator, 224 cGy/min), NV1066 alone or combination therapy using both radiation therapy (RT) and NV1066. NV1066 infection was carried out at multiplicities of infection (MOI: number of viral plaque forming units per tumor cell) of 0.1, 0.2, 0.3, 0.4 or 0.5 in a total volume of 100 μl of medium. Combination therapy was performed using serial dilutions of RT (2, 4, 6, 8, and 10 Gy) and NV1066 (MOI 0.1, 0.2, 0.3, 0.4 and 0.5) in a 1:20 ratio for both cell lines. These ratios were determined by estimating the LD50 for each therapy in initial experiments and by using these doses to determine the ratio of combination therapy. Typically cells were plated overnight, radiated in the morning and infected with virus within two hours of radiation. Percent survival for each group was determined on each day for 7 days after treatment using a standard LDH release bioassay. Results were expressed as surviving fraction, based on the measured absorbance of treated cellular lysates, compared to that of untreated, control cellular lysates. All samples were tested in triplicate. Experiments were repeated at least thrice to ensure reproducibility. Cytotoxicity assays were also performed with A549 and H1299 cells using G207 or NV3616 virus with and without radiation.
Quantitative analysis of synergy between radiotherapy and NV1066
The combination effects of two therapies in terms of synergy, additivity or antagonism were analyzed by the median-effect plot using the multiple therapeutic effect analysis of Chou and Talalay (22). This method defines the expected additive effect of two (or more) agents and then quantifies synergism or antagonism by determining how much the combination effect differs from the expected additive effect. Data were also analyzed by the isobologram technique, which is dose-oriented. Both the median-plot effect and isobologram method are described in detail elsewhere (23). Another calculation available using the combination index method is the dose-reduction index (DRI). The DRI is a determination of the fold of dose reduction allowed for each drug when given in synergistic combination as compared to the concentration of single agent that is needed to achieve the same effect level. A DRI >1 signifies a favorable reduction in toxicity while still maintaining therapeutic efficacy.
In Vitro viral growth analysis
The ability of NV1066 to replicate within A549 and H1299 cells in the presence or absence of radiation was evaluated by viral growth analysis. 5 x 104 cells per well were plated into 6-well plates. Cells were then infected with either NV1066 (MOI 0.01, 0.05 or MOI 0.1) alone, or with NV1066 and RT (2 Gy). Cells and media were harvested at 48, 72, 96, 120 and 144 hours post-infection. After three cycles of freeze-thaw lysis, standard plaque assay was performed on Vero cells to evaluate viral titers. All samples were performed in triplicate.
Vector Spread Assay
Vector propagation as analyzed by EGFP expression was determined by FACS analysis at a viral infective dose of MOI 0.1 and 0.5 after 0, 2.5, 5, 10 Gy radiation. Percentage of GFP-positive live cells at 12, 24, 48, 72, 96 and 108 hours after radiation compared to control cells without radiation was plotted to derive the GFP-expression trend. Cells were harvested with 0.25% trypsin in 0.02% EDTA, centrifuged, washed in PBS, and brought up in 100 μl of PBS. Five μl of 7-amino-actinomycinD (7-AAD, BD Pharmingen, San Diego, CA) was added as an exclusion dye for cell viability. Data for EGFP expression was acquired on a FACS Calibur machine equipped with Cell Quest software (Becton Dickinson, San Jose, CA). Results are reported as percent of live cells expressing EGFP. All samples were performed in triplicate.
Real-time Reverse Transcription-PCR analysis for GADD34 in cells treated with radiation
1 x 105 A549 and H1299 cells per well were plated in six well plates and were incubated for twelve hours. Cells were treated with a radiation dose of 5 Gy. Each sample was done in triplicate. After 48, 72 and 96 hours of incubation, the cells from each well of the plate were collected after washing with cold PBS and frozen for RNA collection. RNA from each sample was collected and isolated with an RNeasy protect kit (QIAGEN Inc., Valencia, CA) using the manufacturer’s protocol. GADD34 in each sample is measured quantitatively by real-time RT-PCR using a SYBR green fluorophores with a Bio-Rad iCycler iQ detection system (Bio-Rad laboratories, Hercules, CA) and normalized by corresponding 18S ribosomal RNA. For GADD-34, the following primers were applied: GADD-34 forward 5′-GGA GGA AGA GAA TCA AGC CA-3′; GADD-34 reverse 5′-TGG GGT CGG AGC CTG AAG AT-3′; For 18-S: 18-S forward 5′-GTA ACC CGT TGA ACC CCA TT-3′; 18-S reverse 5′-CCA TCC AAT CGG TAG TAG CG-3′. A comparison between each treatment sample and the control group, which did not receive any radiation, is made to determine GADD34 upregulation. For ease of interpretation, semi-log plots of actual GADD34 values were omitted and the results were represented as -fold increase in the treatment sample compared to the control group.
GADD34 siRNA transfection
Duplex siRNAs targeting human GADD34 outside the viral homology domain were designed and tested for the ability to decrease GADD34 expression. After preliminary experiments, the following sequence targeting from codon 635 was chosen for further experiments: 5-GUCAAUUUGCAGAUGGCCATTUGGCCAUCUGCAAAUUGACTT- 3. A549 and H1299 cells were plated at a concentration of 5x104 per well in 24 well plates twelve hours prior to transfection in appropriate medium without antibiotics. Standard siRNA transfection protocol as described before was used(24). Cells in the wells that are exposed to only Oligofectamine transfection reagent (Invitrogen, Carlsbad, CA) were used as controls.
Western blot
A549 cells (non-transfected and GADD34 siRNA transfected) were incubated overnight and radiated in the morning with either 5 or 10 Gy. Cells with no radiation served as a control. Cells were lysed and collected with Cell Lysis Buffer (Cell Signaling Technology, Inc., Beverly, Maryland). Equal amounts of proteins were resolved on 10% SDS-polyacrylamide gels (Bio-Rad, Hercules, California) under reducing conditions and blotted on PVDF membrane (Schleicher & Schuell Bioscience, Keene, New Hampshire). Expression of proteins was determined by using primary rabbit polyclonal anti-human GADD34 (Santa Cruz Biotechnology, santa Cruz, California) and primary goat polyclonal anti-human Actin (Santa Cruz Biotechnology). A secondary antibody conjugated to horse radish peroxidase (Santa Cruz Biotechnology) was used to visualize the expression level of GADD34 and Actin on chemiluminescence film (Hyperfilm; Amersham Biosciences, Buckinghamshire, Engalnd) by application of an ECL Plus Western Blotting Detection System (Amersham Biosciences).
Establishment and treatment of flank tumors
Athymic male mice were purchased from the National Cancer Institute (Bethesda, MD) and were provided with food and water ad libitum. All animals received humane care in accordance with the “Guide for the care and use of laboratory animals (NIH)” and the animal protocols were approved by the animal care committee of the institution. Mice were anesthetized with a mixture of 70 mg/kg ketamine and 10 mg/kg xylazine administered intraperitoneally. H1299 flank tumor establishment and tumor measurements were conducted by the antitumor division of Sloan-Kettering Cancer Institute core facility who were blinded to the treatment arms. Mice were examined daily until tumor nodules reached approximately 250 mm volume at which time they were randomized into 4 groups (n = 6/group): (1) untreated control, (2) 5 Gy RT alone, (3) single intratumoral injection of 1 × 107 plaque-forming units (pfu) NV1066 alone, and (4) 5 Gy RT followed by a single intratumoral injection of 1 × 107 pfu NV1066 (24 hours later). Mice were shielded with lead when flank tumors were exposed to external beam radiation. The length and width of the tumors were measured every 3 days for 32 days. Tumor volume was calculated by the formula for an ellipsoid volume, [(4/3)*(π)*(length/2)*(width/2)2]. Animals were sacrificed if the greatest tumor dimension exceeded 4 cm or if there was skin ulceration.
Statistical analysis
All data were expressed as mean ± standard error of the mean. Comparisons between groups were made using the two-tailed Student’s t-test. The ANOVA test, where appropriate, was used to identify statistical significance for multiple comparisons.
Results
In vitro cytotoxicity of radiotherapy and NV1066
Both radiotherapy and NV1066 demonstrate dose-dependent cytotoxicity against A549 and H1299 lung cancer cells. Combination therapy killed more tumor cells than either single agent alone and showed greater efficacy than the expected additive effect by day 7 in both cell lines (p = 0.002). Cytotoxicity derived by LDH release assay on each day up to day 7 is represented for A549 (Fig 1A) and H1299 (Fig 1B) cells. Synergistic cytotoxicity (p < 0.01) is demonstrated with other γ1 34.5 deletion herpes simplex mutants G207, NV3616 in A549 and H1299 cell lines (data not shown).
Figure 1. A&B: Cytotoxic effect of NV1066, radiation therapy, or both on A549 and H1299 cells in vitro.
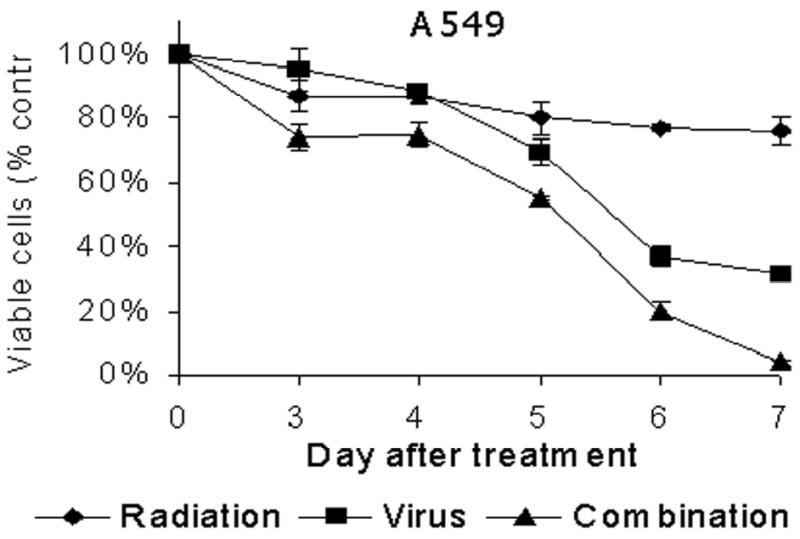
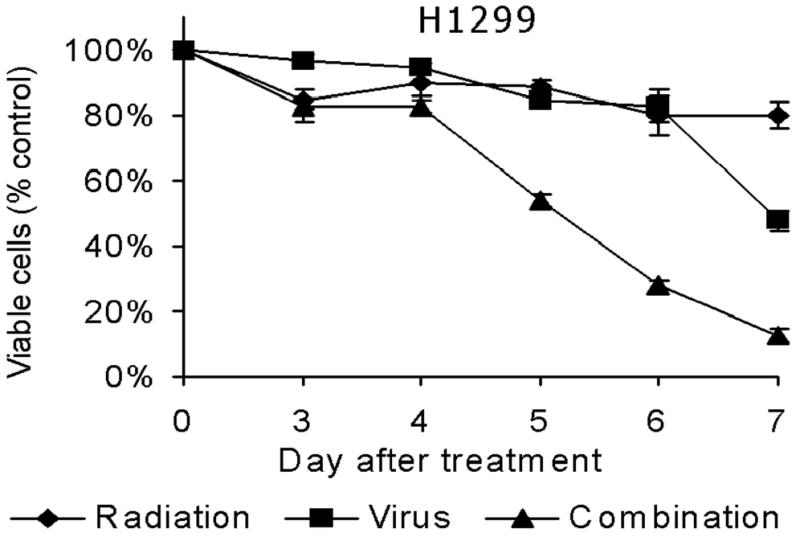
A549 (A) or H1299 (B) cells were treated with radiation therapy (4 Gy), NV1066 (MOI 0.2), or the combination. Results for the treated groups are expressed as cell survival compared to untreated control cells grown under identical conditions. MOI = multiplicity of infection.
Pharmacological analysis of synergy between radiotherapy and NV1066
Chou-Talalay analysis demonstrated that the Combination Index values remained <1 over the entire range of Fa values for both the A549 and H1299 cells (Table 1). Moderate synergism was demonstrated for the H1299 cell line while strong synergism was demonstrated for the A549 cell line. The dose-reduction index (DRI) was calculated for each Fa value. For the A549 cell line the radiation dose could be lowered 1–6160 fold and the NV1066 dose could be lowered 6–11 fold when given as combination therapy. For the H1299 cell line, both radiation and NV1066 dose could be lowered 2–9 fold and still achieve the same tumor killing when given as combination therapy. DRI values >1 indicate that a reduction in toxicity can be achieved without loss of efficacy. Isobolograms were constructed for the doses of radiotherapy and NV1066 necessary to kill 75% of cells (LD75), 50% of cells (LD50) and 25% of cells (LD25) (data not shown). Experimental combination data points at drug and viral concentrations were well below the expected additive effect line for each of these Fa values (0.25, 0.5 and 0.75). These studies confirm synergism between radiotherapy and NV1066 for both cell lines across a wide range of therapeutic doses.
Table 1.
Radiation and viral doses needed to kill various fractions (Fa) of A549 and H1299 cells.
| Fraction Affected (Fa) | Cell line | Radiation alone (Gy) | NV1066 alone (MOI) | Radiation dose reduction index | NV1066 dose reduction index | Combination Index value |
|---|---|---|---|---|---|---|
| LD10 | A549 | 3.2 | 0.0003 | 6160 | 11 | 0.0918 |
| H1299 | 3.6 | 0.14 | 6.2 | 4.9 | 0.3669 | |
| LD20 | A549 | 4.7 | 0.002 | 1161 | 10 | 0.1044 |
| H1299 | 4.8 | 0.28 | 4.6 | 5.4 | 0.4021 | |
| LD30 | A549 | 6 | 0.007 | 383 | 9 | 0.1150 |
| H1299 | 5.8 | 0.45 | 3.8 | 5.8 | 0.4373 | |
| LD40 | A549 | 7.5 | 0.02 | 154 | 8 | 0.1266 |
| H1299 | 6.8 | 0.66 | 3.2 | 6 | 0.4748 | |
| LD50 | A549 | 9 | 0.05 | 67 | 8 | 0.1427 |
| H1299 | 7.9 | 0.93 | 2.8 | 6.5 | 0.5170 | |
| LD60 | A549 | 10.9 | 0.14 | 29 | 7 | 0.1702 |
| H1299 | 9.1 | 1.32 | 2.4 | 6.9 | 0.5679 | |
| LD70 | A549 | 13.4 | 0.4 | 12 | 7 | 0.2306 |
| H1299 | 10.7 | 1.93 | 2 | 7.3 | 0.6348 | |
| LD80 | A549 | 17.3 | 1.4 | 4 | 6 | 0.4165 |
| H1299 | 12.9 | 3.07 | 1.6 | 7.8 | 0.7355 | |
| LD90 | A549 | 25.3 | 9.8 | 0.7 | 6 | 1.5524 |
| H1299 | 17.2 | 6.2 | 1.2 | 8.7 | 0.9360 |
Dose reduction index is the –fold of dose reduction possible to achieve the same cell kill if radiation and virus are used in combination. LDx = Lethal dosex, dose needed to kill x% of cells, MOI = multiplicity of infection.
In vitro viral growth analysis
NV1066 replicated well in both A549 and H1299 cells. In the A549 cell line, after 2Gy radiation, a 16 -fold increase in viral titers compared to NV1066 infection alone (MOI 0.01) was observed over 7 days post-infection. At higher concentrations of virus, there was a 12 -fold (MOI 0.05) and 10 -fold (MOI 0.1) increase in viral yields in the presence of radiation (2 Gy) (p = 0.02). In the H1299 cell line, a 6 -fold increase in viral titers (MOI 0.1) was observed after 2Gy radiation compared to without radiation. Lower fold increases in viral yield measured with combination therapy at higher concentrations (MOI) of virus may be secondary to significant loss of cellular substrate at earlier time points.
Vector spread following NV1066 infection and radiation
Radiation increased the expression of GFP-positive cells indicating better viral propagation among both cell lines. In A549 cells infected at an MOI of 0.5, radiation of 5 Gy increased GFP-positive cells to 37% (±4%) and 10 Gy increased GFP-positive cells to 44% (±5%) when compared to cells infected without radiation increased only 23% (±2%) (p < 0.001) after 12 hours.
Real time RT-PCR analysis for GADD 34
RNA extracted from cells that were not treated with radiation served as negative controls, while RNA extracted from U937 cells radiated at 20 Gy was used as positive controls in real time RT-PCR analysis for GADD34. In A549 cells, 5 Gy radiation increased GADD34 by 1.12-fold (48 hours), 3.49-fold (72 hours) and 5.04-fold (96 hours) (p = 0.02). In H1299 cells, 5 Gy radiation increased GADD34 by 2.92-fold (48 hours), 2.04-fold (72 hours) and 1.69-fold (96 hours) (Fig 2A&B).
Figure 2. A&B: GADD34 elevation following radiation.
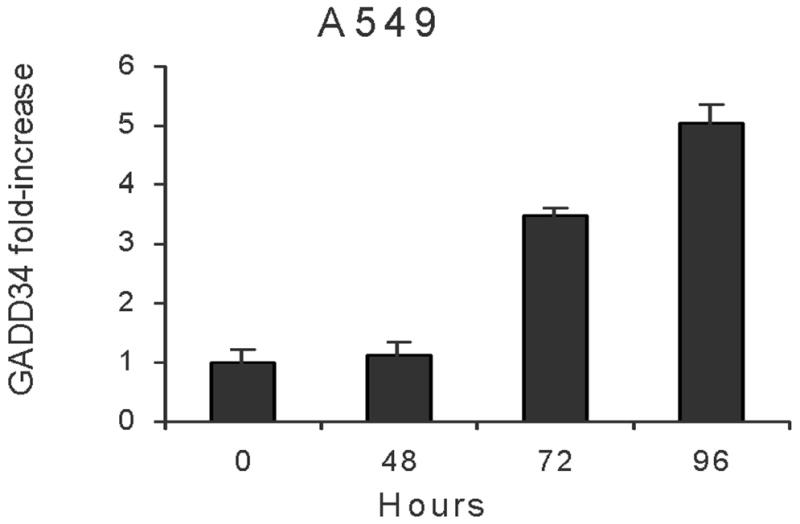
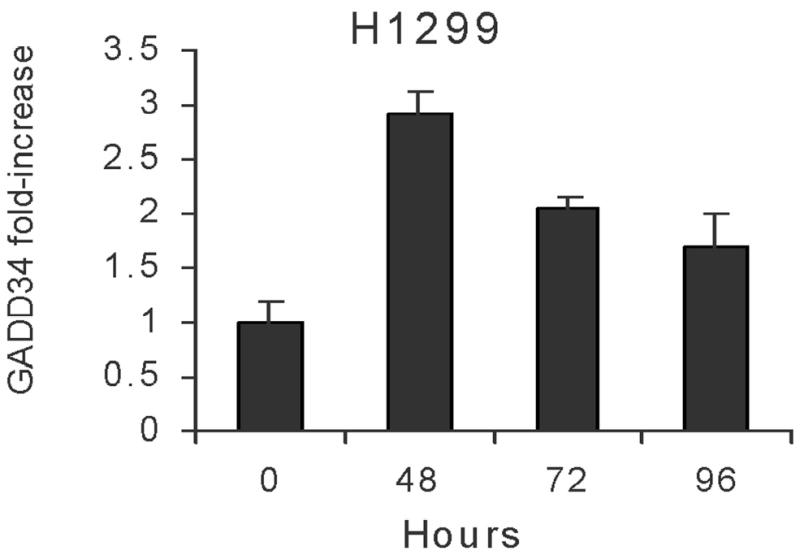
A549 cells (A) or H1299 (B) cells were radiated (5 Gy) and GADD34 expression measured by real time RT-PCR at various time points.
GADD34 knock-down by siRNA eliminates synergistic cytotoxicity
GADD34 is successfully downregulated by siRNA for GADD34 compared to non-transfected A549 cells (Fig. 3A). Downregulation of GADD34 eliminated synergistic cytotoxicity as demonstrated in Fig. 3B. Western blot analysis confirmed radiation induced upregulation of GADD34 protein and its inhibition by GADD34 siRNA (Fig 4).
Figure 3. A&B: Knock-down of GADD34 upregulation by siRNA.
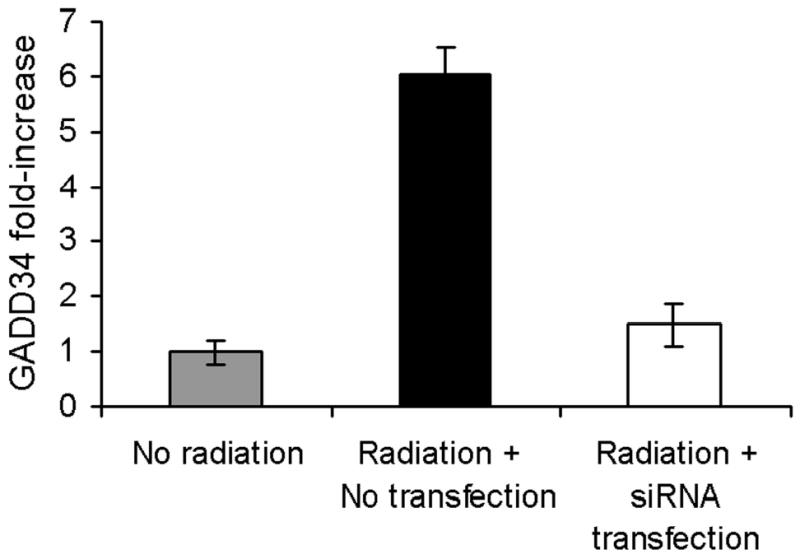
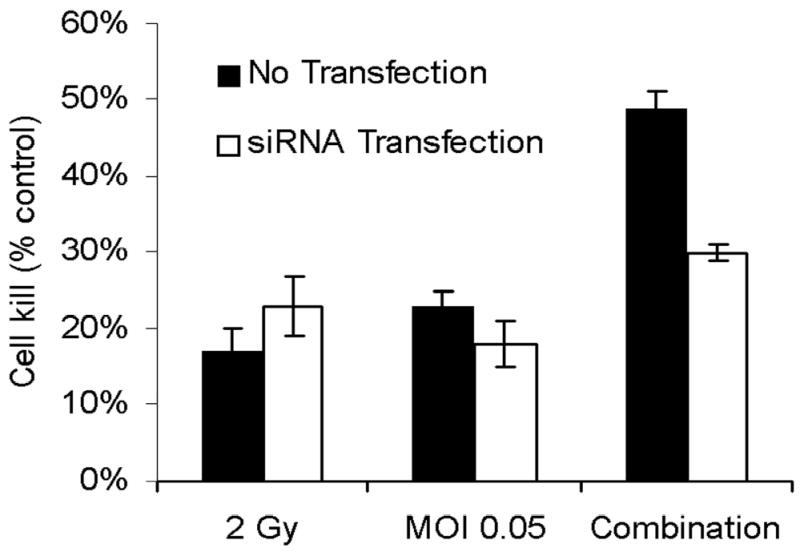
(A) GADD34 levels were determined by real-time RT-PCR in A549 cells with and without siRNA transfection. In cells transfected with siRNA, GADD34 levels were indistinguishable from control (no radiation) cells, while non-transfected cells had a six-fold increase in GADD34 levels. (B) A549 cells were treated with radiation therapy (2 Gy), NV1066 (MOI 0.05), or the combination, with or without transfection with siRNA. Knock-down of GADD34 upregulation by siRNA eliminated the synergistic cytotoxicity in transfected cells. MOI = multiplicity of infection.
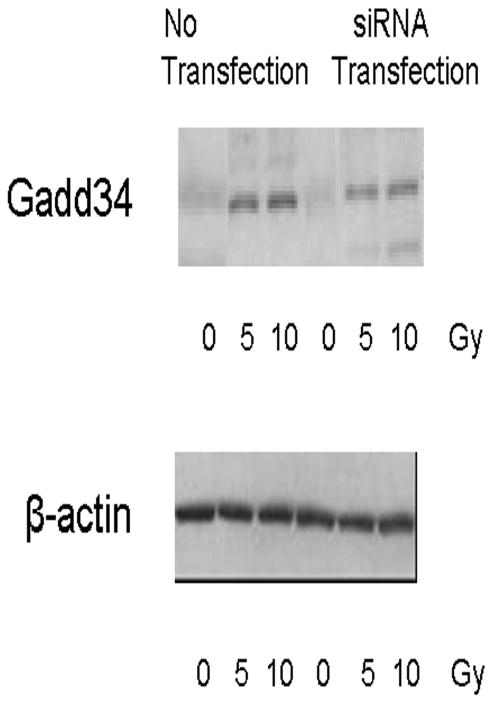
Figure 4. Western blot analysis to confirm GADD34 upregulation following radiation and its inhibition by GADD34 siRNA.
A549 non-transfected and GADD34 siRNA transfected cells were radiated with 5 or 10 Gy. Cells with no radiation served as control. GADD34 protein expression was quantified by western blot analysis. Radiation upregulated GADD34 protein expression and GADD34 siRNA knocked-down the upregulation in siRNA transfected cells.
Antitumor effect of NV1066 and radiation on H1299 flanks tumor volume in vivo:
The H1299 cell line was selected for in vivo flank tumor experiments because this cell line is relatively less sensitive to NV1066 and the synergistic effect is less compared to A549 in vitro. The improved tumor killing by NV1066 and radiation was demonstrated in a xenogenic flank tumor model established in athymic mice after treatment with 5 Gy of radiation and 1x107 pfu of NV1066 (Fig. 5). Combination therapy was significantly better than single agent therapy (p = 0.01). Thirty two days after treatment, in mice with combination therapy, mean flank tumor volume changed to 280 ± 112 mm3 from 250 mm3 compared to mice treated with NV1066 alone (1070 ± 231mm3), radiation alone (1987 ± 312mm3) or the control untreated mice (3879 ± 600mm3). The experiment was terminated after 32 days because of the overgrowth and ulceration of flank tumors.
Figure 5. Antitumor effect of radiation, NV1066 or the combination on H1299 flank tumor volume in vivo.
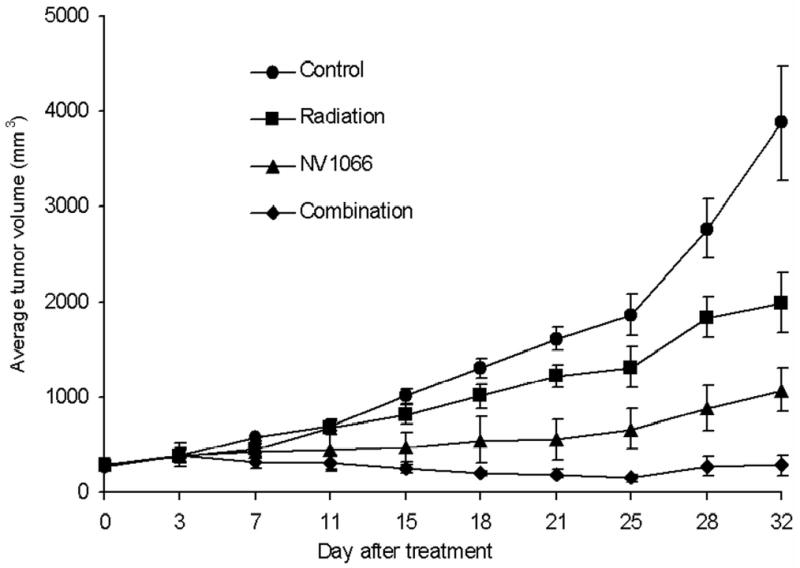
Flank tumors were grown in athymic mice and treated with (1) no treatment, (2) 5 Gy of external beam radiation, (3) single injection of 1 x 107 pfu NV1066 intratumorally, (4) combination of therapies. Tumors treated with combination therapies had smaller average tumor volume than either treatment alone. Results represent the average of the sample group (n = 6 per group) and are shown with standard errors of mean. pfu = plaque forming unit.
Comment
The current study demonstrates that radiation induced GADD34 upregulation greatly enhances replication and antitumor efficacy of a γ134.5-deficient HSV oncolytic virus. The genotoxic response to DNA damage by radiation results in complex cellular events that directs DNA repair. A series of five growth arrest and DNA damage-inducible (GADD) genes have been identified in mammalian cells in response to ionizing radiation, medium-depletion and alkylating agents(17;18). As noted in other studies(18;19), the fact that radiation therapy results in high expression of GADD34 was confirmed in our study and is central to our strategy of combining radiation therapy with oncolytic viral therapy.
NV1066 is an attenuated multimutant HSV-1 that has demonstrated cytotoxic activity against a wide variety of tumor cell types, including lung cancer. Strategic deletions have been made within the genome of NV1066 to improve the safety of this vector. One such deleted gene, γ134.5, is a neurovirulent gene that codes for the protein ICP 34.5, which functions to preclude the shutoff of protein synthesis that occurs as part of the host defense response to cellular infection by viruses. Expression of ICP 34.5 produces a cellular milieu greatly favoring viral replication. There is great homology between the carboxy terminus of the mammalian GADD34 and ICP-34.5(17). The mammalian GADD34 gene has also been shown to complement viral γ134.5 mutants and to permit viral growth in cultured cells. Our hypothesis that radiation therapy and oncolytic viral therapy are synergistic is confirmed by the increased cytotoxicity and the increased viral proliferation seen with combination treatment. The hypothesis that elevated GADD34 is at least partly responsible for such enhanced tumoricidal activity was indeed confirmed by increased GADD34 expression with radiation therapy and by GADD34-inhibition experiments using siRNA. Moreover, similar synergistic tumoricidal activity with radiation therapy was demonstrated for G207 and NV3616, two other viruses with γ134.5 deletions.
Recent attempts have been made to restore the γ134.5 gene into viral mutants since deletion of this gene markedly reduces cytotoxicity(25;26). In particular, investigators have attempted to insert the γ134.5 genes under control of a transcriptional-regulated promoter in order to facilitate selective gene expression in rapidly dividing cells. Although this approach is promising, insertion of the entire γ134.5 gene may theoretically restore neurovirulence and virulence in other non-malignant cells in addition to providing any improvements in tumor cell kill. Our approach of inducing GADD34 function to replace the ICP 34.5 function may provide a superior safety profile. Using radiation therapy in this strategy provides a selective means of restoring the GADD34 phenotype in tumor cells without the potential risk of increasing neurovirulence or risk of enhancing viral replication in non-malignant cells. Furthermore, such combination therapy may prove efficacious for tumor cells that may be resistant to either virus or radiotherapy alone.
Synergy was examined in this study using the combination index and isobologram methods of Chou and Talalay (27;28). This type of analysis is one of the few methods available that determines synergy based on an extrapolated equation. The possibility of predicting false-positive synergistic interactions, a problem inherent in many other methods, is minimized as the analysis takes into account both the potency and shapes of the dose-effect curves in precisely analyzing two therapeutic combinations. Synergistic therapeutic efficacy was demonstrated using two different cell lines with varying sensitivities to virus and radiotherapy. Based on these data, a significant dose-reduction-index (DRI) was also demonstrated. Ultimately, the DRI is the most important parameter in determining the clinical applicability of combination therapy because potential toxicity can be reduced without sacrificing any therapeutic effect.
Higher doses of radiation have been to shown to achieve better local control in advanced lung cancer at the cost of enhanced toxicity. Enhanced acute toxicities can lead to treatment interruptions, delays or alterations in the schedule of radiation delivery resulting in reduction in total dose, thereby potentially compromising the effectiveness of the definitive treatment modality. Dose reduction achieved because of synergistic cytotoxicity may reduce treatment-related toxicities. Studies of malignant glioma in mice have demonstrated that radiation increases viral persistence, furthering the idea that the two therapies can be considered interactive(21;26). Our studies confirmed on a very different tumor type, namely lung cancer, that a significantly greater reduction in volume of tumors can be achieved with such combined radiation and viral therapy. The significantly enhanced oncolytic effects of the combined treatment correlate with enhanced replication of virus in irradiated tumor cells.
In our study, the synergistic efficacy of radiation therapy with oncolytic viral therapy is demonstrated in both p53 positive and p53 negative cell lines. Previous studies have demonstrated in many cancer cell lines that expression of GADD34 is independent of p53 status of the tumor(16;29;30). Therefore, the synergistic efficacy with radiotherapy and oncolytic HSV therapy is independent of p53 status of the tumor, an important limitation in other novel therapies for lung cancer.
The data from these studies provide a cellular basis for the synergistic actions of radiation therapy and oncolytic viral therapy. These data are strong support for future clinical investigation of such combined therapy for lung cancer that aim to increase efficacy while minimizing toxicity.
Acknowledgments
The authors thank Yuhong She, M.D. and Wong Wai, M.S., of the Anti-tumor Core Facility, and Scott Tuorto of the Department of Surgery at Memorial Sloan-Kettering Cancer Center for their assistance with this project. We also thank Brian Horsburgh, Ph.D. and Medigene, Inc. for constructing and providing us with the NV1066 virus.
This project is supported in part by AACR-AstraZeneca Cancer Research and Prevention Foundation fellowship (P.S.A), grants RO1 CA 75416 and RO1 CA/DK80982 (Y.F.) from the National Institutes of Health, grant MBC-99366 (Y.F.) from the American Cancer Society, grant BC024118 from the US Army (Y.F.), grant IMG0402501 from the Susan Komen foundation (Y.F.) and grant 032047 from Flight Attendant Medical Research Institute (Y.F.)
Footnotes
Meeting presentation: Annual meeting of Society of Thoracic Surgeons, San Antonio, Texas, Jan 2004
References
- 1.2004 Cancer Facts & Figures. Available at: http://www.cancer.org/downloads/STT?CAFF_finalPWSecured.pdf Accessed October 30, 2004.
- 2.Sirzen F, Kjellen E, Sorenson S, Cavallin-Stahl E. A systematic overview of radiation therapy effects in non-small cell lung cancer. Acta Oncol. 2003;42(5–6):493–515. doi: 10.1080/02841860310014453. [DOI] [PubMed] [Google Scholar]
- 3.Cox JD, Sause WT, Byhardt RW, Komaki R, Perez CA, Pajak TF. Dose intensity of radiation therapy in non-small cell carcinoma of the lung: a review of RTOG data and strategies. Lung Cancer. 1994;10 (Suppl 1):S161–S166. doi: 10.1016/0169-5002(94)91678-0. [DOI] [PubMed] [Google Scholar]
- 4.Perez CA, Pajak TF, Rubin P, Simpson JR, Mohiuddin M, Brady LW, et al. Long-term observations of the patterns of failure in patients with unresectable non-oat cell carcinoma of the lung treated with definitive radiotherapy. Report by the Radiation Therapy Oncology Group Cancer. 1987;59(11):1874–1881. doi: 10.1002/1097-0142(19870601)59:11<1874::aid-cncr2820591106>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 5.Rosenzweig KE, Yorke E, Jackson A, Nayak R, Rusch VW, Kris MG, et al. Results of a phase I dose escalation study in the treatment of inoperable non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2003;57(2 Suppl):S417–S418. [Google Scholar]
- 6.Mineta T, Rabkin SD, Yazaki T, Hunter WD, Martuza RL. Attenuated multi-mutated herpes simplex virus-1 for the treatment of malignant gliomas. Nat Med. 1995;1(9):938–943. doi: 10.1038/nm0995-938. [DOI] [PubMed] [Google Scholar]
- 7.Fong Y, Kemeny N, Jarnagin S, Stanziale S, Guilfoyle B, Gusani N et al. Phase 1 study of a replication-competent Herpes Simplex oncolytic virus for treatment of hepatic colorectal metastases. ASCO. 2002.
- 8.Stiles BM, Bhargava A, Adusumilli PS, Stanziale SF, Kim TH, Rusch VW, et al. The replication-competent oncolytic herpes simplex mutant virus NV1066 is effective in the treatment of esophageal cancer. Surgery. 2003;134(2):357–364. doi: 10.1067/msy.2003.244. [DOI] [PubMed] [Google Scholar]
- 9.Stanziale SF, Stiles BM, Bhargava A, Kerns SA, Kalakonda N, Fong Y. Oncolytic herpes simplex virus-1 mutant expressing green fluorescent protein can detect and treat peritoneal cancer. Hum Gene Ther. 2004;15(6):609–618. doi: 10.1089/104303404323142051. [DOI] [PubMed] [Google Scholar]
- 10.Stanziale SF, Fong Y. Novel approaches to cancer therapy using oncolytic viruses. Current Molecular Medicine. 2003;3(1):61–71. doi: 10.2174/1566524033361663. [DOI] [PubMed] [Google Scholar]
- 11.Kooby DA, Carew JF, Halterman MW, Mack JE, Bertino JR, Blumgart LH, et al. Oncolytic viral therapy for human colorectal cancer and liver metastases using a multi-mutated herpes simplex virus type-1 (G207) Faseb Journal. 1999;13(11):1325–1334. doi: 10.1096/fasebj.13.11.1325. [DOI] [PubMed] [Google Scholar]
- 12.Ebright MI, Zager JS, Malhotra S, Delman KA, Weigel TL, Rusch VW, et al. Replication-competent herpes virus NV1020 as direct treatment of pleural cancer in a rat model. Journal of Thoracic and Cardiovascular Surgery. 2002;124(1):123–129. doi: 10.1067/mtc.2002.122297. [DOI] [PubMed] [Google Scholar]
- 13.Stiles BM, Adusumilli PS, Bhargava A, Stanziale SF, Wong R, Rusch V, Fong Y. Minimally-invasive In Vivo imaging of an oncolytic herpes simplex mutant expressing green fluorescent protein. Molecular Therapy. 2003;7(5):S453. [Google Scholar]
- 14.Stiles BM, Bhargava A, Adusumilli PS, Stanziale SF, Kim TH, Rusch VW, et al. The replication-competent oncolytic herpes simplex mutant virus NV1066 is effective in the treatment of esophageal cancer. Surgery. 2003;134(2):357–364. doi: 10.1067/msy.2003.244. [DOI] [PubMed] [Google Scholar]
- 15.Wong RJ, Joe JK, Kim SH, Shah JP, Horsburgh B, Fong Y. Oncolytic herpesvirus effectively treats murine squamous cell carcinoma and spreads by natural lymphatics to treat sites of lymphatic metastases. Hum Gene Ther. 2002;13(10):1213–1223. doi: 10.1089/104303402320138998. [DOI] [PubMed] [Google Scholar]
- 16.Hollander MC, Zhan Q, Bae I, Fornace AJ., Jr Mammalian GADD34, an apoptosis- and DNA damage-inducible gene. J Biol Chem. 1997;272(21):13731–13737. doi: 10.1074/jbc.272.21.13731. [DOI] [PubMed] [Google Scholar]
- 17.Hollander MC, Poola-Kella S, Fornace AJ., Jr Gadd34 functional domains involved in growth suppression and apoptosis. Oncogene. 2003;22(25):3827–3832. doi: 10.1038/sj.onc.1206567. [DOI] [PubMed] [Google Scholar]
- 18.Hollander MC, Sheikh MS, Yu K, Zhan Q, Iglesias M, Woodworth C, et al. Activation of Gadd34 by diverse apoptotic signals and suppression of its growth inhibitory effects by apoptotic inhibitors. Int J Cancer. 2001;96(1):22–31. doi: 10.1002/1097-0215(20010220)96:1<22::aid-ijc3>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 19.Stanziale SF, Petrowsky H, Joe JK, Roberts GD, Zager JS, Gusani NJ, et al. Ionizing radiation potentiates the antitumor efficacy of oncolytic herpes simplex virus G207 by upregulating ribonucleotide reductase. Surgery. 2002;132(2):353–359. doi: 10.1067/msy.2002.125715. [DOI] [PubMed] [Google Scholar]
- 20.Bennett JJ, Delman KA, Burt BM, Mariotti A, Malhotra S, Zager J, et al. Comparison of safety, delivery, and efficacy of two oncolytic herpes viruses (G207 and NV1020) for peritoneal cancer. Cancer Gene Therapy. 2002;9(11):935–945. doi: 10.1038/sj.cgt.7700510. [DOI] [PubMed] [Google Scholar]
- 21.Advani SJ, Chung SM, Yan SY, Gillespie GY, Markert JM, Whitley RJ, et al. Replication-competent, nonneuroinvasive genetically engineered herpes virus is highly effective in the treatment of therapy-resistant experimental human tumors. Cancer Res. 1999;59(9):2055–2058. [PubMed] [Google Scholar]
- 22.Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 23.Bennett JJ, Adusumilli P, Petrowsky H, Burt BM, Roberts G, Delman KA, et al. Up-regulation of GADD34 mediates the synergistic anticancer activity of mitomycin C and a gamma134. 5 deleted oncolytic herpes virus (G207) FASEB J. 2004;18(9):1001–1003. doi: 10.1096/fj.02-1080fje. [DOI] [PubMed] [Google Scholar]
- 24.2004 The siRNA user guide. Available at: http://www.rockefeller.edu/labheads/tuschl/sirna.html Accessed October 30, 2004.
- 25.Kramm CM, Chase M, Herrlinger U, Jacobs A, Pechan PA, Rainov NG, et al. Therapeutic efficiency and safety of a second-generation replication-conditional HSV1 vector for brain tumor gene therapy. Hum Gene Ther. 1997;8(17):2057–2068. doi: 10.1089/hum.1997.8.17-2057. [DOI] [PubMed] [Google Scholar]
- 26.Chung RY, Saeki Y, Chiocca EA. B-myb promoter retargeting of herpes simplex virus gamma34.5 gene-mediated virulence toward tumor and cycling cells. J Virol. 1999;73(9):7556–7564. doi: 10.1128/jvi.73.9.7556-7564.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chou TC. Assessment of synergistic and antagonistic effects of chemotherapeutic agents in vitro. Contrib Gynecol Obstet. 1994;19:91–107. [PubMed] [Google Scholar]
- 28.Chou TC. Quantitation of synergism and antagonism of two or more drugs by computerized analysis (Chou, T.C., ed). Academic press, New York, 2004: 223–244.
- 29.Amundson SA, Myers TG, Scudiero D, Kitada S, Reed JC, Fornace AJ., Jr An informatics approach identifying markers of chemosensitivity in human cancer cell lines. Cancer Res. 2000;60(21):6101–6110. [PubMed] [Google Scholar]
- 30.Fornace AJ, Jr, Amundson SA, Bittner M, Myers TG, Meltzer P, Weinsten JN, et al. The complexity of radiation stress responses: analysis by informatics and functional genomics approaches. Gene Expr. 1999;7(4–6):387–400. [PMC free article] [PubMed] [Google Scholar]