Abstract
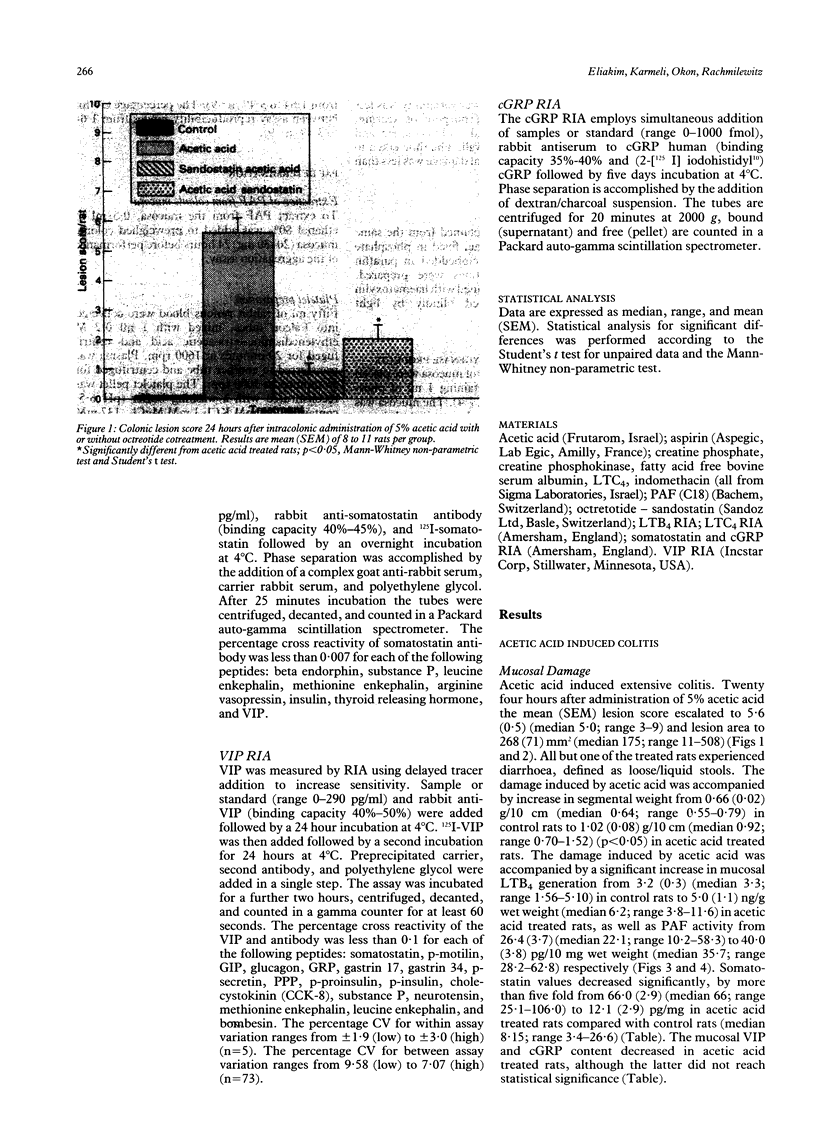
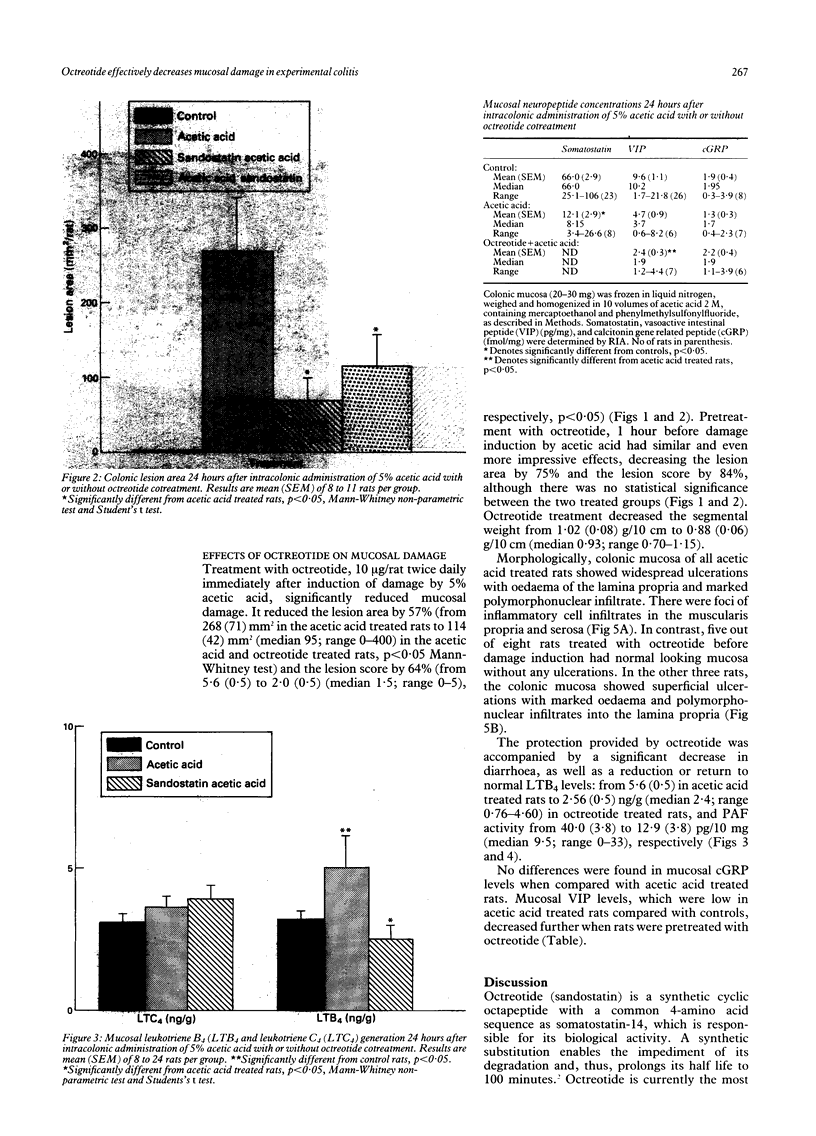
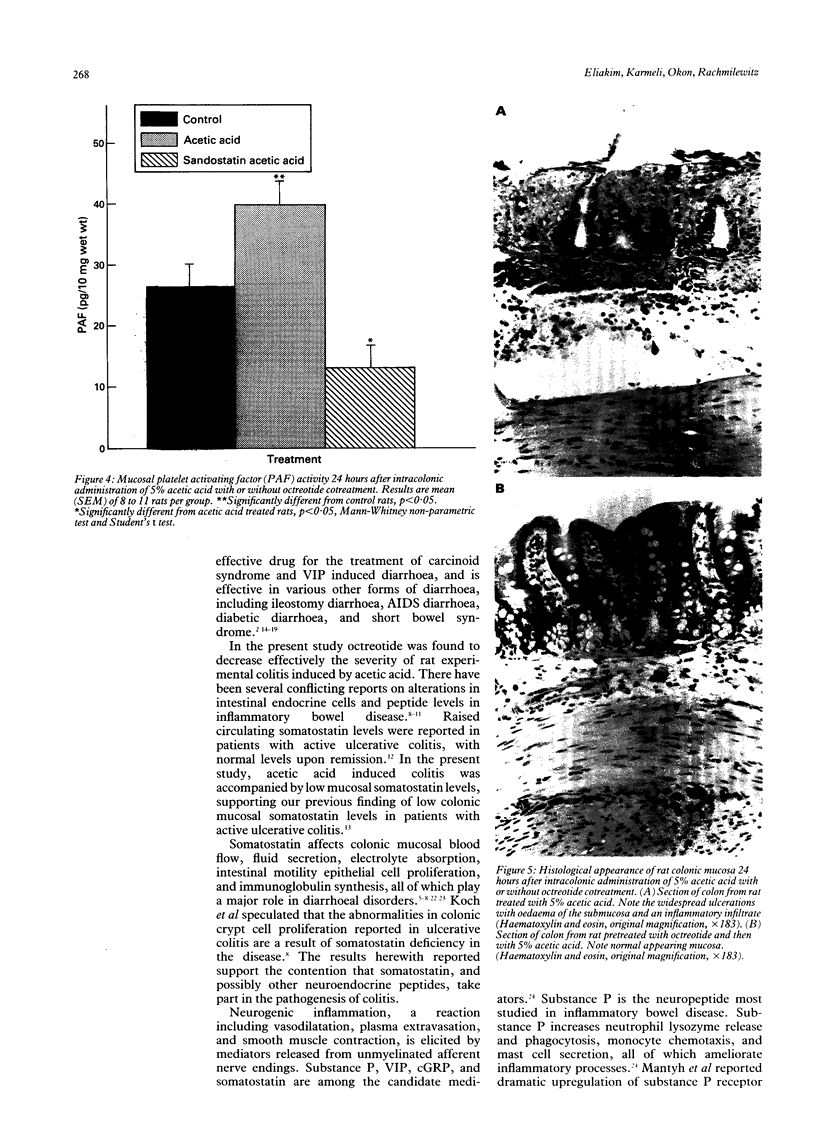
The effect of octreotide, a synthetic analogue of somatostatin, on the modulation of the acetic acid model of experimental colitis was examined. Colitis was induced by intracolonic administration of 2 ml of 5% acetic acid. The inflammatory response elicited by the acetic acid resulted in increased colonic synthesis of platelet activating factor, leukotriene B4 and decreased mucosal somatostatin levels. Subcutaneous administration of octreotide (10 micrograms/rat) 1 hour before or immediately after damage induction, as well as 1 and 23 hours after acetic acid application, resulted in a significant reduction in mucosal damage. The protective effect was accompanied by a significant reduction in platelet activating factor activity, leukotriene B4, and vasoactive intestinal peptide concentrations. There were no significant changes in mucosal leukotriene C4 and calcitonin gene related peptide levels. This study shows that acetic acid induced colitis is pharmacologically manipulated by octreotide. The mechanism of action of octreotide has not yet been fully determined. The potential use of octreotide in treating active inflammatory bowel disease remains to be evaluated.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agerskov K., Bousfield R., Mortensen P. E., Olsen J., Christiansen J. Effect of somatostatin on 133Xe clearance from colonic mucosa before and after local nervous blockade in unanaesthetized man. Scand J Gastroenterol. 1986 Oct;21(8):951–954. doi: 10.3109/00365528608996401. [DOI] [PubMed] [Google Scholar]
- Ahonen A., Kyösola K., Penttilä O. Enterochromaffin cells in macrophages in ulcerative colitis and irritable colon. Ann Clin Res. 1976 Feb;8(1):1–7. [PubMed] [Google Scholar]
- Binimelis J., Webb S. M., Monés J., Serrano J., Casamitjana R., Elena M., Peinado M. A., Vilardell F., De Leiva A. Circulating immunoreactive somatostatin in gastrointestinal diseases. Decrease after vagotomy and enhancement in active ulcerative colitis, irritable bowel syndrome, and duodenal ulcer. Scand J Gastroenterol. 1987 Oct;22(8):931–937. doi: 10.3109/00365528708991938. [DOI] [PubMed] [Google Scholar]
- Bishop A. E., Polak J. M., Bryant M. G., Bloom S. R., Hamilton S. Abnormalities of vasoactive intestinal polypeptide-containing nerves in Crohn's disease. Gastroenterology. 1980 Nov;79(5 Pt 1):853–860. [PubMed] [Google Scholar]
- Cello J. P., Grendell J. H., Basuk P., Simon D., Weiss L., Wittner M., Rood R. P., Wilcox C. M., Forsmark C. E., Read A. E. Effect of octreotide on refractory AIDS-associated diarrhea. A prospective, multicenter clinical trial. Ann Intern Med. 1991 Nov 1;115(9):705–710. doi: 10.7326/0003-4819-115-9-705. [DOI] [PubMed] [Google Scholar]
- Cooper J. C., Williams N. S., King R. F., Barker M. C. Effects of a long-acting somatostatin analogue in patients with severe ileostomy diarrhoea. Br J Surg. 1986 Feb;73(2):128–131. doi: 10.1002/bjs.1800730219. [DOI] [PubMed] [Google Scholar]
- Dharmsathaphorn K., Binder H. J., Dobbins J. W. Somatostatin stimulates sodium and chloride absorption in the rabbit ileum. Gastroenterology. 1980 Jun;78(6):1559–1565. [PubMed] [Google Scholar]
- Dharmsathaphorn K., Gorelick F. S., Sherwin R. S., Cataland S., Dobbins J. W. Somatostatin decreases diarrhea in patients with the short-bowel syndrome. J Clin Gastroenterol. 1982 Dec;4(6):521–524. doi: 10.1097/00004836-198212000-00008. [DOI] [PubMed] [Google Scholar]
- Dharmsathaphorn K., Sherwin R. S., Dobbins J. W. Somatostatin inhibits fluid secretion in the rat jejunum. Gastroenterology. 1980 Jun;78(6):1554–1558. [PubMed] [Google Scholar]
- Duffy L. C., Zielezny M. A., Riepenhoff-Talty M., Byers T. E., Marshall J., Weiser M. M., Graham S., Ogra P. L. Vasoactive intestinal peptide as a laboratory supplement to clinical activity index in inflammatory bowel disease. Dig Dis Sci. 1989 Oct;34(10):1528–1535. doi: 10.1007/BF01537105. [DOI] [PubMed] [Google Scholar]
- Ferrar J. A., Cuthbert A. W., Cox H. M. The antisecretory effects of somatostatin and analogues in rat descending colon mucosa. Eur J Pharmacol. 1990 Aug 10;184(2-3):295–303. doi: 10.1016/0014-2999(90)90621-c. [DOI] [PubMed] [Google Scholar]
- Geerdsen J. P., Pedersen V. M., Kjaergård H. K. Small bowel fistulas treated with somatostatin: preliminary results. Surgery. 1986 Nov;100(5):811–814. [PubMed] [Google Scholar]
- Goldin E., Karmeli F., Selinger Z., Rachmilewitz D. Colonic substance P levels are increased in ulcerative colitis and decreased in chronic severe constipation. Dig Dis Sci. 1989 May;34(5):754–757. doi: 10.1007/BF01540348. [DOI] [PubMed] [Google Scholar]
- Grosman I., Simon D. Potential gastrointestinal uses of somatostain and its synthetic analogue octreotide. Am J Gastroenterol. 1990 Sep;85(9):1061–1072. [PubMed] [Google Scholar]
- Koch T. R., Carney J. A., Morris V. A., Go V. L. Somatostatin in the idiopathic inflammatory bowel diseases. Dis Colon Rectum. 1988 Mar;31(3):198–203. doi: 10.1007/BF02552546. [DOI] [PubMed] [Google Scholar]
- Kyösola K., Penttilä O., Salaspuro M. Rectal mucosal adrenergic innervation and enterochromaffin cells in ulcerative colitis and irritable colon. Scand J Gastroenterol. 1977;12(3):363–367. doi: 10.3109/00365527709180942. [DOI] [PubMed] [Google Scholar]
- Lehy T., Dubrasquet M., Bonfils S. Effect of somatostatin on normal and gastric-stimulated cell proliferation in the gastric and intestinal mucosae of the rat. Digestion. 1979;19(2):99–109. doi: 10.1159/000198330. [DOI] [PubMed] [Google Scholar]
- Lucey M. R., Yamada T. Biochemistry and physiology of gastrointestinal somatostatin. Dig Dis Sci. 1989 Mar;34(3 Suppl):5S–13S. doi: 10.1007/BF01536041. [DOI] [PubMed] [Google Scholar]
- Mantyh C. R., Gates T. S., Zimmerman R. P., Welton M. L., Passaro E. P., Jr, Vigna S. R., Maggio J. E., Kruger L., Mantyh P. W. Receptor binding sites for substance P, but not substance K or neuromedin K, are expressed in high concentrations by arterioles, venules, and lymph nodules in surgical specimens obtained from patients with ulcerative colitis and Crohn disease. Proc Natl Acad Sci U S A. 1988 May;85(9):3235–3239. doi: 10.1073/pnas.85.9.3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason R. T., Shulkes A., Zajac J. D., Fletcher A. E., Hardy K. J., Martin T. J. Basal and stimulated release of calcitonin gene-related peptide (CGRP) in patients with medullary thyroid carcinoma. Clin Endocrinol (Oxf) 1986 Dec;25(6):675–685. doi: 10.1111/j.1365-2265.1986.tb03623.x. [DOI] [PubMed] [Google Scholar]
- Maton P. N., O'Dorisio T. M., Howe B. A., McArthur K. E., Howard J. M., Cherner J. A., Malarkey T. B., Collen M. J., Gardner J. D., Jensen R. T. Effect of a long-acting somatostatin analogue (SMS 201-995) in a patient with pancreatic cholera. N Engl J Med. 1985 Jan 3;312(1):17–21. doi: 10.1056/NEJM198501033120104. [DOI] [PubMed] [Google Scholar]
- McCulloch C. R., Cooke H. J. Human alpha-calcitonin gene-related peptide influences colonic secretion by acting on myenteric neurons. Regul Pept. 1989 Jan;24(1):87–96. doi: 10.1016/0167-0115(89)90214-0. [DOI] [PubMed] [Google Scholar]
- Reasbeck P. G., Burns S. M., Shulkes A. Calcitonin gene-related peptide: enteric and cardiovascular effects in the dog. Gastroenterology. 1988 Oct;95(4):966–971. doi: 10.1016/0016-5085(88)90170-9. [DOI] [PubMed] [Google Scholar]
- Sharon P., Stenson W. F. Metabolism of arachidonic acid in acetic acid colitis in rats. Similarity to human inflammatory bowel disease. Gastroenterology. 1985 Jan;88(1 Pt 1):55–63. doi: 10.1016/s0016-5085(85)80132-3. [DOI] [PubMed] [Google Scholar]
- Stanisz A. M., Befus D., Bienenstock J. Differential effects of vasoactive intestinal peptide, substance P, and somatostatin on immunoglobulin synthesis and proliferations by lymphocytes from Peyer's patches, mesenteric lymph nodes, and spleen. J Immunol. 1986 Jan;136(1):152–156. [PubMed] [Google Scholar]
- Tien X. Y., Wallace L. J., Kachur J. F., Won-Kim S., Gaginella T. S. Neurokinin A increases short-circuit current across rat colonic mucosa: a role for vasoactive intestinal polypeptide. J Physiol. 1991 Jun;437:341–350. doi: 10.1113/jphysiol.1991.sp018599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinik A., Moattari A. R. Use of somatostatin analog in management of carcinoid syndrome. Dig Dis Sci. 1989 Mar;34(3 Suppl):14S–27S. doi: 10.1007/BF01536042. [DOI] [PubMed] [Google Scholar]
- Wallace J. L., Braquet P., Ibbotson G. C., MacNaughton W. K., Cirino G. Assessment of the role of platelet-activating factor in an animal model of inflammatory bowel disease. J Lipid Mediat. 1989 Jan-Feb;1(1):13–23. [PubMed] [Google Scholar]