Abstract
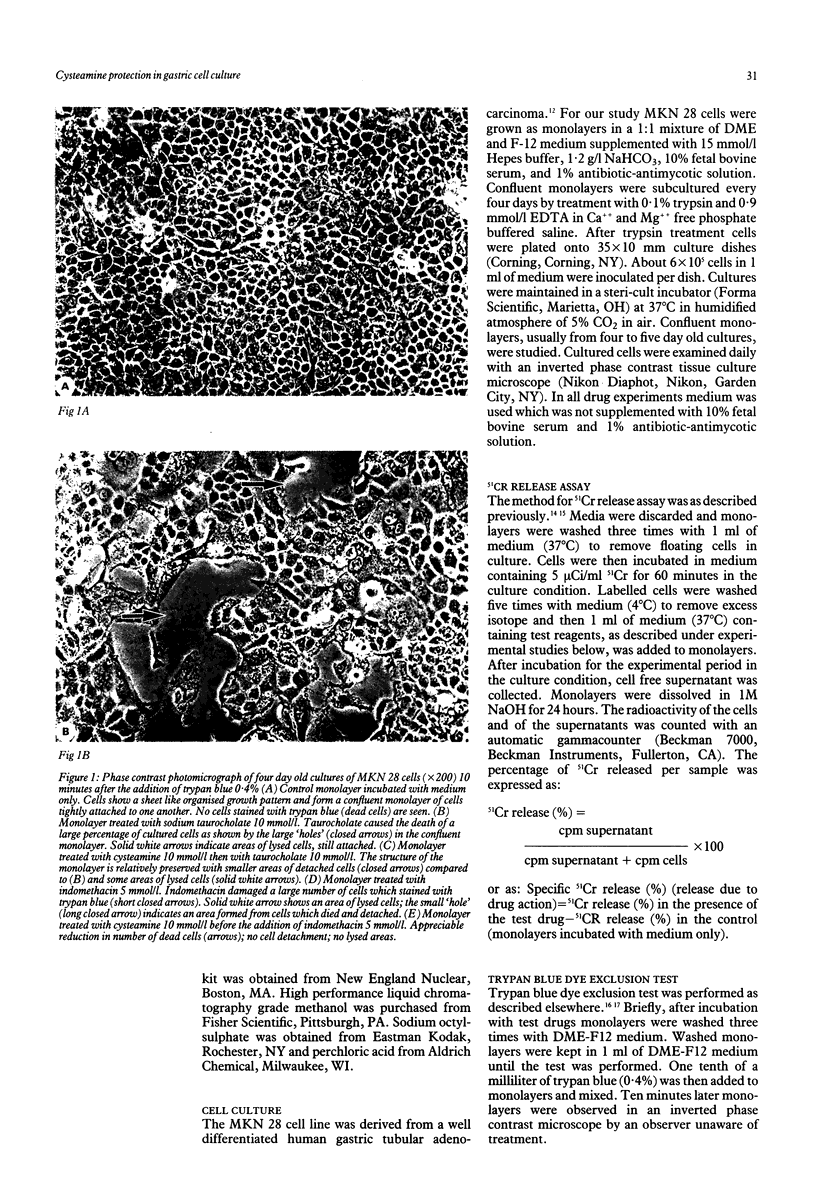
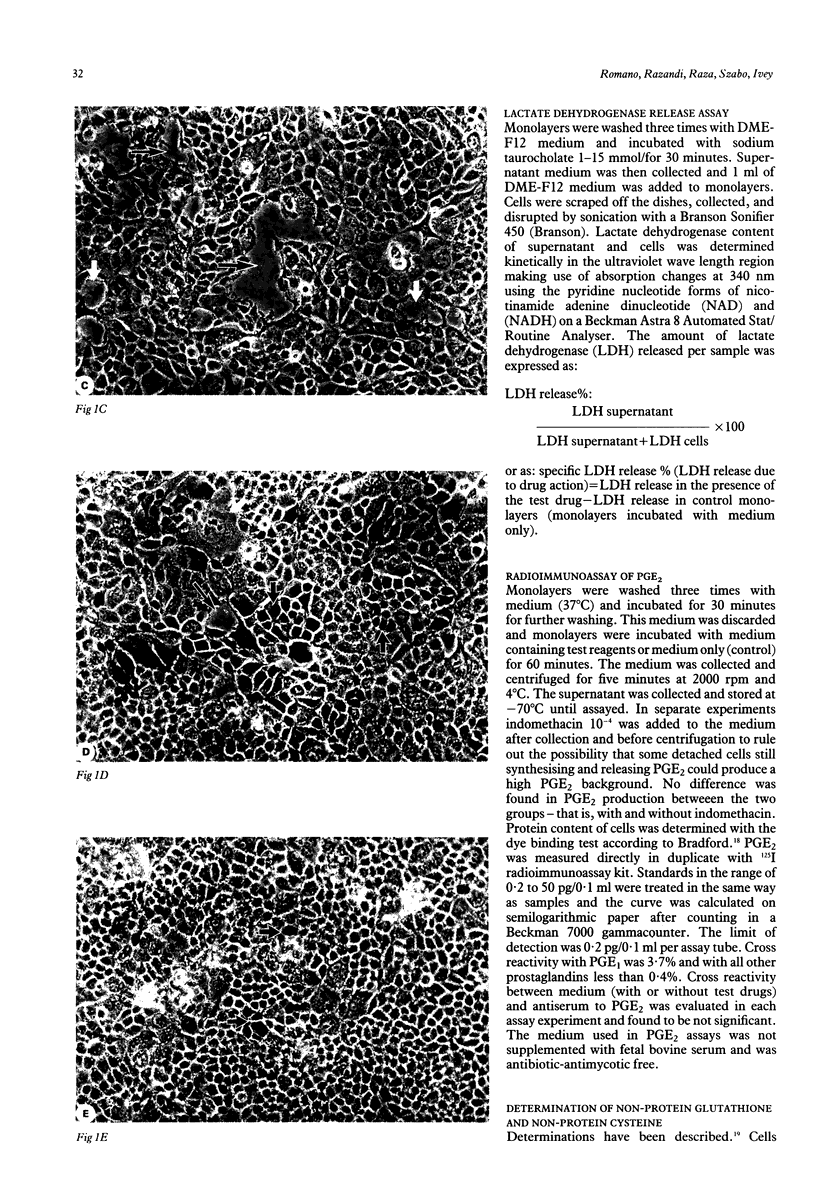
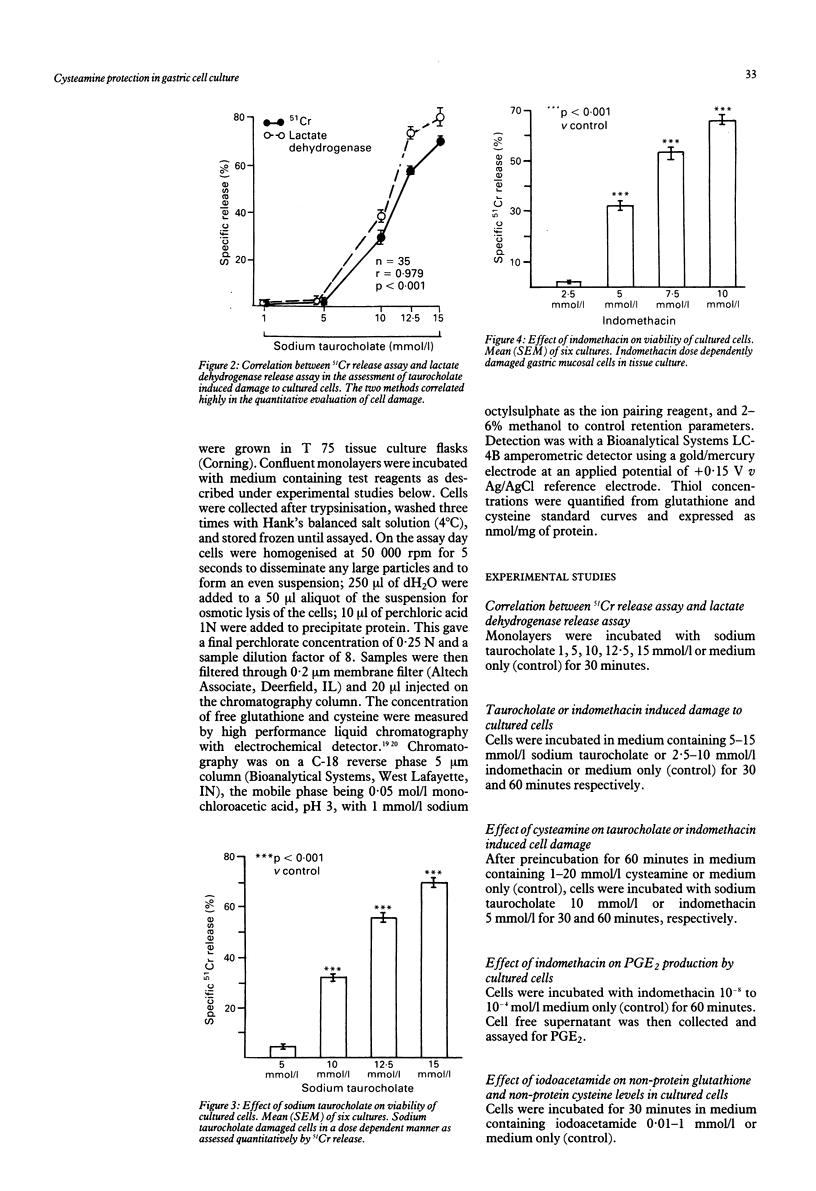
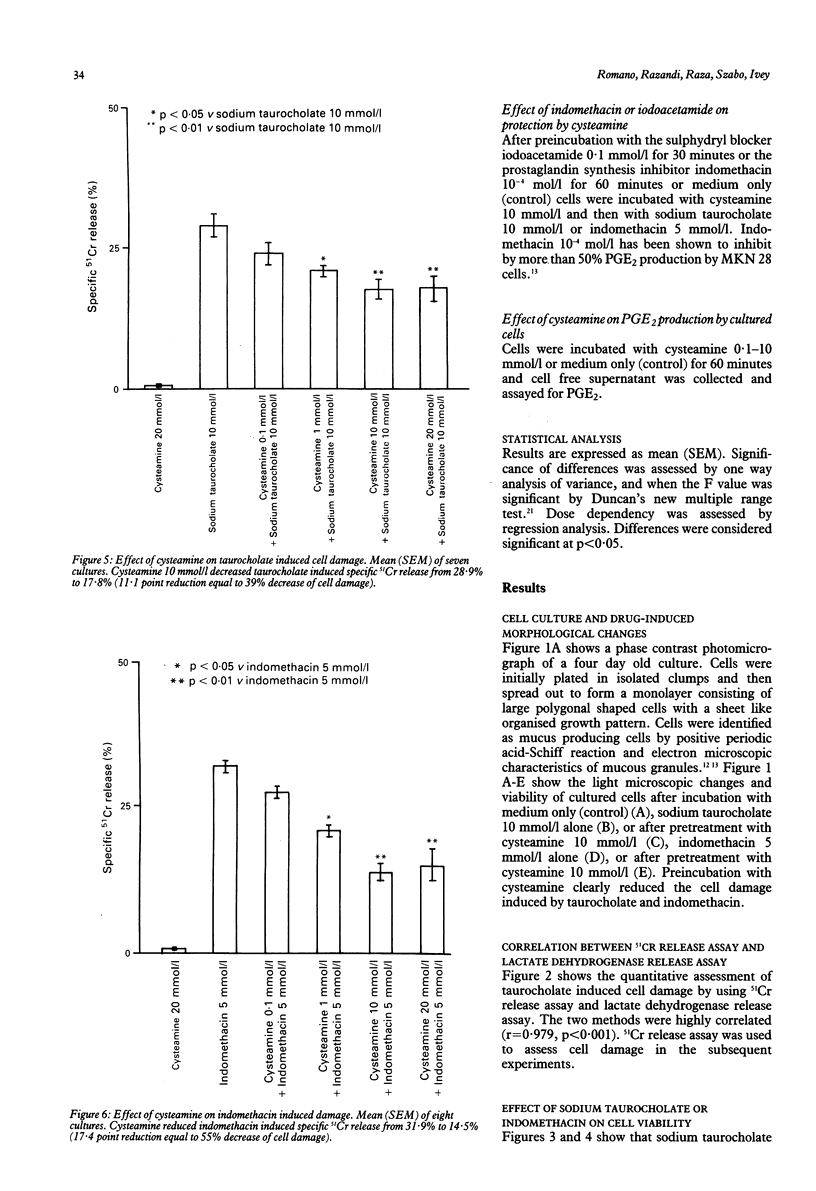
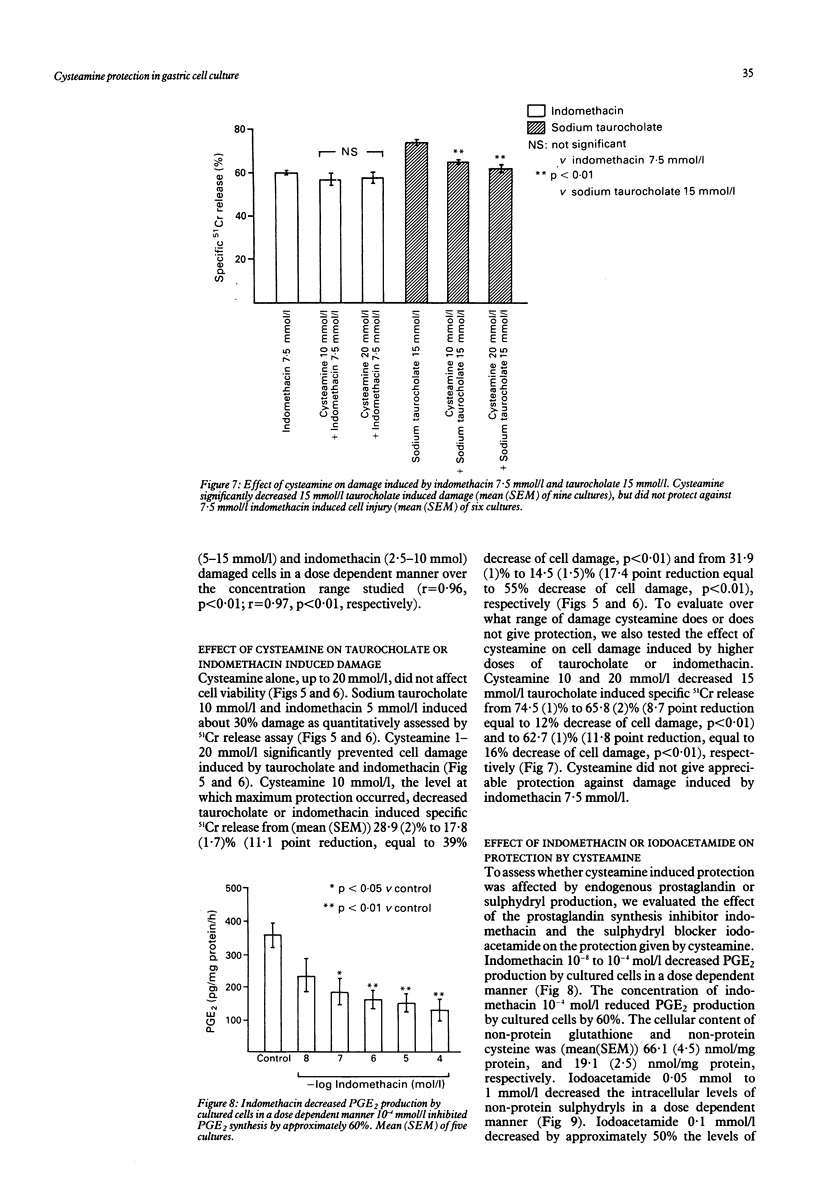
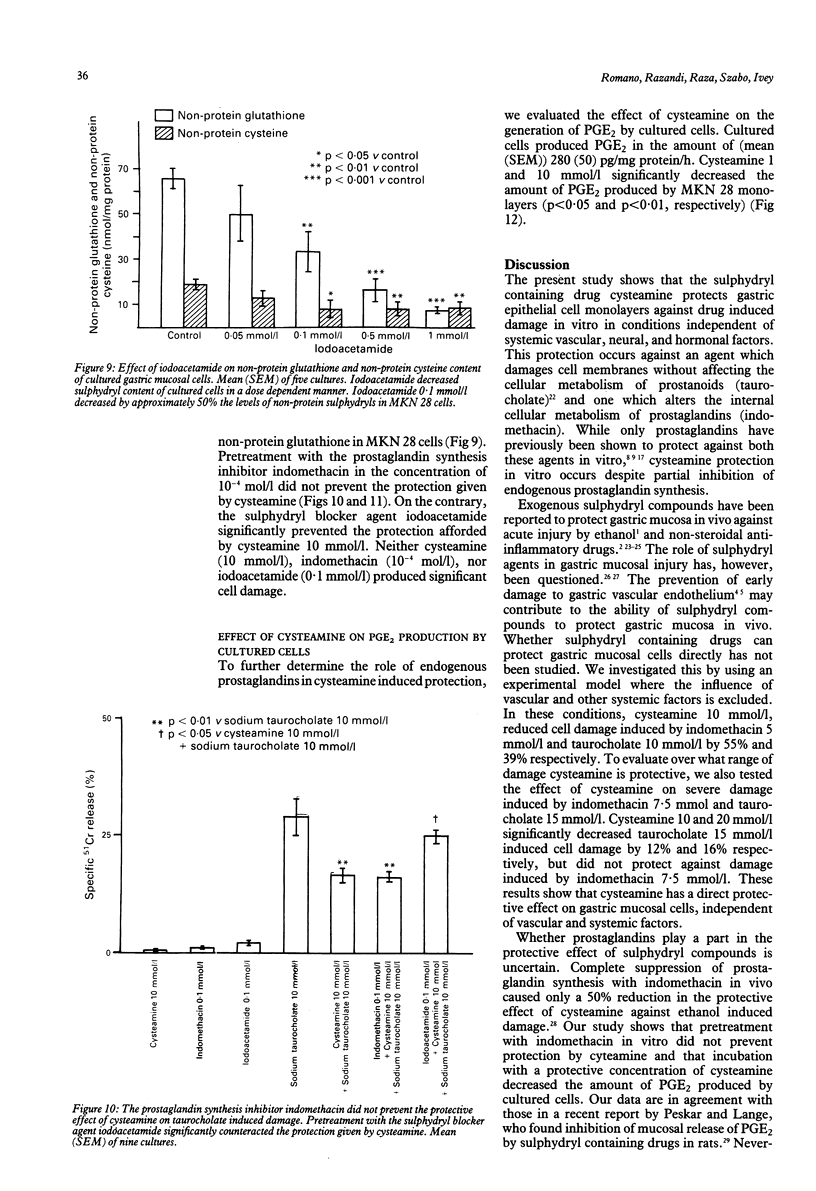
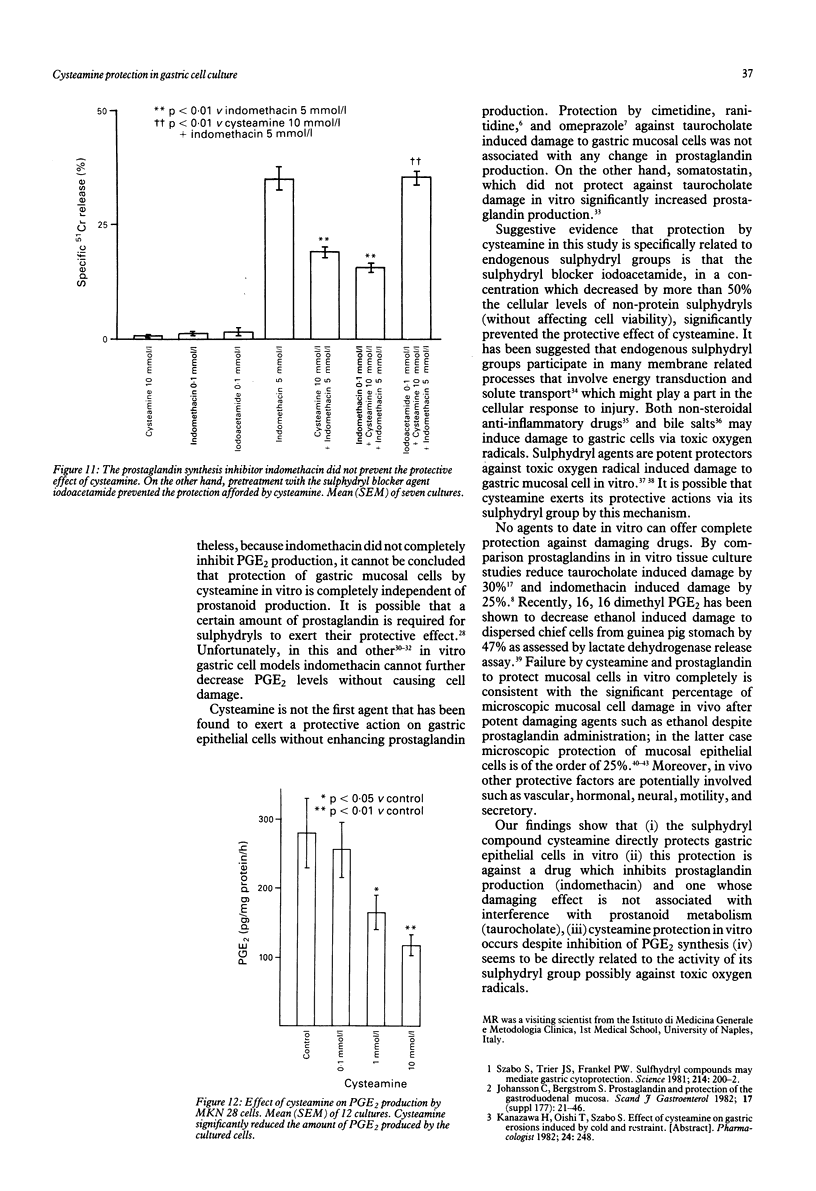
The sulphydryl containing drug cysteamine protects gastric mucosa in vivo against acute injury. It is not known whether this protection includes a direct effect on gastric cells. Using gastric epithelial cell monolayers derived from a well differentiated human cell line, we evaluated whether cysteamine protects against taurocholate or indomethacin induced damage in conditions which completely exclude the influence of vascular, hormonal, and neural factors. The effect of cysteamine on prostaglandin production by monolayer cells in vitro was also assessed. Cysteamine decreased damage brought about by sodium taurocholate and indomethacin by 40% (p less than 0.01) and 50% (p less than 0.01) respectively. The sulphydryl blocker iodoacetamide prevented the protective effect of cysteamine. Pretreatment with indomethacin, which inhibited prostaglandin E2 output by 60%, did not prevent protection by cysteamine; incubation with cysteamine decreased prostaglandin E2 production by cultured cells. We conclude that (i) cysteamine directly protected gastric epithelial cells in vitro (ii) this protection occurred with indomethacin, which interferes with cellular metabolism of prostaglandins, and taurocholate, whose damaging action at neutral pH is unrelated to interference with prostanoid metabolism, (iii) cysteamine protection in vitro is unrelated to endogenous prostaglandins and is probably mediated by endogenous sulphydryl compounds.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bálint G. A., Varró V. On the cytoprotective action of sulfhydryl-containing substances. Acta Physiol Acad Sci Hung. 1982;60(3):139–142. [PubMed] [Google Scholar]
- Cherner J. A., Naik L., Tarnawski A., Brzozowski T., Stachura J., Singh G. Ability of prostaglandin to reduce ethanol injury to dispersed chief cells from guinea pig stomach. Am J Physiol. 1989 Apr;256(4 Pt 1):G704–G714. doi: 10.1152/ajpgi.1989.256.4.G704. [DOI] [PubMed] [Google Scholar]
- Craven P. A., Pfanstiel J., DeRubertis F. R. Role of reactive oxygen in bile salt stimulation of colonic epithelial proliferation. J Clin Invest. 1986 Mar;77(3):850–859. doi: 10.1172/JCI112382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fumagalli R., Caponi R., Corsini A., Brambilla A., Palmira M., Bernini F., Valcavi U. Nonprotein sulfhydryls as possible components of the protective effect of rosaprostol on the rat gastric mucosa. Prostaglandins. 1985 Mar;29(3):467–474. doi: 10.1016/0090-6980(85)90104-2. [DOI] [PubMed] [Google Scholar]
- Guth P. H., Paulsen G., Nagata H. Histologic and microcirculatory changes in alcohol-induced gastric lesions in the rat: effect of prostaglandin cytoprotection. Gastroenterology. 1984 Nov;87(5):1083–1090. [PubMed] [Google Scholar]
- Hiraishi H., Terano A., Ota S., Ivey K. J., Sugimoto T. Effect of cimetidine on indomethacin-induced damage in cultured rat gastric mucosal cells; comparison with prostaglandin. J Lab Clin Med. 1986 Dec;108(6):608–615. [PubMed] [Google Scholar]
- Johansson C., Bergström S. Prostaglandin and protection of the gastroduodenal mucosa. Scand J Gastroenterol Suppl. 1982;77:21–46. [PubMed] [Google Scholar]
- Konturek S. J., Brzozowski T., Radecki T., Dobrzańska M. Generation of endogenous prostaglandins and thromboxanes in taurocholate-induced gastric mucosal lesions. Scand J Gastroenterol Suppl. 1984;92:91–96. [PubMed] [Google Scholar]
- Miller T. A., Li D., Kuo Y. J., Schmidt K. L., Shanbour L. L. Nonprotein sulfhydryl compounds in canine gastric mucosa: effects of PGE2 and ethanol. Am J Physiol. 1985 Jul;249(1 Pt 1):G137–G144. doi: 10.1152/ajpgi.1985.249.1.G137. [DOI] [PubMed] [Google Scholar]
- Mutoh H., Hiraishi H., Ota S., Ivey K. J., Terano A., Sugimoto T. Role of oxygen radicals in ethanol-induced damage to cultured gastric mucosal cells. Am J Physiol. 1990 Apr;258(4 Pt 1):G603–G609. doi: 10.1152/ajpgi.1990.258.4.G603. [DOI] [PubMed] [Google Scholar]
- Mutoh H., Hiraishi H., Ota S., Yoshida H., Ivey K. J., Terano A., Sugimoto T. Protective role of intracellular glutathione against ethanol-induced damage in cultured rat gastric mucosal cells. Gastroenterology. 1990 Jun;98(6):1452–1459. doi: 10.1016/0016-5085(90)91075-h. [DOI] [PubMed] [Google Scholar]
- Peskar B. M. On the synthesis of prostaglandins by human gastric mucosa and its modification by drugs. Biochim Biophys Acta. 1977 May 25;487(2):307–314. doi: 10.1016/0005-2760(77)90007-8. [DOI] [PubMed] [Google Scholar]
- Postius S., Ruoff H. J., Szelenyi I. Prostaglandin formation by isolated gastric parietal and nonparietal cells of the rat. Br J Pharmacol. 1985 Apr;84(4):871–877. doi: 10.1111/j.1476-5381.1985.tb17381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert A., Eberle D., Kaplowitz N. Role of glutathione in gastric mucosal cytoprotection. Am J Physiol. 1984 Sep;247(3 Pt 1):G296–G304. doi: 10.1152/ajpgi.1984.247.3.G296. [DOI] [PubMed] [Google Scholar]
- Robillard G. T., Konings W. N. A hypothesis for the role of dithiol-disulfide interchange in solute transport and energy-transducing processes. Eur J Biochem. 1982 Oct;127(3):597–604. doi: 10.1111/j.1432-1033.1982.tb06914.x. [DOI] [PubMed] [Google Scholar]
- Romano M., Razandi M., Ivey K. J. Effect of cimetidine and ranitidine on drug induced damage to gastric epithelial cell monolayers in vitro. Gut. 1989 Oct;30(10):1313–1322. doi: 10.1136/gut.30.10.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano M., Razandi M., Ivey K. J. Protection of gastric epithelial cell monolayers from a human cell line by omeprazole in vitro. Scand J Gastroenterol. 1989 Jun;24(5):513–521. doi: 10.3109/00365528909093082. [DOI] [PubMed] [Google Scholar]
- Romano M., Razandi M., Ivey K. J. Somatostatin stimulates prostaglandin production by rat gastric epithelial cells in vitro, but is not cytoprotective. Dig Dis Sci. 1988 Nov;33(11):1435–1440. doi: 10.1007/BF01536999. [DOI] [PubMed] [Google Scholar]
- Romano M., Razandi M., Sekhon S., Krause W. J., Ivey K. J. Human cell line for study of damage to gastric epithelial cells in vitro. J Lab Clin Med. 1988 Apr;111(4):430–440. [PubMed] [Google Scholar]
- Schmidt K. L., Henagan J. M., Smith G. S., Hilburn P. J., Miller T. A. Prostaglandin cytoprotection against ethanol-induced gastric injury in the rat. A histologic and cytologic study. Gastroenterology. 1985 Mar;88(3):649–659. doi: 10.1016/0016-5085(85)90132-5. [DOI] [PubMed] [Google Scholar]
- Strubelt O., Hoppenkamps R. Relations between gastric glutathione and the ulcerogenic action of non-steroidal anti-inflammatory drugs. Arch Int Pharmacodyn Ther. 1983 Apr;262(2):268–278. [PubMed] [Google Scholar]
- Szabo S., Trier J. S., Brown A., Schnoor J. Early vascular injury and increased vascular permeability in gastric mucosal injury caused by ethanol in the rat. Gastroenterology. 1985 Jan;88(1 Pt 2):228–236. doi: 10.1016/s0016-5085(85)80176-1. [DOI] [PubMed] [Google Scholar]
- Szabo S., Trier J. S., Frankel P. W. Sulfhydryl compounds may mediate gastric cytoprotection. Science. 1981 Oct 9;214(4517):200–202. doi: 10.1126/science.7280691. [DOI] [PubMed] [Google Scholar]
- Takeuchi K., Okada M., Niida H., Okabe S. Role of sulfhydryls in mucosal injury caused by ethanol: relation to microvascular permeability, gastric motility and cytoprotection. J Pharmacol Exp Ther. 1989 Feb;248(2):836–841. [PubMed] [Google Scholar]
- Tarnawski A., Brzozowski T., Sarfeh I. J., Krause W. J., Ulich T. R., Gergely H., Hollander D. Prostaglandin protection of human isolated gastric glands against indomethacin and ethanol injury. Evidence for direct cellular action of prostaglandin. J Clin Invest. 1988 Apr;81(4):1081–1089. doi: 10.1172/JCI113420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarnawski A., Hollander D., Stachura J., Krause W. J., Gergely H. Prostaglandin protection of the gastric mucosa against alcohol injury--a dynamic time-related process. Role of the mucosal proliferative zone. Gastroenterology. 1985 Jan;88(1 Pt 2):334–352. doi: 10.1016/s0016-5085(85)80188-8. [DOI] [PubMed] [Google Scholar]
- Terano A., Mach T., Stachura J., Tarnawski A., Ivey K. J. Effect of 16,16 dimethyl prostaglandin E2 on aspirin induced damage to rat gastric epithelial cells in tissue culture. Gut. 1984 Jan;25(1):19–25. doi: 10.1136/gut.25.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terano A., Ota S., Mach T., Hiraishi H., Stachura J., Tarnawski A., Ivey K. J. Prostaglandin protects against taurocholate-induced damage to rat gastric mucosal cell culture. Gastroenterology. 1987 Mar;92(3):669–677. doi: 10.1016/0016-5085(87)90016-3. [DOI] [PubMed] [Google Scholar]
- WIGZELL H. QUANTITATIVE TITRATIONS OF MOUSE H-2 ANTIBODIES USING CR-51-LABELLED TARGET CELLS. Transplantation. 1965 May;3:423–431. doi: 10.1097/00007890-196505000-00011. [DOI] [PubMed] [Google Scholar]
- Whittle B. J., Steel G. Evaluation of the protection of rat gastric mucosa by a prostaglandin analogue using cellular enzyme marker and histologic techniques. Gastroenterology. 1985 Jan;88(1 Pt 2):315–327. doi: 10.1016/s0016-5085(85)80186-4. [DOI] [PubMed] [Google Scholar]




