Abstract
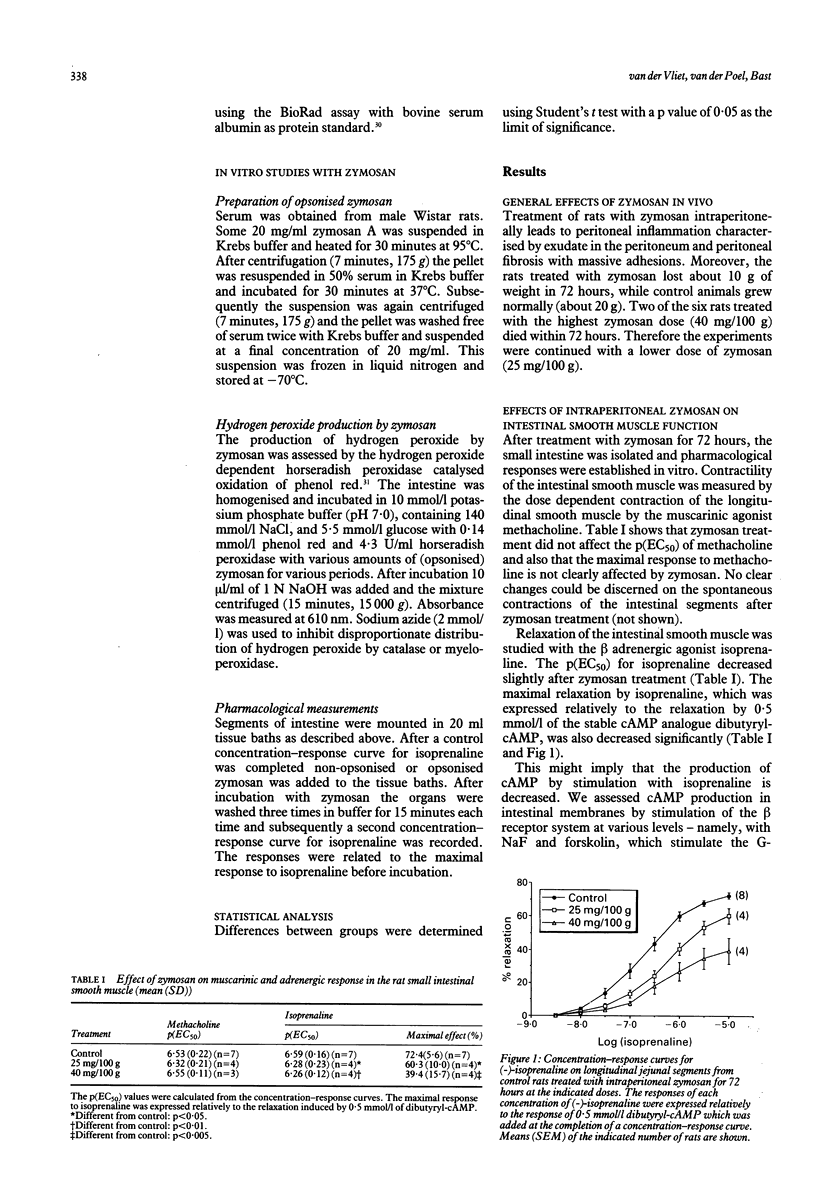
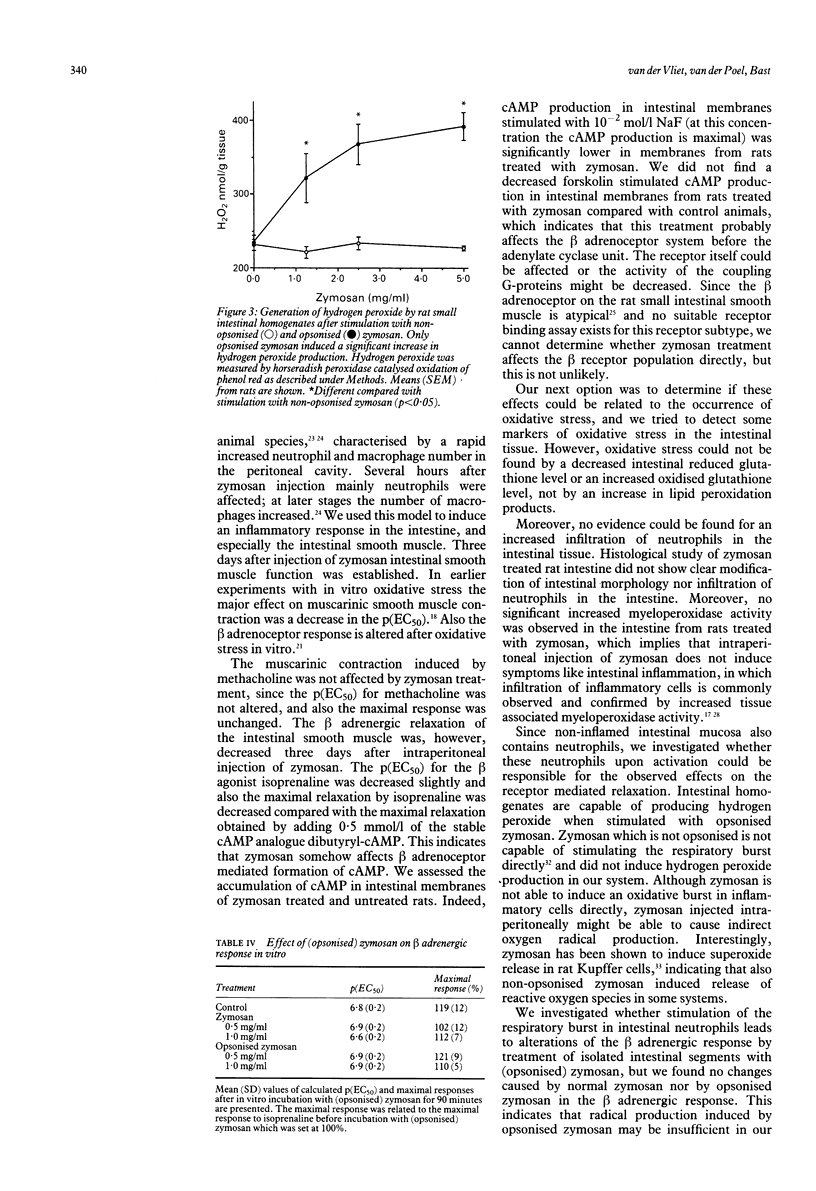
Zymosan is frequently used as an activator of granulocytes to study inflammatory responses. We used zymosan as a model to understand the mechanisms involved in intestinal inflammatory diseases, and our special interest was focused on the smooth muscle function. Moreover, we investigated the role of oxidative stress in intestinal pathology after inflammatory processes. Intraperitoneal injection of zymosan induces a peritoneal inflammation, characterised by exudate in the peritoneum and peritoneal fibrosis. Three days after injection of zymosan (25-40 mg/100 g) we measured a decreased beta adrenergic smooth muscle response, while the muscarinic receptor-mediated contraction was not significantly affected. Efforts were made to correlate these observations with the development of oxidative stress; however, the intestinal glutathione balance remained undisturbed and no increase in lipid peroxidation products in the intestine was observed. Our conclusion is the peritoneal inflammation will lead to a release of various mediators, which may destroy receptor systems, among which are beta adrenoceptors. There was no evidence of an important role for reactive oxygen metabolites in this effect.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bhatnagar R., Schirmer R., Ernst M., Decker K. Superoxide release by zymosan-stimulated rat Kupffer cells in vitro. Eur J Biochem. 1981 Sep;119(1):171–175. doi: 10.1111/j.1432-1033.1981.tb05590.x. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Doherty N. S., Poubelle P., Borgeat P., Beaver T. H., Westrich G. L., Schrader N. L. Intraperitoneal injection of zymosan in mice induces pain, inflammation and the synthesis of peptidoleukotrienes and prostaglandin E2. Prostaglandins. 1985 Nov;30(5):769–789. doi: 10.1016/0090-6980(85)90006-1. [DOI] [PubMed] [Google Scholar]
- Forrest M. J., Jose P. J., Williams T. J. Kinetics of the generation and action of chemical mediators in zymosan-induced inflammation of the rabbit peritoneal cavity. Br J Pharmacol. 1986 Dec;89(4):719–730. doi: 10.1111/j.1476-5381.1986.tb11176.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goris R. J., Boekholtz W. K., van Bebber I. P., Nuytinck J. K., Schillings P. H. Multiple-organ failure and sepsis without bacteria. An experimental model. Arch Surg. 1986 Aug;121(8):897–901. doi: 10.1001/archsurg.1986.01400080039006. [DOI] [PubMed] [Google Scholar]
- Grisham M. B., Granger D. N. Neutrophil-mediated mucosal injury. Role of reactive oxygen metabolites. Dig Dis Sci. 1988 Mar;33(3 Suppl):6S–15S. doi: 10.1007/BF01538126. [DOI] [PubMed] [Google Scholar]
- Grisham M. B., Hernandez L. A., Granger D. N. Xanthine oxidase and neutrophil infiltration in intestinal ischemia. Am J Physiol. 1986 Oct;251(4 Pt 1):G567–G574. doi: 10.1152/ajpgi.1986.251.4.G567. [DOI] [PubMed] [Google Scholar]
- Haenen G. R., Bast A. Protection against lipid peroxidation by a microsomal glutathione-dependent labile factor. FEBS Lett. 1983 Aug 8;159(1-2):24–28. doi: 10.1016/0014-5793(83)80409-8. [DOI] [PubMed] [Google Scholar]
- Haenen G. R., Van Dansik P., Vermeulen N. P., Timmerman H., Bast A. The effect of hydrogen peroxide on beta-adrenoceptor function in the heart. Free Radic Res Commun. 1988;4(4):243–249. doi: 10.3109/10715768809055149. [DOI] [PubMed] [Google Scholar]
- Halliwell B., Hoult J. R., Blake D. R. Oxidants, inflammation, and anti-inflammatory drugs. FASEB J. 1988 Oct;2(13):2867–2873. doi: 10.1096/fasebj.2.13.2844616. [DOI] [PubMed] [Google Scholar]
- Koch T. R., Carney J. A., Go V. L., Szurszewski J. H. Spontaneous contractions and some electrophysiologic properties of circular muscle from normal sigmoid colon and ulcerative colitis. Gastroenterology. 1988 Jul;95(1):77–84. doi: 10.1016/0016-5085(88)90293-4. [DOI] [PubMed] [Google Scholar]
- Krawisz J. E., Sharon P., Stenson W. F. Quantitative assay for acute intestinal inflammation based on myeloperoxidase activity. Assessment of inflammation in rat and hamster models. Gastroenterology. 1984 Dec;87(6):1344–1350. [PubMed] [Google Scholar]
- Otamiri T., Tagesson C. Role of phospholipase A2 and oxygenated free radicals in mucosal damage after small intestinal ischemia and reperfusion. Am J Surg. 1989 Jun;157(6):562–566. doi: 10.1016/0002-9610(89)90699-5. [DOI] [PubMed] [Google Scholar]
- Parks D. A., Bulkley G. B., Granger D. N., Hamilton S. R., McCord J. M. Ischemic injury in the cat small intestine: role of superoxide radicals. Gastroenterology. 1982 Jan;82(1):9–15. [PubMed] [Google Scholar]
- Parks D. A., Granger D. N. Xanthine oxidase: biochemistry, distribution and physiology. Acta Physiol Scand Suppl. 1986;548:87–99. [PubMed] [Google Scholar]
- Pick E., Keisari Y. A simple colorimetric method for the measurement of hydrogen peroxide produced by cells in culture. J Immunol Methods. 1980;38(1-2):161–170. doi: 10.1016/0022-1759(80)90340-3. [DOI] [PubMed] [Google Scholar]
- Raaijmakers J. A., Beneker C., van Geffen E. C., Meisters T. M., Pover P. Inflammatory mediators and beta-adrenoceptor function. Agents Actions. 1989 Jan;26(1-2):45–47. doi: 10.1007/BF02126558. [DOI] [PubMed] [Google Scholar]
- Roldán E. J., Pinus C. R., Turrens J. F., Boveris A. Chemiluminescence of ischaemic and reperfused intestine in vivo. Gut. 1989 Feb;30(2):184–187. doi: 10.1136/gut.30.2.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos D., Bot A. A., van Schaik M. L., de Boer M., Daha M. R. Interaction between human neutrophils and zymosan particles: the role of opsonins and divalent cations. J Immunol. 1981 Feb;126(2):433–440. [PubMed] [Google Scholar]
- Salim A. S. The significance of removing oxygen-derived free radicals in the treatment of acute and chronic duodenal ulceration in the rat. J Pharm Pharmacol. 1990 Jan;42(1):64–67. doi: 10.1111/j.2042-7158.1990.tb05354.x. [DOI] [PubMed] [Google Scholar]
- Scott R. B., Gall D. G., Diamant S. C. Intestinal motility during acute Yersinia enterocolitica enteritis in rabbits. Can J Physiol Pharmacol. 1989 Jun;67(6):553–560. doi: 10.1139/y89-089. [DOI] [PubMed] [Google Scholar]
- Sjogren R. W., Sherman P. M., Boedeker E. C. Altered intestinal motility precedes diarrhea during Escherichia coli enteric infection. Am J Physiol. 1989 Nov;257(5 Pt 1):G725–G731. doi: 10.1152/ajpgi.1989.257.5.G725. [DOI] [PubMed] [Google Scholar]
- Smith S. M., Holm-Rutili L., Perry M. A., Grisham M. B., Arfors K. E., Granger D. N., Kvietys P. R. Role of neutrophils in hemorrhagic shock-induced gastric mucosal injury in the rat. Gastroenterology. 1987 Sep;93(3):466–471. doi: 10.1016/0016-5085(87)90907-3. [DOI] [PubMed] [Google Scholar]
- Suematsu M., Suzuki M., Kitahora T., Miura S., Suzuki K., Hibi T., Watanabe M., Nagata H., Asakura H., Tsuchiya M. Increased respiratory burst of leukocytes in inflammatory bowel diseases--the analysis of free radical generation by using chemiluminescence probe. J Clin Lab Immunol. 1987 Nov;24(3):125–128. [PubMed] [Google Scholar]
- Suzuki K., Ota H., Sasagawa S., Sakatani T., Fujikura T. Assay method for myeloperoxidase in human polymorphonuclear leukocytes. Anal Biochem. 1983 Jul 15;132(2):345–352. doi: 10.1016/0003-2697(83)90019-2. [DOI] [PubMed] [Google Scholar]
- Van der Vliet A., Bast A. Hydrogen peroxide reduces beta-adrenoceptor function in the rat small intestine. Eur J Pharmacol. 1991 Jun 25;199(2):153–156. doi: 10.1016/0014-2999(91)90452-v. [DOI] [PubMed] [Google Scholar]
- Van der Vliet A., Tuinstra T. J., Rademaker B., Bast A. Intestinal motility disorder induced by peroxides: possible role of lipid peroxidation. Res Commun Chem Pathol Pharmacol. 1990 Nov;70(2):227–243. [PubMed] [Google Scholar]
- Younes M., Schoenberg M. H., Jung H., Fredholm B. B., Haglund U., Schildberg F. W. Oxidative tissue damage following regional intestinal ischemia and reperfusion in the cat. Res Exp Med (Berl) 1984;184(4):259–264. doi: 10.1007/BF01852385. [DOI] [PubMed] [Google Scholar]
- van der Vliet A., Rademaker B., Bast A. A beta adrenoceptor with atypical characteristics is involved in the relaxation of the rat small intestine. J Pharmacol Exp Ther. 1990 Oct;255(1):218–226. [PubMed] [Google Scholar]
- van der Vliet A., Tuinstra T. J., Bast A. Modulation of oxidative stress in the gastrointestinal tract and effect on rat intestinal motility. Biochem Pharmacol. 1989 Sep 1;38(17):2807–2818. doi: 10.1016/0006-2952(89)90435-8. [DOI] [PubMed] [Google Scholar]



