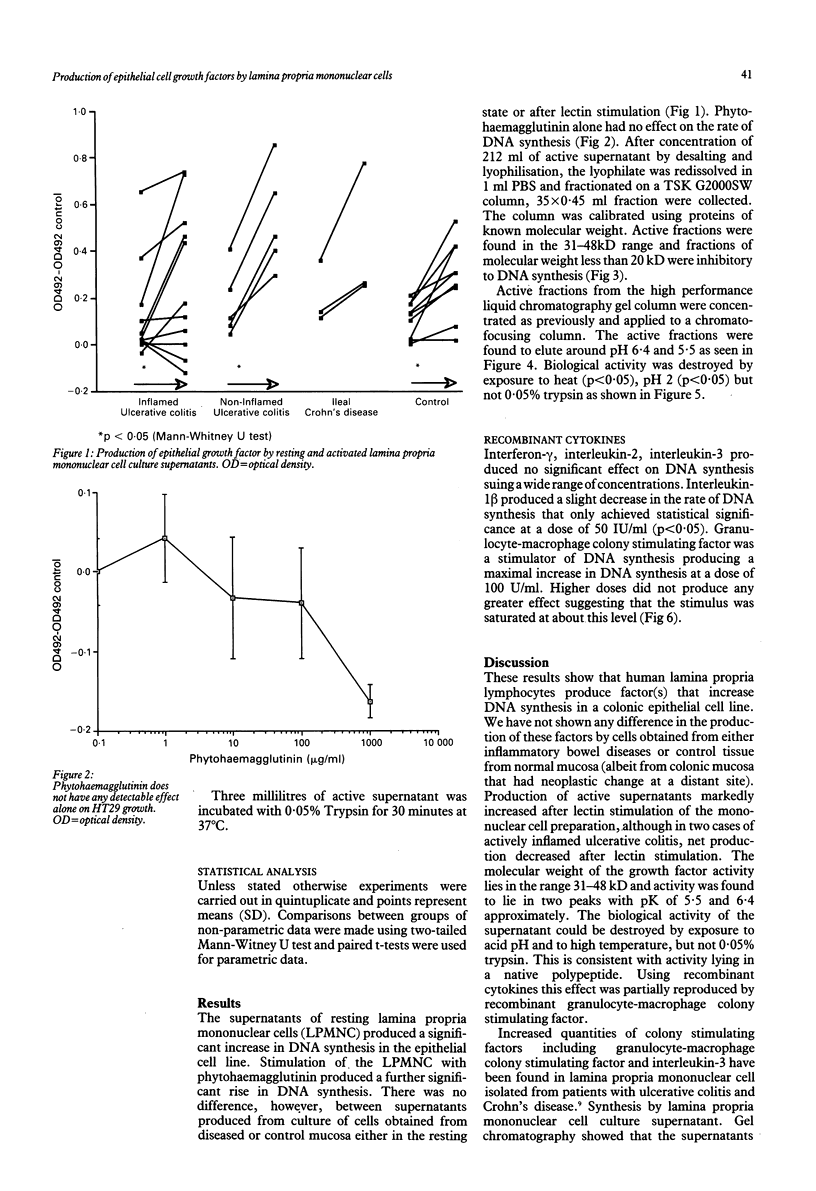
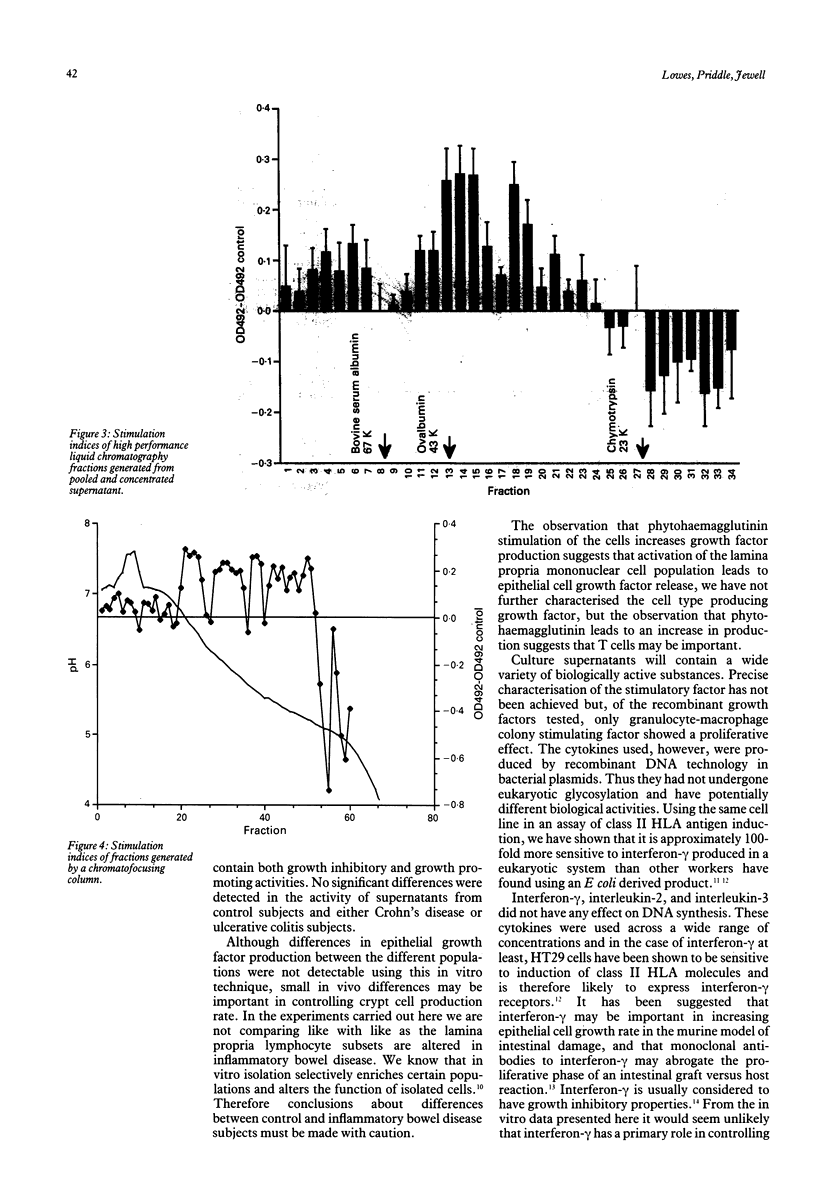
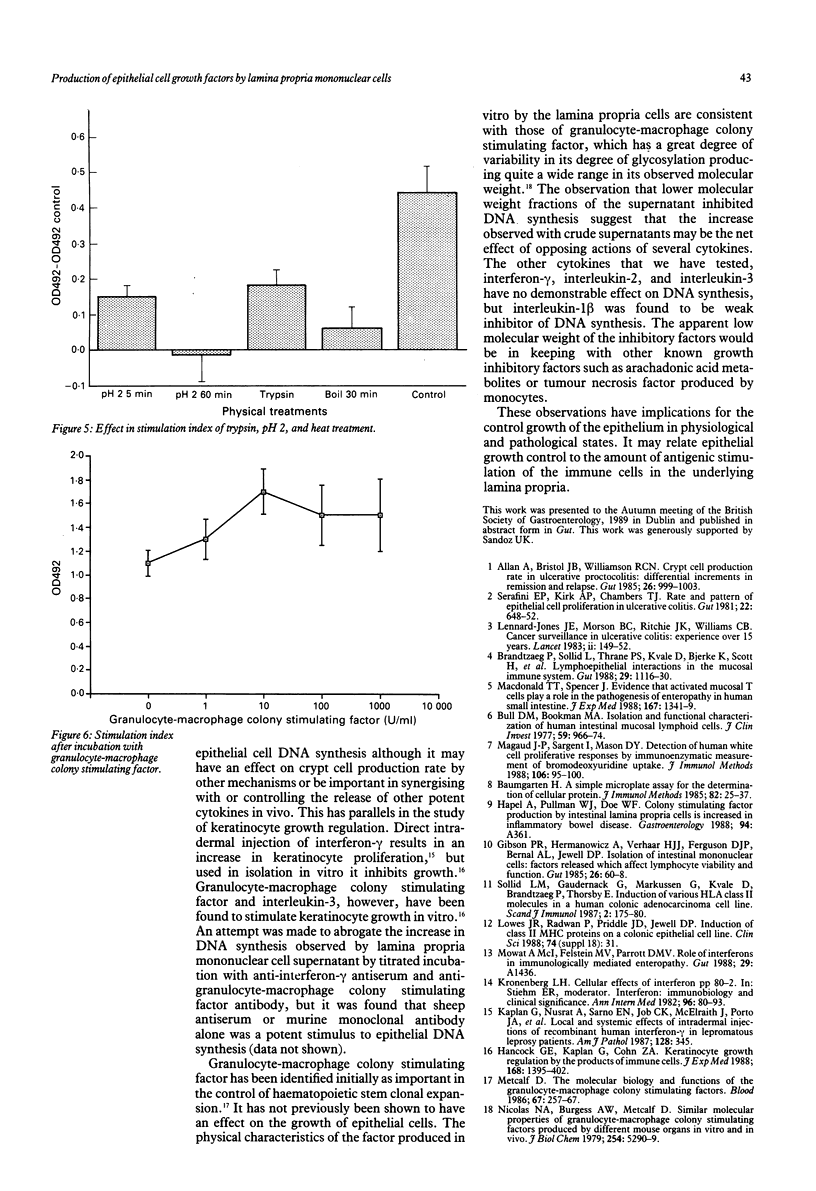
Abstract
The effects of lamina propria mononuclear cell culture supernatant on epithelial cell DNA synthesis were studied using cells isolated from patients with inflammatory bowel disease and normal controls. Supernatants from resting and phytohaemagglutinin stimulated cells were studied and supernatants that strongly promoted DNA synthesis were pooled, and growth factor activity partially characterised. The effects of recombinant interleukins-1 beta,2,3,interferon-gamma, and granulocyte macrophage colony stimulating factor were tested in the same system. Resting lamina propria mononuclear cells produce factors that increase DNA synthesis. Production of these factors is increased by phytohaemagglutinin stimulation. No significant differences were found in production of these factors between patients with inflammatory bowel disease and normal controls. The molecular weight of the active factor(s) lies in the region 31-48 kD. Chromatofocusing produced two peaks of activity, one in the region pk 5.5 and one around pk 6.4. The activity was heat and acid pH labile. Activity was not destroyed, however, by 0.05% trypsin. Recombinant granulocyte macrophage colony stimulating factor was a weak stimulus to epithelial DNA synthesis, interleukin-1 beta was weakly inhibitory but other cytokines tested did not have any effect. Granulocyte macrophage colony stimulating factor is probably important in controlling epithelial cell growth.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allan A., Bristol J. B., Williamson R. C. Crypt cell production rate in ulcerative proctocolitis: differential increments in remission and relapse. Gut. 1985 Oct;26(10):999–1003. doi: 10.1136/gut.26.10.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgarten H. A simple microplate assay for the determination of cellular protein. J Immunol Methods. 1985 Sep 3;82(1):25–37. doi: 10.1016/0022-1759(85)90221-2. [DOI] [PubMed] [Google Scholar]
- Brandtzaeg P., Sollid L. M., Thrane P. S., Kvale D., Bjerke K., Scott H., Kett K., Rognum T. O. Lymphoepithelial interactions in the mucosal immune system. Gut. 1988 Aug;29(8):1116–1130. doi: 10.1136/gut.29.8.1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bull D. M., Bookman M. A. Isolation and functional characterization of human intestinal mucosal lymphoid cells. J Clin Invest. 1977 May;59(5):966–974. doi: 10.1172/JCI108719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson P. R., Hermanowicz A., Verhaar H. J., Ferguson D. J., Bernal A. L., Jewell D. P. Isolation of intestinal mononuclear cells: factors released which affect lymphocyte viability and function. Gut. 1985 Jan;26(1):60–68. doi: 10.1136/gut.26.1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock G. E., Kaplan G., Cohn Z. A. Keratinocyte growth regulation by the products of immune cells. J Exp Med. 1988 Oct 1;168(4):1395–1402. doi: 10.1084/jem.168.4.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan G., Nusrat A., Sarno E. N., Job C. K., McElrath J., Porto J. A., Nathan C. F., Cohn Z. A. Cellular responses to the intradermal injection of recombinant human gamma-interferon in lepromatous leprosy patients. Am J Pathol. 1987 Aug;128(2):345–353. [PMC free article] [PubMed] [Google Scholar]
- Lennard-Jones J. E., Morson B. C., Ritchie J. K., Williams C. B. Cancer surveillance in ulcerative colitis. Experience over 15 years. Lancet. 1983 Jul 16;2(8342):149–152. doi: 10.1016/s0140-6736(83)90129-0. [DOI] [PubMed] [Google Scholar]
- MacDonald T. T., Spencer J. Evidence that activated mucosal T cells play a role in the pathogenesis of enteropathy in human small intestine. J Exp Med. 1988 Apr 1;167(4):1341–1349. doi: 10.1084/jem.167.4.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magaud J. P., Sargent I., Mason D. Y. Detection of human white cell proliferative responses by immunoenzymatic measurement of bromodeoxyuridine uptake. J Immunol Methods. 1988 Jan 21;106(1):95–100. doi: 10.1016/0022-1759(88)90276-1. [DOI] [PubMed] [Google Scholar]
- Metcalf D. The molecular biology and functions of the granulocyte-macrophage colony-stimulating factors. Blood. 1986 Feb;67(2):257–267. [PubMed] [Google Scholar]
- Nicola N. A., Burgess A. W., Metcalf D. Similar molecular properties of granulocyte-macrophage colony-stimulating factors produced by different mouse organs in vitro and in vivo. J Biol Chem. 1979 Jun 25;254(12):5290–5299. [PubMed] [Google Scholar]
- Serafini E. P., Kirk A. P., Chambers T. J. Rate and pattern of epithelial cell proliferation in ulcerative colitis. Gut. 1981 Aug;22(8):648–652. doi: 10.1136/gut.22.8.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sollid L. M., Gaudernack G., Markussen G., Kvale D., Brandtzaeg P., Thorsby E. Induction of various HLA class II molecules in a human colonic adenocarcinoma cell line. Scand J Immunol. 1987 Feb;25(2):175–180. doi: 10.1111/j.1365-3083.1987.tb01061.x. [DOI] [PubMed] [Google Scholar]
- Stiehm E. R., Kronenberg L. H., Rosenblatt H. M., Bryson Y., Merigan T. C. UCLA conference. Interferon: immunobiology and clinical significance. Ann Intern Med. 1982 Jan;96(1):80–93. doi: 10.7326/0003-4819-96-1-80. [DOI] [PubMed] [Google Scholar]


