Abstract
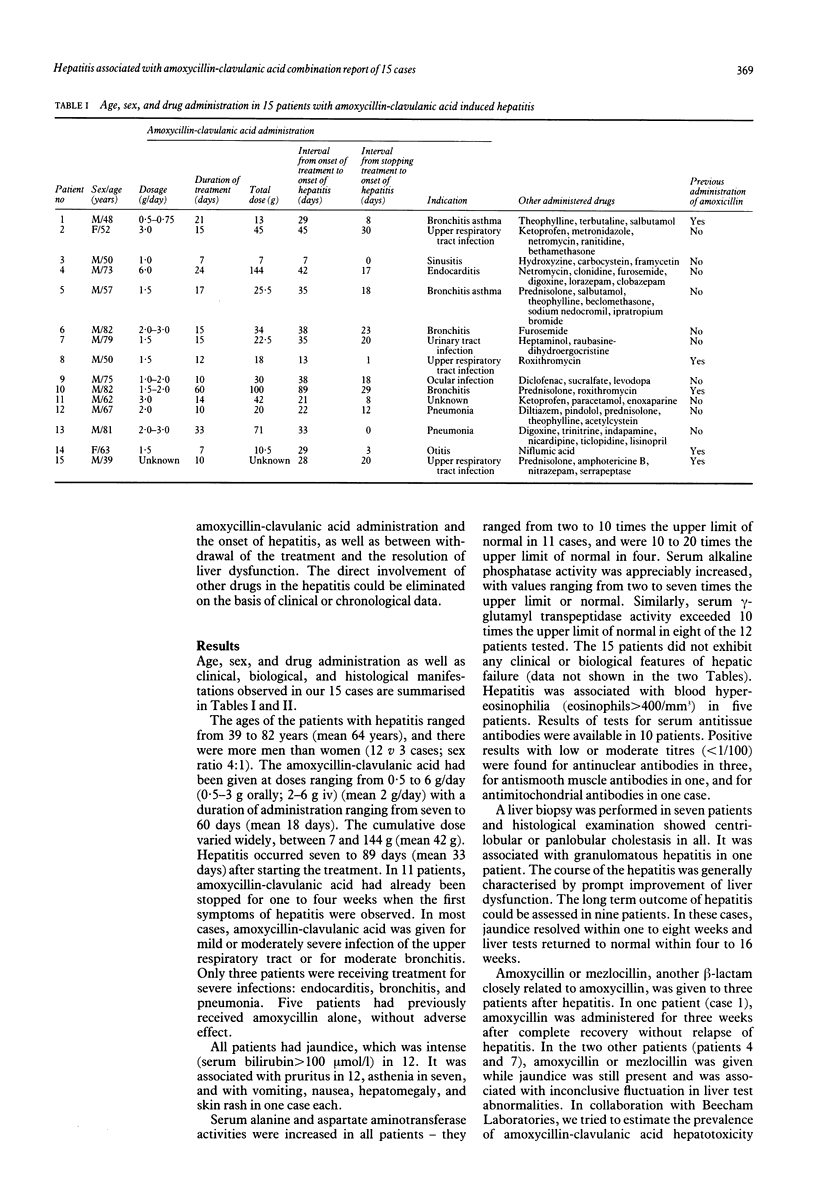
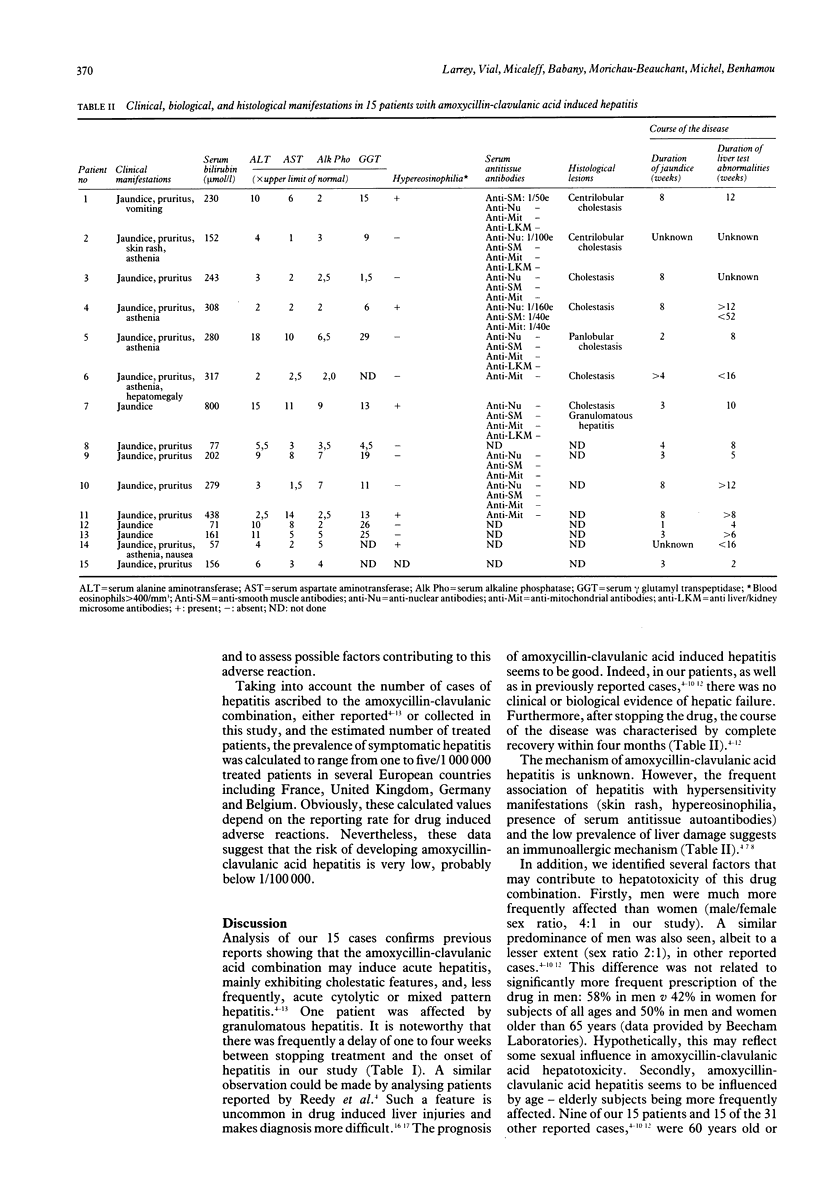
Fifteen cases of hepatitis related to a combination of amoxycillin and clavulanic acid are reported. Most patients were aged 60 years or more and there were more men than women (sex ratio 4:1). The amoxycillin-clavulanic acid had been given at doses ranging from 0.5 to 6 g/day (mean 2 g/day) for seven to 60 days (mean 18 days). In 11 cases, the first symptoms appeared one to four weeks after stopping treatment. Jaundice was observed in all patients and was frequently associated with pruritus. Serum aminotransferase activities were increased in all patients and were generally two to 10 times the upper limit of normal. Serum alkaline phosphatase activity was considerably increased, from two to seven times the upper limit of normal. Histological examination of the liver, performed in seven patients, showed centri- or panlobular cholestasis in all cases, associated with granulomatous hepatitis in one. The prognosis of amoxycillin-clavulanic acid induced hepatitis seemed to be good. None of the patients exhibited biological or clinical features of hepatic failure and the course of the disease was characterised by the resolution of jaundice within one to eight weeks and a complete recovery within four to 16 weeks. Taking into account the number of treated subjects and reported cases, we estimated the risk of developing hepatitis with this drug combination to be very low, probably below 1/100,000. Our data suggest that the risk of hepatotoxicity may be increased in elderly men given lengthy treatment. The association of hepatitis and signs of hypersensitivity may suggest an immunoallergic mechanism of hepatotoxicity in some patients.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bénichou C. Criteria of drug-induced liver disorders. Report of an international consensus meeting. J Hepatol. 1990 Sep;11(2):272–276. doi: 10.1016/0168-8278(90)90124-a. [DOI] [PubMed] [Google Scholar]
- Cleau D., Jobard J. M., Alves T., Gury S., Rey B., Vuillemard M., Noirot A., Floriot C., Wagschal G., Vieille J. Hépatite cholestatique due à l'association amoxicilline-acide clavulanique. Un cas et revue de la littérature. Gastroenterol Clin Biol. 1990;14(12):1007–1009. [PubMed] [Google Scholar]
- Dowsett J. F., Gillow T., Heagerty A., Radcliffe M., Toadi R., Isle I., Russell R. C. Amoxycillin/clavulanic acid (Augmentin)-induced intrahepatic cholestasis. Dig Dis Sci. 1989 Aug;34(8):1290–1293. doi: 10.1007/BF01537281. [DOI] [PubMed] [Google Scholar]
- Iravani A., Richard G. A. Amoxicillin-clavulanic acid versus cefaclor in the treatment of urinary tract infections and their effects on the urogenital and rectal flora. Antimicrob Agents Chemother. 1986 Jan;29(1):107–111. doi: 10.1128/aac.29.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michielsen P. P., Van Outryve M. J., Van Marck E. A., De Maeyer M. H., Pelckmans P. A., Van Maercke Y. M. Amoxycillin/clavulanic acid induced cholestasis. J Hepatol. 1990 Nov;11(3):392–392. doi: 10.1016/0168-8278(90)90230-o. [DOI] [PubMed] [Google Scholar]
- Pelletier G., Ink O., Fabre M., Hagège H. Hépatite cholestatique probablement due à l'association d'amoxicilline et d'acide clavulanique. Gastroenterol Clin Biol. 1990;14(6-7):601–601. [PubMed] [Google Scholar]
- Reading C., Cole M. Clavulanic acid: a beta-lactamase-inhiting beta-lactam from Streptomyces clavuligerus. Antimicrob Agents Chemother. 1977 May;11(5):852–857. doi: 10.1128/aac.11.5.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy K. R., Brillant P., Schiff E. R. Amoxicillin-clavulanate potassium-associated cholestasis. Gastroenterology. 1989 Apr;96(4):1135–1141. doi: 10.1016/0016-5085(89)91633-8. [DOI] [PubMed] [Google Scholar]
- Royer R. J. Pharmacovigilance. The French system. Drug Saf. 1990;5 (Suppl 1):137–140. doi: 10.2165/00002018-199000051-00021. [DOI] [PubMed] [Google Scholar]
- Schneider J. E., Kleinman M. S., Kupiec J. W. Cholestatic hepatitis after therapy with amoxicillin/clavulanate potassium. N Y State J Med. 1989 Jun;89(6):355–356. [PubMed] [Google Scholar]
- Stricker B. H., Van den Broek J. W., Keuning J., Eberhardt W., Houben H. G., Johnson M., Blok A. P. Cholestatic hepatitis due to antibacterial combination of amoxicillin and clavulanic acid (augmentin) Dig Dis Sci. 1989 Oct;34(10):1576–1580. doi: 10.1007/BF01537113. [DOI] [PubMed] [Google Scholar]
- Van der Auwera P., Legrand J. C. Ticarcillin-clavulanic acid therapy in severe infections. Drugs Exp Clin Res. 1985;11(11):805–813. [PubMed] [Google Scholar]
- Verhamme M., Ramboer C., Van de Bruaene P., Inderadjaja N. Cholestatic hepatitis due to an amoxycillin/clavulanic acid preparation. J Hepatol. 1989 Sep;9(2):260–264. doi: 10.1016/0168-8278(89)90061-5. [DOI] [PubMed] [Google Scholar]


