Abstract
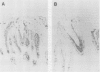
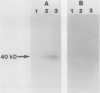
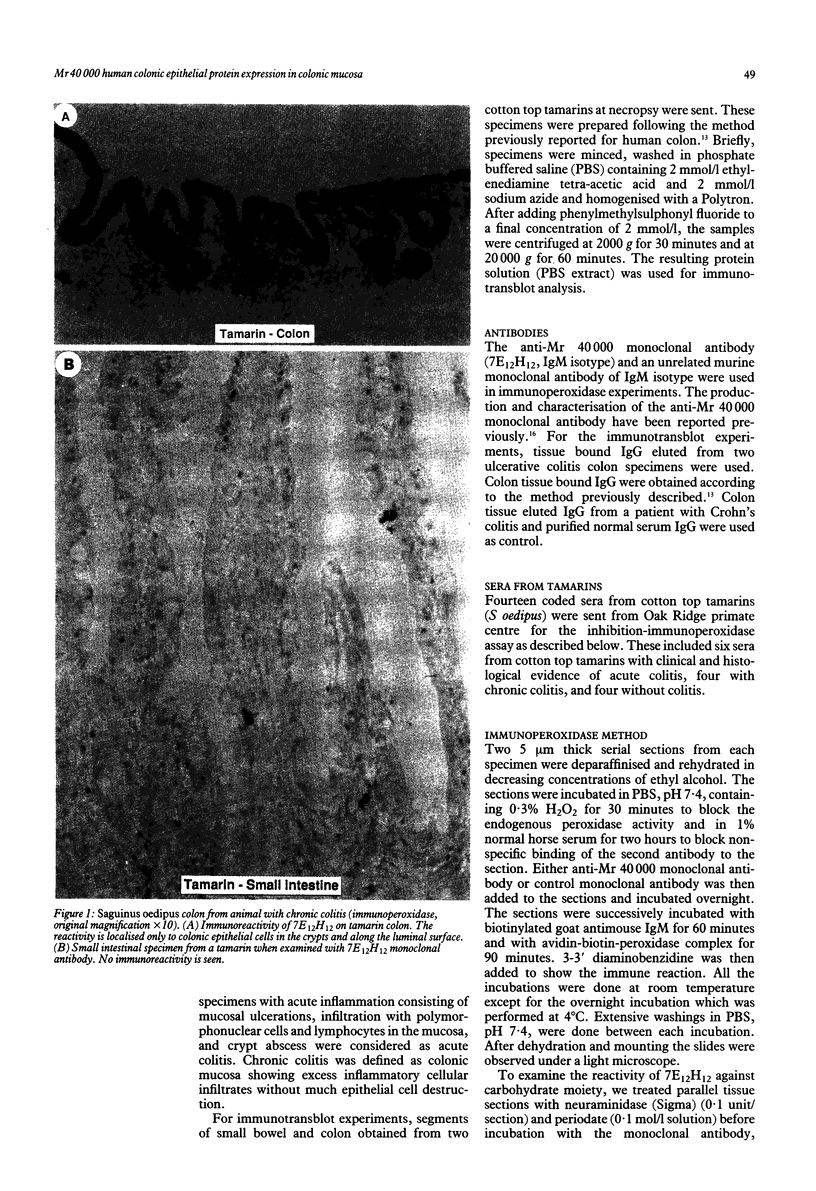
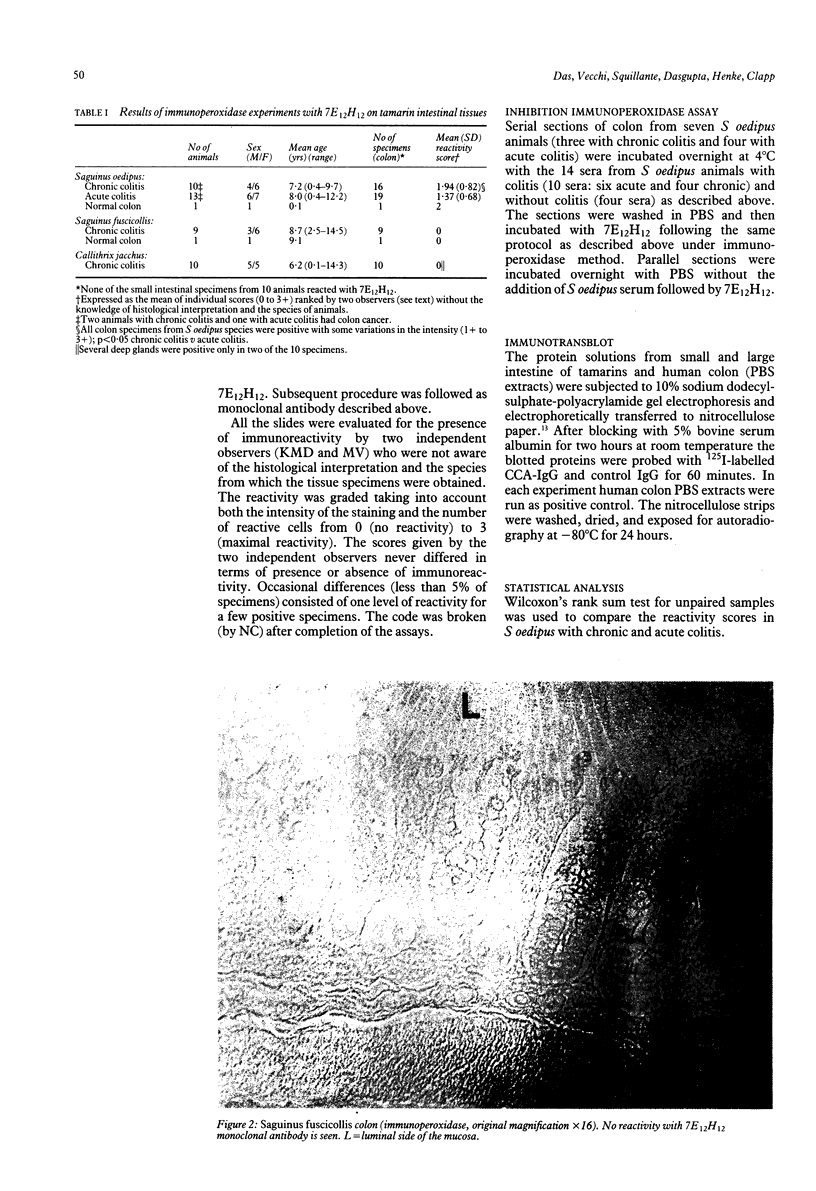
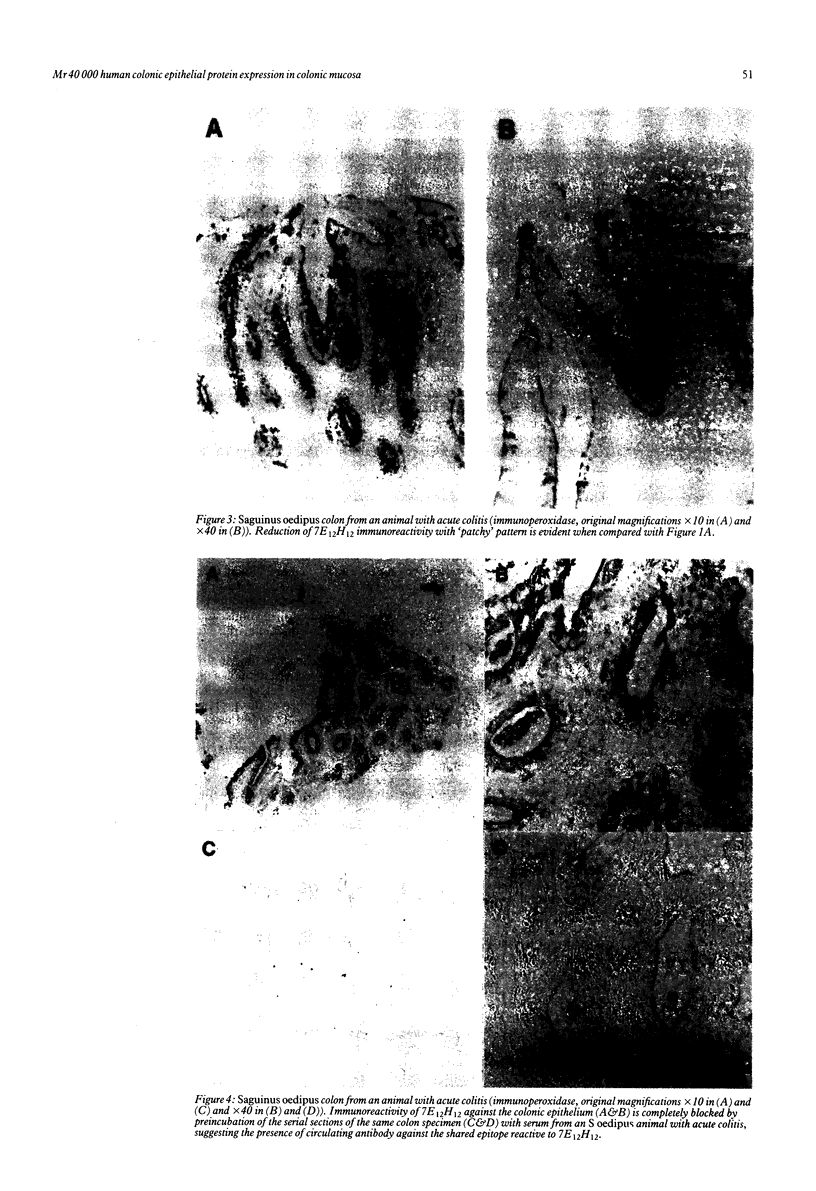
Saguinus oedipus, Callithrix jacchus, and Saguinus fuscicollis are three species of New World monkeys which develop a form of colitis that is similar to human ulcerative colitis. Only S oedipus, however, develop colon cancer. We examined intestinal tissues from these animals for the presence of an antigen cross reacting to the Mr 40,000 human colonic epithelial protein that acts as an autoantigen in ulcerative colitis. Using an anti-Mr 40,000 monoclonal antibody (7E12H12, IgM isotype), by an immunoperoxidase assay we showed that all colon specimens from S oedipus reacted with 7E12H12; however, the colonic tissue from C jacchus and S fuscicollis did not. In immunotransblot analysis eluted IgG antibody bound to human ulcerative colitis colon (CCA-IgG) reacted with Mr 40,000 protein(s) present in the extracts of colon from S oedipus animals and humans. Small intestinal tissue reacted neither with 7E12H12 nor with CCA-IgG. In S oedipus, the Mr 40,000 protein was localised exclusively to colonic epithelial cells. Preincubation of seven S oedipus colon specimens with eight of 10 sera from animals with acute or chronic colitis and 0 of four sera from animals without colitis almost completely inhibited the binding of 7E12H12 to the colonic epithelium. Four of these 10 sera inhibited the binding of 7E12H12 to the autologous colon. These results show the presence of circulating autoantibodies in S oedipus with colitis against an epitope(s) on Mr 40,000 protein shared by human and S oedipus colon.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Biancone L., Wise L. S., Das K. M. The presence in experimental animals of a colon specific Mr 40,000 protein(s) with relevance to ulcerative colitis. Gut. 1991 May;32(5):504–508. doi: 10.1136/gut.32.5.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boland C. R., Clapp N. K. Glycoconjugates in the colons of New World monkeys with spontaneous colitis. Association between inflammation and neoplasia. Gastroenterology. 1987 Mar;92(3):625–634. doi: 10.1016/0016-5085(87)90010-2. [DOI] [PubMed] [Google Scholar]
- Chalifoux L. V., Bronson R. T. Colonic adenocarcinoma associated with chronic colitis in cotton top marmosets, Saguinus oedipus. Gastroenterology. 1981 May;80(5 Pt 1):942–946. [PubMed] [Google Scholar]
- Clapp N. K., Henke M. L., Lushbaugh C. C., Humason G. L., Gangaware B. L. Effect of various biological factors on spontaneous marmoset and tamarin colitis. A retrospective histopathologic study. Dig Dis Sci. 1988 Aug;33(8):1013–1019. doi: 10.1007/BF01535999. [DOI] [PubMed] [Google Scholar]
- Clapp N. K., McArthur A. H., Carson R. L., Henke M. A., Peck O. C., Wood J. D. Visualization and biopsy of the colon in tamarins and marmosets by endoscopy. Lab Anim Sci. 1987 Apr;37(2):217–219. [PubMed] [Google Scholar]
- Das K. M., Sakamaki S., Vecchi M., Diamond B. The production and characterization of monoclonal antibodies to a human colonic antigen associated with ulcerative colitis: cellular localization of the antigen by using the monoclonal antibody. J Immunol. 1987 Jul 1;139(1):77–84. [PubMed] [Google Scholar]
- Das K. M., Sakamaki S., Vecchi M. Ulcerative colitis: specific antibodies against a colonic epithelial Mr 40,000 protein. Immunol Invest. 1989 Jan-May;18(1-4):459–472. doi: 10.3109/08820138909112256. [DOI] [PubMed] [Google Scholar]
- Lushbaugh C., Humason G., Clapp N. Histology of colitis: Saguinus oedipus oedipus and other marmosets. Dig Dis Sci. 1985 Dec;30(12 Suppl):45S–51S. doi: 10.1007/BF01296974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madara J. L., Podolsky D. K., King N. W., Sehgal P. K., Moore R., Winter H. S. Characterization of spontaneous colitis in cotton-top tamarins (Saguinus oedipus) and its response to sulfasalazine. Gastroenterology. 1985 Jan;88(1 Pt 1):13–19. doi: 10.1016/s0016-5085(85)80126-8. [DOI] [PubMed] [Google Scholar]
- Moore R., King N., Alroy J. Characterization of colonic cellular glycoconjugates in colitis and cancer-prone tamarins versus colitis and cancer-resistant primates. Am J Pathol. 1988 Jun;131(3):477–483. [PMC free article] [PubMed] [Google Scholar]
- Moore R., King N., Alroy J. Differences in cellular glycoconjugates of quiescent, inflamed, and neoplastic colonic epithelium in colitis and cancer-prone tamarins. Am J Pathol. 1988 Jun;131(3):484–489. [PMC free article] [PubMed] [Google Scholar]
- Nagai T., Das K. M. Detection of colonic antigen(s) in tissues from ulcerative colitis using purified colitis colon tissue-bound IgG (CCA-IgG). Gastroenterology. 1981 Sep;81(3):463–470. [PubMed] [Google Scholar]
- Podolsky D. K., Madara J. L., King N., Sehgal P., Moore R., Winter H. S. Colonic mucin composition in primates. Selective alterations associated with spontaneous colitis in the cotton-top tamarin. Gastroenterology. 1985 Jan;88(1 Pt 1):20–25. doi: 10.1016/s0016-5085(85)80127-x. [DOI] [PubMed] [Google Scholar]
- Takahashi F., Das K. M. Isolation and characterization of a colonic autoantigen specifically recognized by colon tissue-bound immunoglobulin G from idiopathic ulcerative colitis. J Clin Invest. 1985 Jul;76(1):311–318. doi: 10.1172/JCI111963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahasi F., Shah H. S., Wise L. S., Das K. M. Circulating antibodies against human colonic extract enriched with a 40 kDa protein in patients with ulcerative colitis. Gut. 1990 Sep;31(9):1016–1020. doi: 10.1136/gut.31.9.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vecchi M., Sakamaki S., Diamond B., Novikoff A. B., Novikoff P. M., Das K. M. Development of a monoclonal antibody specifically reactive to gastrointestinal goblet cells. Proc Natl Acad Sci U S A. 1987 May;84(10):3425–3429. doi: 10.1073/pnas.84.10.3425. [DOI] [PMC free article] [PubMed] [Google Scholar]