Abstract
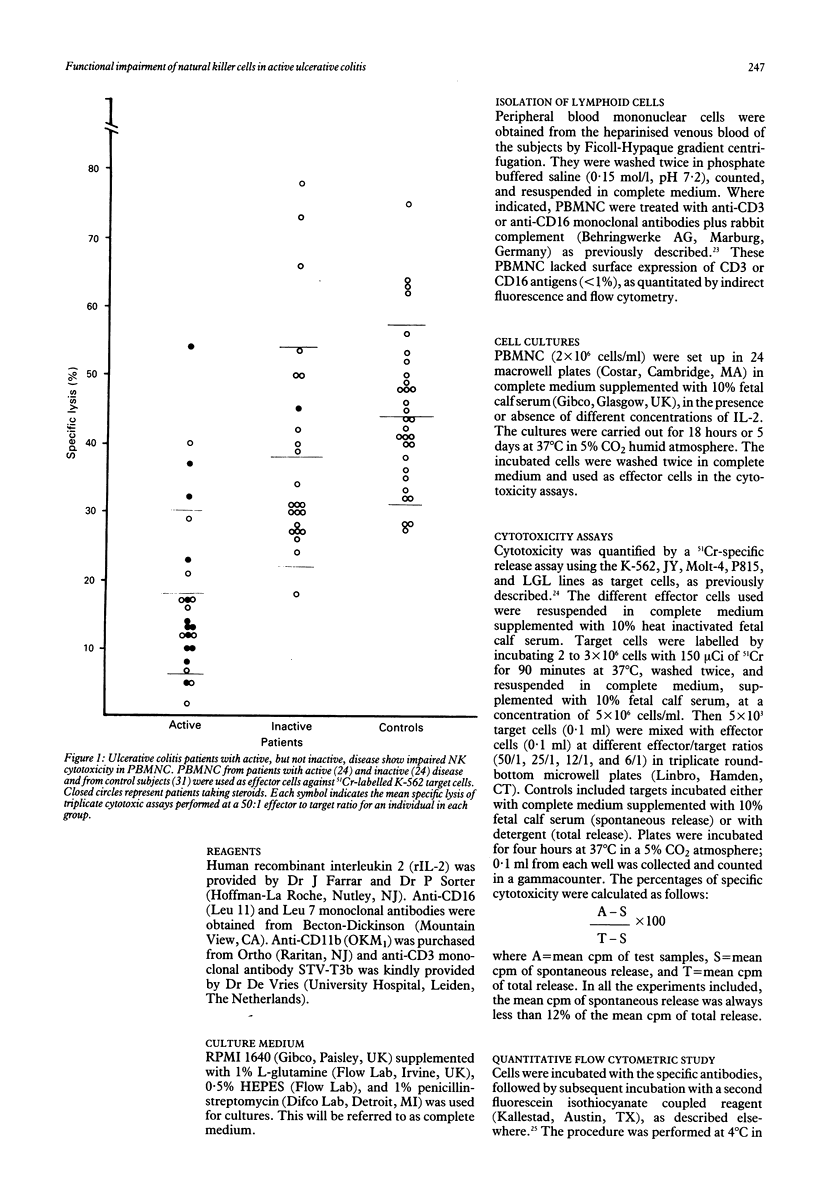
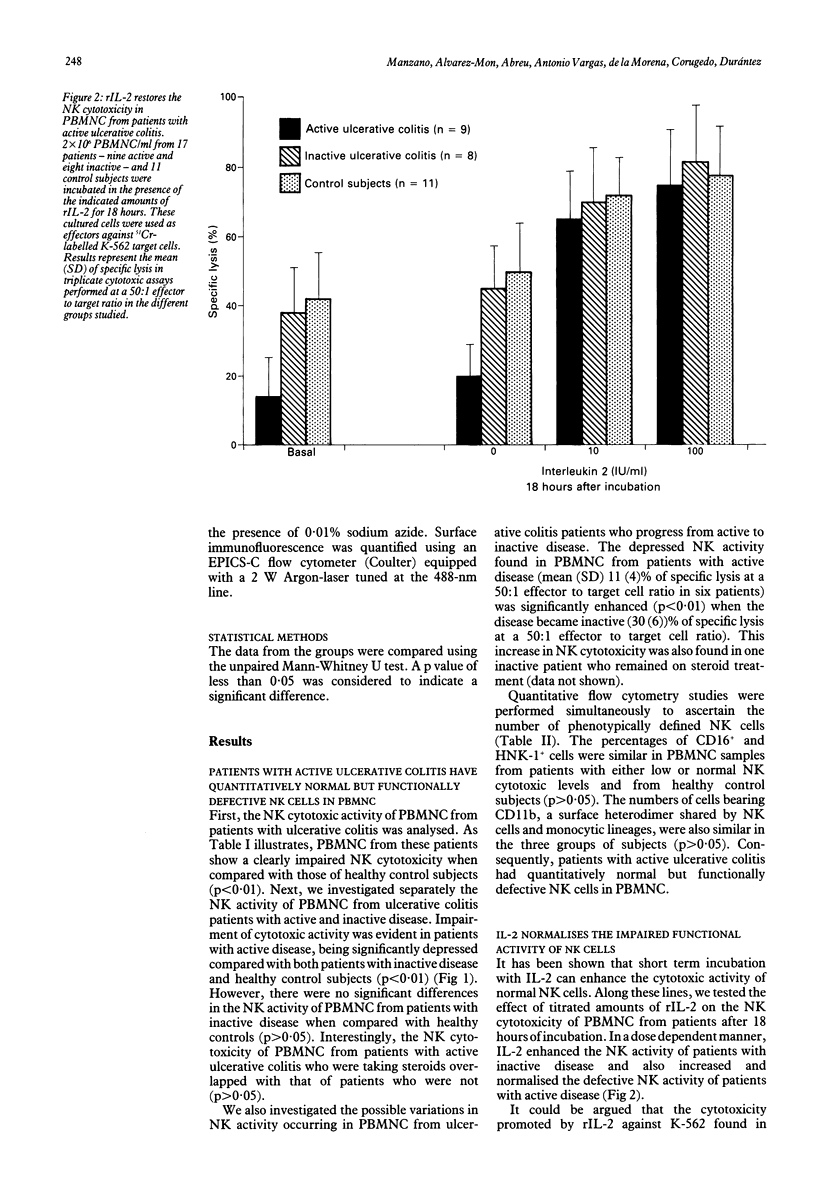
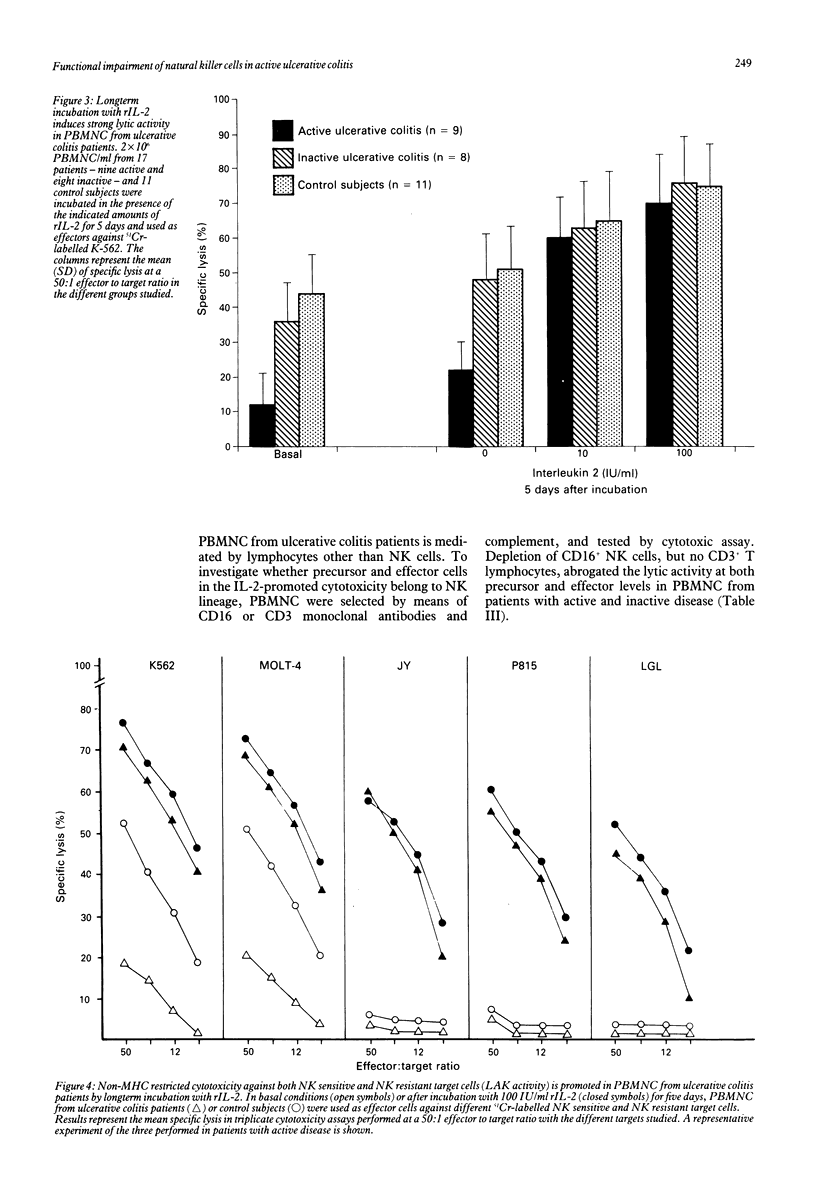
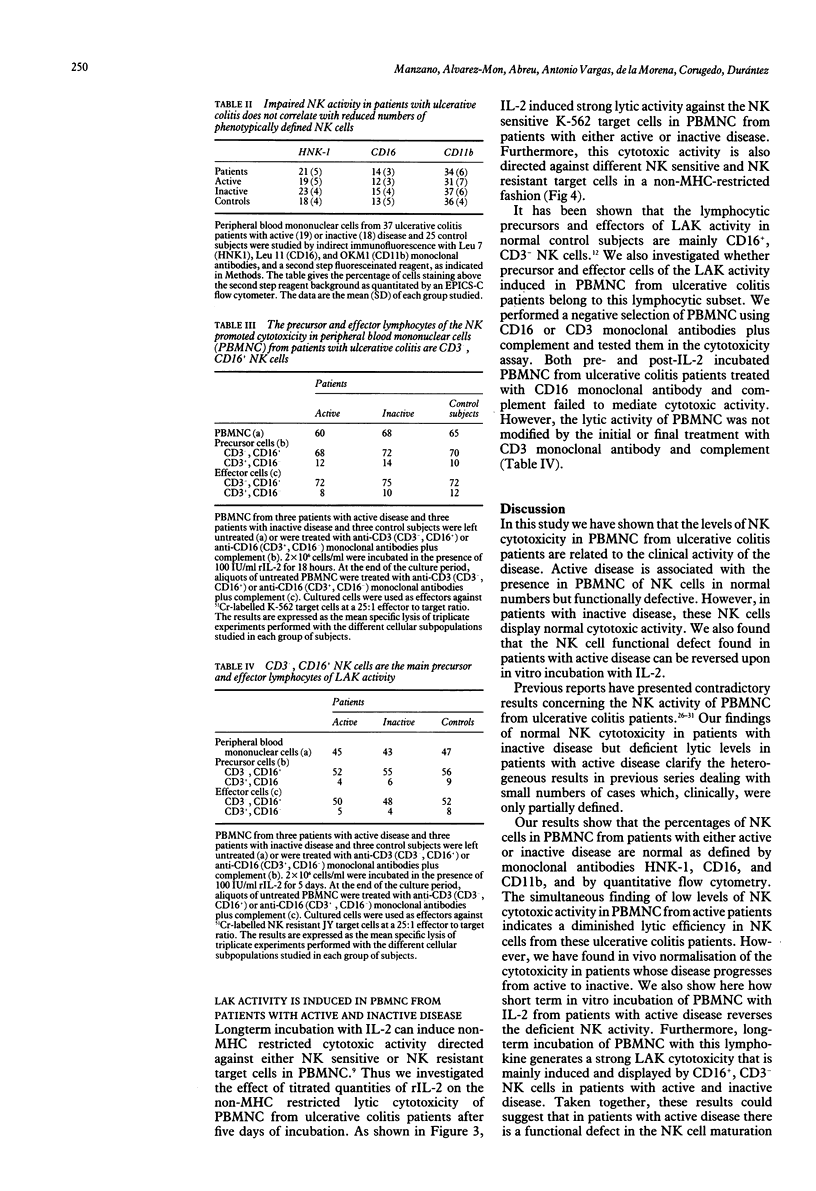
We have studied the functional characteristics and clinical importance of the natural killer (NK) cytotoxicity of peripheral blood mononuclear cells (PBMNC) from patients with ulcerative colitis. Normal NK activity was observed in PBMNC from patients with inactive disease, but a pronounced decrease was found in those with active disease. Clinical change from active to inactive disease was associated with enhancement of the depressed NK activity. The impairment of NK cytotoxicity found in patients with active disese could not be ascribed to a deficient number of NK cells as the amounts of HNK-1+, CD16+ (Leu 11), and CD11b (OKM1) cells in PBMNC were within normal ranges. This defective cytotoxic PBMNC activity was normalised by short term (18 hour) incubation with recombinant interleukin 2 (rIL-2). Moreover, long term (5 day) incubation of these effector cells with rIL-2 induced strong cytotoxic activity against NK resistant and NK sensitive target cells in patients with active and inactive disease. We also found that both precursors and effectors of cytotoxic activity promoted by short term and long term incubation with rIL-2 of PBMNC from the patients showed the phenotype of NK cells (CD16+, CD3-). Taken together, these results show that active ulcerative colitis is associated with a defective function of NK cells that is found to be normal in the inactive stage of the disease. The possible pathogenic and therapeutic implications of these findings are discussed.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alvarez de Mon M., Casas J., Laguna R., Toribio M. L., de Landázuri M. O., Durántez A. Lymphokine induction of NK-like cytotoxicity in T cells from B-CLL. Blood. 1986 Jan;67(1):228–232. [PubMed] [Google Scholar]
- Alvarez-Mon M., Casas J., Laguna R., Jordá J., Durantez A. Clinical signification of natural killer activity in B-cell chronic lymphocytic leukemia. Eur J Haematol. 1987 Mar;38(3):268–273. doi: 10.1111/j.1600-0609.1987.tb01175.x. [DOI] [PubMed] [Google Scholar]
- Alvarez-Mon M., de la Hera A., Gaspar M. L., Orfao A., Casas J., Jordá J., Durantez A. Proliferation of B cells from chronic lymphocytic leukemia is selectively promoted by B cell growth factor. Acta Haematol. 1989;81(2):91–97. doi: 10.1159/000205533. [DOI] [PubMed] [Google Scholar]
- Biron C. A., Byron K. S., Sullivan J. L. Severe herpesvirus infections in an adolescent without natural killer cells. N Engl J Med. 1989 Jun 29;320(26):1731–1735. doi: 10.1056/NEJM198906293202605. [DOI] [PubMed] [Google Scholar]
- Bonilla F., Alvarez-Mon M., Merino F., de la Hera A., Alés J. E., España P., Durántez A. Interleukin-2 induces cytotoxic activity in lymphocytes from regional axillary nodes of breast cancer patients. Cancer. 1988 Feb 15;61(4):629–634. doi: 10.1002/1097-0142(19880215)61:4<629::aid-cncr2820610402>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Carballido J., Alvarez-Mon M., Solovera O. J., Menéndez-Ondina L., Durántez A. Clinical significance of natural killer activity in patients with transitional cell carcinoma of the bladder. J Urol. 1990 Jan;143(1):29–33. doi: 10.1016/s0022-5347(17)39854-3. [DOI] [PubMed] [Google Scholar]
- Cave D. R., Mitchell D. N., Brooke B. N. Evidence of an agent transmissible from ulcerative colitis tissue. Lancet. 1976 Jun 19;1(7973):1311–1315. doi: 10.1016/s0140-6736(76)92649-0. [DOI] [PubMed] [Google Scholar]
- Dickinson R. J., Varian S. A., Axon A. T., Cooke E. M. Increased incidence of faecal coliforms with in vitro adhesive and invasive properties in patients with ulcerative colitis. Gut. 1980 Sep;21(9):787–792. doi: 10.1136/gut.21.9.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egawa S., Hiwatashi N. Natural killer cell activity in patients with inflammatory bowel disease. J Clin Lab Immunol. 1986 Aug;20(4):187–192. [PubMed] [Google Scholar]
- Fiocchi C., Tubbs R. R., Youngman K. R. Human intestinal mucosal mononuclear cells exhibit lymphokine-activated killer cell activity. Gastroenterology. 1985 Mar;88(3):625–637. doi: 10.1016/0016-5085(85)90130-1. [DOI] [PubMed] [Google Scholar]
- Garcia-Peñarrubia P., Koster F. T., Kelley R. O., McDowell T. D., Bankhurst A. D. Antibacterial activity of human natural killer cells. J Exp Med. 1989 Jan 1;169(1):99–113. doi: 10.1084/jem.169.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaspar M. L., Alvarez-Mon M., Gutiérrez C. Role of interleukin 2 in inducing normalization of natural killer activity in systemic lupus erythematosus. Clin Immunol Immunopathol. 1988 Nov;49(2):204–214. doi: 10.1016/0090-1229(88)90110-9. [DOI] [PubMed] [Google Scholar]
- Gibson P. R., Jewell D. P. Local immune mechanisms in inflammatory bowel disease and colorectal carcinoma. Natural killer cells and their activity. Gastroenterology. 1986 Jan;90(1):12–19. doi: 10.1016/0016-5085(86)90068-5. [DOI] [PubMed] [Google Scholar]
- Ginsburg C. H., Dambrauskas J. T., Ault K. A., Falchuk Z. M. Impaired natural killer cell activity in patients with inflammatory bowel disease: evidence for a qualitative defect. Gastroenterology. 1983 Oct;85(4):846–851. [PubMed] [Google Scholar]
- Gitnick G. L., Rosen V. J., Arthur M. H., Hertweck S. A. Evidence for the isolation of a new virus from ulcerative colitis patients. Comparison with virus derived from Crohn's disease. Dig Dis Sci. 1979 Aug;24(8):609–619. doi: 10.1007/BF01333705. [DOI] [PubMed] [Google Scholar]
- Grimm E. A., Mazumder A., Zhang H. Z., Rosenberg S. A. Lymphokine-activated killer cell phenomenon. Lysis of natural killer-resistant fresh solid tumor cells by interleukin 2-activated autologous human peripheral blood lymphocytes. J Exp Med. 1982 Jun 1;155(6):1823–1841. doi: 10.1084/jem.155.6.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harries A. D., Myers B., Cook G. C. Inflammatory bowel disease: a common cause of bloody diarrhoea in visitors to the tropics. Br Med J (Clin Res Ed) 1985 Dec 14;291(6510):1686–1687. doi: 10.1136/bmj.291.6510.1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser S. L., Ault K. A., Levin M. J., Garovoy M. R., Weiner H. L. Natural killer cell activity in multiple sclerosis. J Immunol. 1981 Sep;127(3):1114–1117. [PubMed] [Google Scholar]
- Hercend T., Schmidt R. E. Characteristics and uses of natural killer cells. Immunol Today. 1988 Oct;9(10):291–293. doi: 10.1016/0167-5699(88)91317-5. [DOI] [PubMed] [Google Scholar]
- Janeway C. A. Natural killer cells: a primitive immune system. Nature. 1989 Sep 14;341(6238):108–108. doi: 10.1038/341108a0. [DOI] [PubMed] [Google Scholar]
- Kanof M. E., Strober W. Lymphokine-activated killer-cell cytotoxicity in the intestinal immune system. Gastroenterology. 1989 Jul;97(1):222–224. doi: 10.1016/0016-5085(89)91441-8. [DOI] [PubMed] [Google Scholar]
- Kemler B. J., Alpert E. Inflammatory bowel disease: study of cell mediated cytotoxicity for isolated human colonic epithelial cells. Gut. 1980 May;21(5):353–359. doi: 10.1136/gut.21.5.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsner J. B., Shorter R. G. Recent developments in nonspecific inflammatory bowel disease (second of two parts). N Engl J Med. 1982 Apr 8;306(14):837–848. doi: 10.1056/NEJM198204083061404. [DOI] [PubMed] [Google Scholar]
- MacDermott R. P., Bragdon M. J., Kodner I. J., Bertovich M. J. Deficient cell-mediated cytotoxicity and hyporesponsiveness to interferon and mitogenic lectin activation by inflammatory bowel disease peripheral blood and intestinal mononuclear cells. Gastroenterology. 1986 Jan;90(1):6–11. doi: 10.1016/0016-5085(86)90067-3. [DOI] [PubMed] [Google Scholar]
- MacDermott R. P., Stenson W. F. Alterations of the immune system in ulcerative colitis and Crohn's disease. Adv Immunol. 1988;42:285–328. doi: 10.1016/s0065-2776(08)60848-2. [DOI] [PubMed] [Google Scholar]
- Merino F., Alvarez-Mon M., de la Hera A., Alés J. E., Bonilla F., Durantez A. Regulation of natural killer cytotoxicity by 1,25-dihydroxyvitamin D3. Cell Immunol. 1989 Feb;118(2):328–336. doi: 10.1016/0008-8749(89)90381-x. [DOI] [PubMed] [Google Scholar]
- Ortaldo J. R., Longo D. L. Human natural lymphocyte effector cells: definition, analysis of activity, and clinical effectiveness. J Natl Cancer Inst. 1988 Sep 7;80(13):999–1010. doi: 10.1093/jnci/80.13.999. [DOI] [PubMed] [Google Scholar]
- Phillips J. H., Lanier L. L. Dissection of the lymphokine-activated killer phenomenon. Relative contribution of peripheral blood natural killer cells and T lymphocytes to cytolysis. J Exp Med. 1986 Sep 1;164(3):814–825. doi: 10.1084/jem.164.3.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinnan G. V., Jr, Kirmani N., Rook A. H., Manischewitz J. F., Jackson L., Moreschi G., Santos G. W., Saral R., Burns W. H. Cytotoxic t cells in cytomegalovirus infection: HLA-restricted T-lymphocyte and non-T-lymphocyte cytotoxic responses correlate with recovery from cytomegalovirus infection in bone-marrow-transplant recipients. N Engl J Med. 1982 Jul 1;307(1):7–13. doi: 10.1056/NEJM198207013070102. [DOI] [PubMed] [Google Scholar]
- Ritz J. The role of natural killer cells in immune surveillance. N Engl J Med. 1989 Jun 29;320(26):1748–1749. doi: 10.1056/NEJM198906293202608. [DOI] [PubMed] [Google Scholar]
- Sancho L., Martinez C., Nogales A., de la Hera A. Reconstitution of natural-killer-cell activity in the newborn by interleukin-2. N Engl J Med. 1986 Jan 2;314(1):57–58. doi: 10.1056/NEJM198601023140115. [DOI] [PubMed] [Google Scholar]
- Shorter R. G., McGill D. B., Bahn R. C. Cytotoxicity of mononuclear cells for autologous colonic epithelial cells in colonic diseases. Gastroenterology. 1984 Jan;86(1):13–22. [PubMed] [Google Scholar]
- Solovera J. J., Alvarez-Mon M., Casas J., Carballido J., Durantez A. Inhibition of human natural killer (NK) activity by calcium channel modulators and a calmodulin antagonist. J Immunol. 1987 Aug 1;139(3):876–880. [PubMed] [Google Scholar]
- TRUELOVE S. C., WITTS L. J. Cortisone in ulcerative colitis; final report on a therapeutic trial. Br Med J. 1955 Oct 29;2(4947):1041–1048. doi: 10.1136/bmj.2.4947.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor-Robinson S., Miles R., Whitehead A., Dickinson R. J. Salmonella infection and ulcerative colitis. Lancet. 1989 May 20;1(8647):1145–1145. doi: 10.1016/s0140-6736(89)92428-8. [DOI] [PubMed] [Google Scholar]
- Trinchieri G., Matsumoto-Kobayashi M., Clark S. C., Seehra J., London L., Perussia B. Response of resting human peripheral blood natural killer cells to interleukin 2. J Exp Med. 1984 Oct 1;160(4):1147–1169. doi: 10.1084/jem.160.4.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]


