Abstract
Neurons in the brain of individuals with focal epilepsy exhibit sustained discharges, called paroxysmal depolarization shifts. Unexpected new evidence indicates that glutamate release from glia can generate these events, and may serve to synchronize the activity of neurons.
One of the most venerable dogmas of neuroscience is that signaling in the nervous system is a function of neurons and that glial cells serve a subsidiary role, only providing such prosaic functions as structural and nutritional support. In the past decade, this doctrine has become increasingly obsolete, as evidence has mounted for various forms of bidirectional communication between neurons and glia 1. Astrocytes and oligodendrocytes are beginning to be viewed as active components of neural networks.
Reinforcing the rising status of the once unassuming glial cell is the growing acceptance of the notion of the tripartite synapse—in which glial processes share the work of signal processing with presynaptic and postsynaptic neuronal elements. The most intensively characterized example of fast signaling between glia and neurons is that mediated by the release of the amino acid glutamate 2, which is also the chemical neurotransmitter for fast excitatory transmission between neurons.
Recently, several groups have reported that glutamate released from glial cells generates slow transient inward currents in nearby neurons through the activation of NMDA receptors 3. A single glial cell appears to be capable of evoking simultaneous transient currents in adjacent neurons, which could be a mechanism for synchronizing neuronal activity4.
In this issue, Tian and Azmi et al. 5 have extended the recent discoveries on glianeuronal signaling to epilepsy, often viewed as a disorder of abnormal, excessive neuronal synchrony. How synchronization occurs in focal epilepsies is largely a mystery and the new results suggest that astrocytes could have a key role. This revelation is sure to shake up the neuron-centric world of epilepsy physiology—where a bedrock concept is that the underlying electrophysiological events in focal seizure disorders involve pathological neuron-to-neuron signaling.
Although the neurological function of most individuals with focal epilepsy is normal between seizures, the disorder is often betrayed by electroencephalogram (EEG) recordings from the scalp, which reveal electrophysiological disturbances—spikes and sharp waves—in the underlying cortex. In the early 1960s, studies in experimental models of focal cortical epilepsy showed that the EEG sike was associated with a sudden, slow depolarization that occurred synchronously in virtually all neurons within the epileptic zone6. This 50-200 millisecond event is called the paroxysmal depolarization shift (PDS).
Later observations led to the now universally accepted view that the PDS is a synaptic potential that is slower and larger in amplitude than ordinary excitatory synaptic potentials, but is generated by the same ionic mechanisms7. AMPA receptors (glutamate-gated ion channels that are the main mediators of excitatory neurotransmission) trigger the PDS, whereas NMDA receptors have a prominent role in the late phase of the response8.
Similar mechanisms are believed to generate seizure discharges, except that there is failure of inhibitory processes that terminate the PDS. In this view of the PDS, astrocytes are passive bystanders. Intracellular recordings from glia in the vicinity of neurons in the epileptic focus indicate that each PDS is associated with a slow and prolonged depolarization— reflecting an increase in extracellular potassium resulting from the synchronous activation of a large population of neurons9.
Even though glia are absent from recent theories of epileptogenesis, glial pathology is a universal feature of focal epilepsy 10. Astrocytes are hypertrophied, change shape and may increase in number. This gliosis is not simply a response to neuronal degeneration—hypertrophy of astrocytes during the process of epileptogenesis has been observed before the development of seizures and in the absence of other pathological changes.
Early epilepsy researchers noted the prominent gliosis in epileptic foci and attempted to draw a mechanistic link to the pathological electrical events of the disorder. Various theories were advanced, ranging from a proposal that reactive astrocytes ‘irritate’ neurons to the suggestion that gliosis is associated with impaired clearance of potassium, allowing extracellular potassium concentrations to rise to levels sufficient to excite neurons. None of these ideas received much experimental support nor have they adequately explained how neurons are synchronized.
The report of Tian et al. suggests a radically different view. The authors propose that astrocytic release of glutamate—acting on AMPA and NMDA receptors—can trigger PDS-like events in populations of neurons, even in the absence of synaptic interactions among the neurons (Fig. 1).
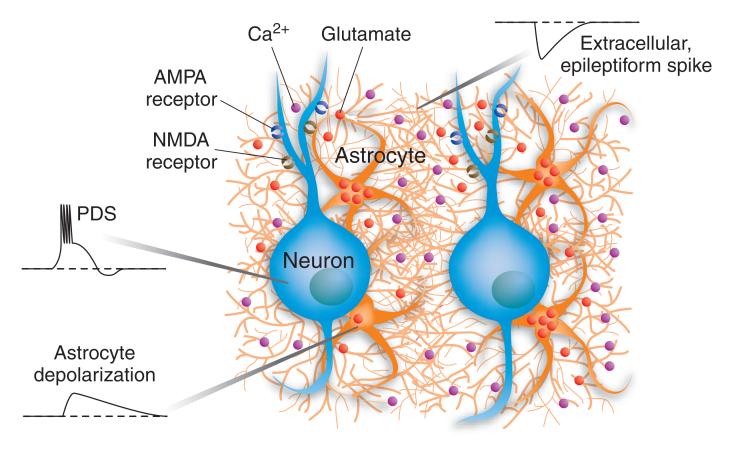
Figure 1.
Glutamate released from astrocytes generates a paroxysmal depolarization shift (PDS), the elemental electrophysiological event in focal epilepsy. Astrocytes form a multicellular functional syncytium as a result of gap-junctional coupling between processes of the same and neighboring astrocytes. Calcium waves within the astrocytic syncytium induce release of glutamate from astrocytes. Glutamate diffuses to dendritic AMPA and NMDA receptors of nearby neurons, inducing a depolarizing (excitatory) response. The glutamate release from astrocytes could occur as a result of calcium-dependent exocytosis, or diffusion through ionotropic purinergic receptors or gap junction hemichannels. In an experimental epilepsy model, the glutamate release is sufficient to elicit a PDS-like membrane depolarization that evokes a train of action potentials and is followed by membrane hyperpolarization. Recording from an astrocyte reveals a slow depolarization that coincides with the PDS but is more prolonged in duration. An extracellular electrode records a negative-going field potential (similar to an electroencepalographic ‘spike’) representing summated dendritic inward currents of the synchronously occurring PDSs in many neurons in the aggregate. Artist: Katie Ris.
In the central experiment in this study, the authors induced the release of glutamate and other amino acids from a single astrocyte by experimentally elevating intracellular calcium levels. To do this, they exposed astrocytes in hippocampal slices loaded with the caged calcium buffer NP-EGTA to the beam of a UV laser, which liberates calcium by UV photocleavage. The elevations in amino acids were associated with PDS-like epileptiform responses of neighboring neurons. Exposure of neurons to the UV beam did not result in PDS-like neuronal activity.
In additional experiments, the authors blocked synaptic transmission among neurons in in vitro epilepsy models—and found that PDS-like events could still occur. Several models were studied and in each case glial calcium waves and oscillations were found to immediately precede epileptiform events in nearby neurons. Overall, the data point to one conclusion: astrocytes can generate PDS-like depolarizations in neurons.
The question now is how important these glial mechanisms are for epilepsy—especially because the experiments used artificial methods of inducing epilepsy-like events and were carried out under conditions where synaptic interactions were eliminated. Most likely, an interplay occurs between astrocytic and neuronal mechanisms. For example, epileptiform activity in neurons may initially activate astrocytes, but then astrocytes could maintain and propagate the activity in the neurons independent of neuronal synaptic interactions.
The concept that astrocytes contribute to the generation of epileptic discharges is appealing because it can explain how neurons are synchronized during focal seizures—an important outstanding problem in epileptology. There is evidence that mutual synaptic excitation accounts for the synchronization in some brain regions 11. However, in other brain areas synchronized activity can occur in the absence of chemical synaptic transmission12.
Astrocytes are well positioned for the task of synchronization because the extensions of a single astrocyte can contact many neurons, and astrocytes form a functional syncytium because of extensive gap-junctional coupling. However, signaling within the astrocytic syncytium is thought to be confined to nonoverlapping microdomains 13; determining how a sufficiently large mass of astrocytes is recruited to generate epileptiform events will be a challenge.
The new role of astrocytes in the generation of epileptic activity has implications for understanding the actions of antiepileptic drugs. Indeed, Tian et al. found that such drugs suppress glial calcium events in an epilepsy model and also when calcium signaling is activated in astrocytes directly. This raises the possibility that the inhibition of seizure discharges occurs to some extent through astrocytes. It is too soon to conclude that actions of antiepileptic drugs on neurons are irrelevant. Nevertheless, it now seems that astrocytes are potential new cellular targets for antiepileptic drugs. The search is on for agents that selectively influence glial signaling and that might be useful in treating epilepsy.
REFERENCES
- Araque A, Perea G. Glial modulation of synaptic transmission in culture. Glia. 2004;47:241–248. doi: 10.1002/glia.20026. [DOI] [PubMed] [Google Scholar]
- Parpura V, Basarsky TA, Liu F, Jeftinija K, Jeftinija S, Haydon PG. Glutamate-mediated astrocyte-neuron signalling. Nature. 1994;369:744–747. doi: 10.1038/369744a0. [DOI] [PubMed] [Google Scholar]
- Fellin T, Pascual O, Gobbo S, Pozzan T, Haydon PG, Carmignoto G. Neuronal synchrony mediated by astrocytic glutamate through activation of extrasynaptic NMDA receptors. Neuron. 2004;43:729–743. doi: 10.1016/j.neuron.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Angulo MC, Kozlov AS, Charpak S. Audinat E.Glutamate released from glial cells synchronizes neuronal activity in the hippocampus. J Neurosci. 2004;24:6920–6927. doi: 10.1523/JNEUROSCI.0473-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian GF, Azmi H, Takano T, Xu Q, Peng W, Lin J, Oberheim N, Lou N, Wang X, Zielke HR, Kang J, Nedergaard M. An astrocytic basis of epilepsy. Nat Med. 2005;11:973–981. doi: 10.1038/nm1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto H, Ajmone-Marsan C. Cortical cellular phenomena in experimental epilepsy: interictal manifestations. Exp Neurol. 1964;80:286–304. doi: 10.1016/0014-4886(64)90025-1. [DOI] [PubMed] [Google Scholar]
- Johnston D, Brown TH. Giant synaptic potential hypothesis for epileptiform activity. Science. 1981;211:294–297. doi: 10.1126/science.7444469. [DOI] [PubMed] [Google Scholar]
- Dingledine R, Hynes MA, King GL. Involvement of N-methyl-D-aspartate receptors in epileptiform bursting in the rat hippocampal slice. J Physiol (Lond) 1986;380:175–189. doi: 10.1113/jphysiol.1986.sp016279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutnick MJ, Connors BW, Prince DA. Mechanisms of neocortical epileptogenesis in vitro. J Neurophysiol. 1982;48:1321–1335. doi: 10.1152/jn.1982.48.6.1321. [DOI] [PubMed] [Google Scholar]
- Khurgel M, Ivy GO. Astrocytes in kindling: relevance to epileptogenesis. Epilepsy Res. 1996;26:163–175. doi: 10.1016/s0920-1211(96)00051-4. [DOI] [PubMed] [Google Scholar]
- Traub RD, Wong RK. Cellular mechanism of neuronal synchronization in epilepsy. Science. 1982;216:745–747. doi: 10.1126/science.7079735. [DOI] [PubMed] [Google Scholar]
- Dudek FE, Yasumura T, Rash JE. ‘Non-synaptic’ mechanisms in seizures and epileptogenesis. Cell Biol Int. 1998;22:793–805. doi: 10.1006/cbir.1999.0397. [DOI] [PubMed] [Google Scholar]
- Nett WJ, Oloff SH, McCarthy KD. Hippocampal astrocytes in situ exhibit calcium oscillations that occur independent of neuronal activity. J Neurophysiol. 2002;87:528–537. doi: 10.1152/jn.00268.2001. [DOI] [PubMed] [Google Scholar]