Abstract
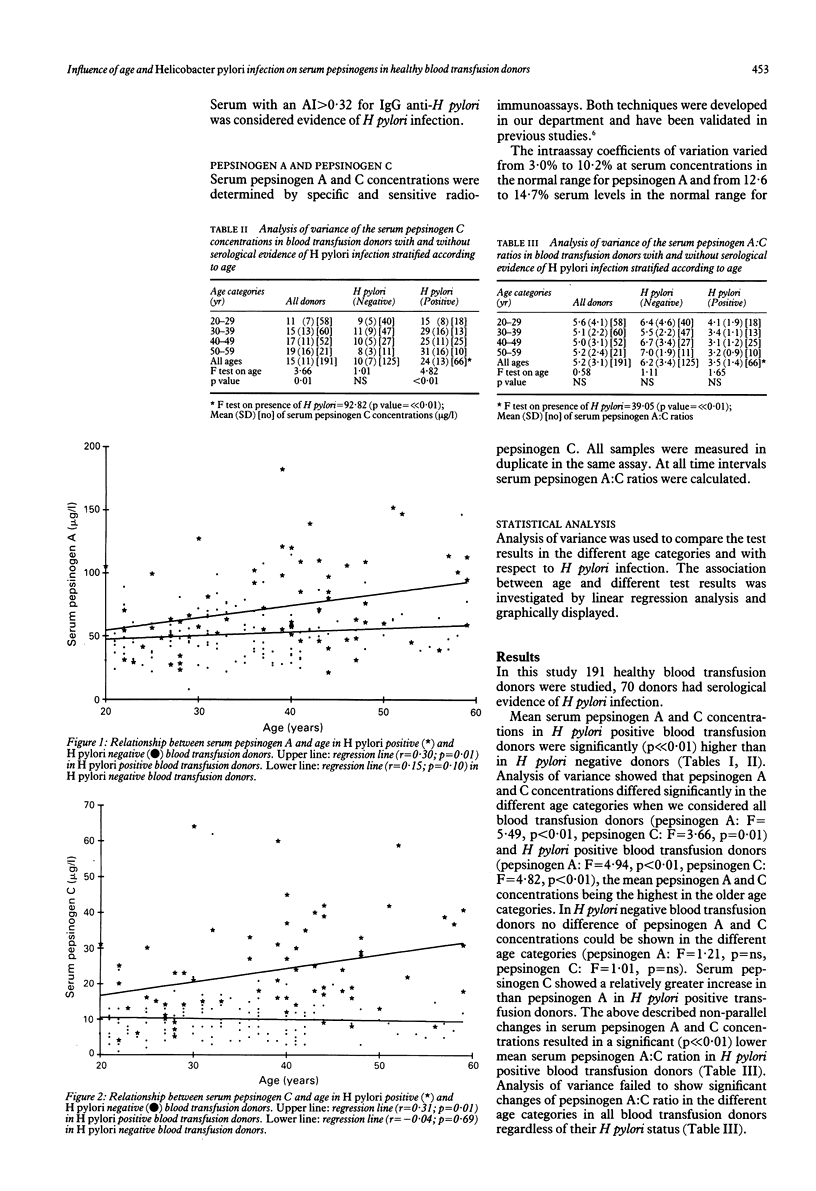
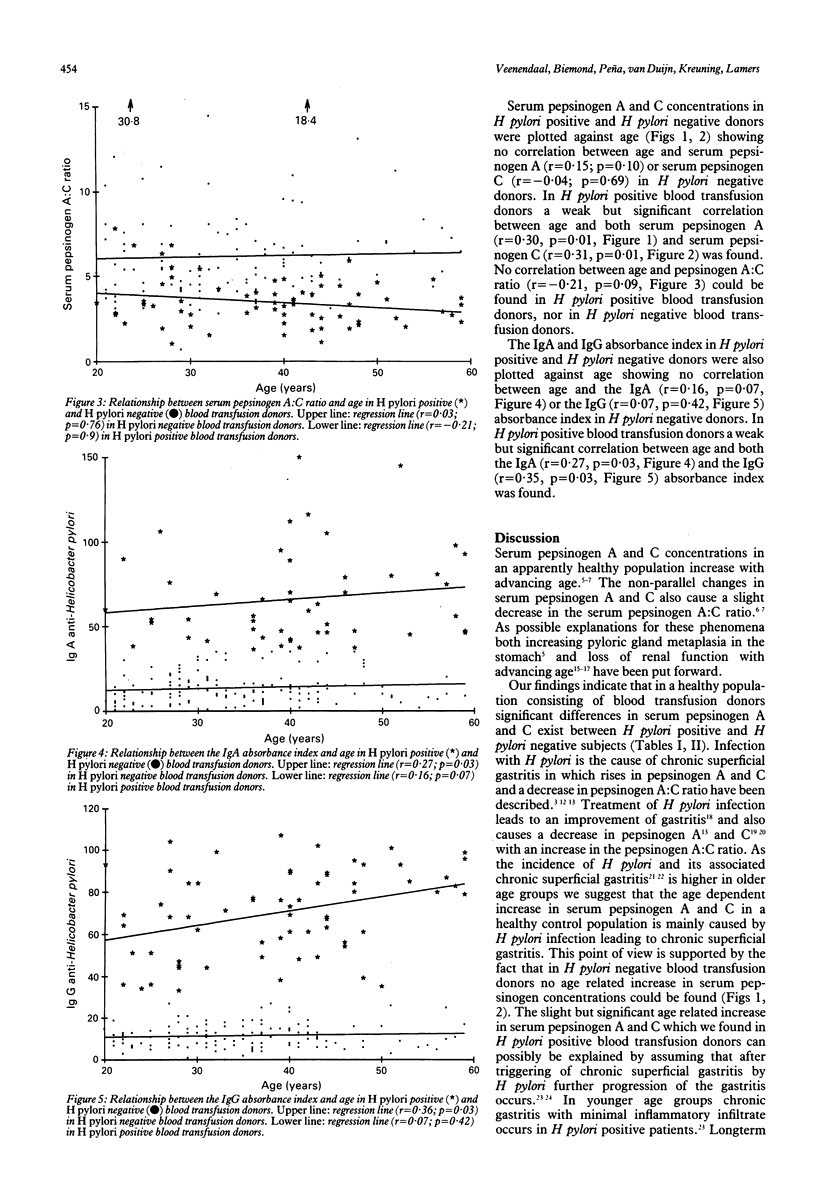
In a healthy population pepsinogen A and pepsinogen C increase with advancing age. As pepsinogen A and C are raised in chronic superficial gastritis which is caused by H pylori infection, we investigated whether H pylori is responsible for the age related increase of pepsinogen A and C. In H pylori positive blood transfusion donors serum pepsinogen A (mean (SD) 73 (35) micrograms/ml v 52 (19) micrograms/ml, p much less than 0.01) and C (mean (SD) 24 (13) micrograms/ml v 10 (7) micrograms/ml, p much less than 0.01) concentrations were significantly higher than in H pylori negative blood transfusion donors, while the serum pepsinogen A:C ratio mean (SD) 3.5 (1.4) v 6.2 (3.4), p much less than 0.01) was significantly decreased because of a relative greater increase in serum pepsinogen C in H pylori positive blood transfusion donors. Analysis of variance showed that pepsinogen A and C concentrations differed significantly in the different age groups (p much less than 0.01) when we considered all blood transfusion donors and H pylori positive blood transfusion donors, the mean pepsinogen levels being highest in the older age categories. In H pylori negative blood transfusion donors no such age related difference in pepsinogen A and C could be shown. In H pylori positive blood transfusion donors a weak positive but significant correlation between pepsinogen A and C and age could be shown (r = 0.30; p = 0.01 and r = 0.31; p = 0.01 respectively). In H pylori negative blood transfusion donors no correlation between serum pepsinogens and age was found. We conclude that the age related increase in serum pepsinogen A and C described in healthy control populations is caused by an increasing prevalence of H pylori infection. Serum pepsinogen A and C concentrations in patients should therefore be related to the presence or absence of H pylori infection.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Biemond I., Jansen J. B., Crobach L. F., Kreuning J., Lamers C. B. Radioimmunoassay of human pepsinogen A and pepsinogen C. J Clin Chem Clin Biochem. 1989 Jan;27(1):19–25. doi: 10.1515/cclm.1989.27.1.19. [DOI] [PubMed] [Google Scholar]
- Blaser M. J. Helicobacter pylori and the pathogenesis of gastroduodenal inflammation. J Infect Dis. 1990 Apr;161(4):626–633. doi: 10.1093/infdis/161.4.626. [DOI] [PubMed] [Google Scholar]
- Borch K., Axelsson C. K., Halgreen H., Damkjaer Nielsen M. D., Ledin T., Szesci P. B. The ratio of pepsinogen A to pepsinogen C: a sensitive test for atrophic gastritis. Scand J Gastroenterol. 1989 Sep;24(7):870–876. doi: 10.3109/00365528909089228. [DOI] [PubMed] [Google Scholar]
- Dooley C. P., Cohen H., Fitzgibbons P. L., Bauer M., Appleman M. D., Perez-Perez G. I., Blaser M. J. Prevalence of Helicobacter pylori infection and histologic gastritis in asymptomatic persons. N Engl J Med. 1989 Dec 7;321(23):1562–1566. doi: 10.1056/NEJM198912073212302. [DOI] [PubMed] [Google Scholar]
- Greenberg R. E., Bank S. The prevalence of Helicobacter pylori in nonulcer dyspepsia. Importance of stratification according to age. Arch Intern Med. 1990 Oct;150(10):2053–2055. [PubMed] [Google Scholar]
- Ichinose M., Miki K., Furihata C., Kageyama T., Hayashi R., Niwa H., Oka H., Matsushima T., Takahashi K. Radioimmunoassay of serum group I and group II pepsinogens in normal controls and patients with various disorders. Clin Chim Acta. 1982 Dec 9;126(2):183–191. doi: 10.1016/0009-8981(82)90034-1. [DOI] [PubMed] [Google Scholar]
- Jones D. M., Eldridge J., Fox A. J., Sethi P., Whorwell P. J. Antibody to the gastric campylobacter-like organism ("Campylobacter pyloridis")--clinical correlations and distribution in the normal population. J Med Microbiol. 1986 Aug;22(1):57–62. doi: 10.1099/00222615-22-1-57. [DOI] [PubMed] [Google Scholar]
- Marshall B. J., Warren J. R. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet. 1984 Jun 16;1(8390):1311–1315. doi: 10.1016/s0140-6736(84)91816-6. [DOI] [PubMed] [Google Scholar]
- Mitchell H. M., Bohane T. D., Berkowicz J., Hazell S. L., Lee A. Antibody to Campylobacter pylori in families of index children with gastrointestinal illness due to C pylori. Lancet. 1987 Sep 19;2(8560):681–682. doi: 10.1016/s0140-6736(87)92459-7. [DOI] [PubMed] [Google Scholar]
- Niedobitek F., Grosse G., Hammer M., Bonk G., Nehls R., Volkheimer G. Gastritis and bacterial colonization of the gastric mucosa in adolescents. Am J Gastroenterol. 1989 Mar;84(3):239–244. [PubMed] [Google Scholar]
- Oderda G., Vaira D., Holton J., Ainley C., Altare F., Ansaldi N. Amoxycillin plus tinidazole for Campylobacter pylori gastritis in children: assessment by serum IgG antibody, pepsinogen I, and gastrin levels. Lancet. 1989 Apr 1;1(8640):690–692. doi: 10.1016/s0140-6736(89)92206-x. [DOI] [PubMed] [Google Scholar]
- Oderda G., Vaira D., Holton J., Ainley C., Altare F., Boero M., Smith A., Ansaldi N. Helicobacter pylori in children with peptic ulcer and their families. Dig Dis Sci. 1991 May;36(5):572–576. doi: 10.1007/BF01297021. [DOI] [PubMed] [Google Scholar]
- Perez-Perez G. I., Dworkin B. M., Chodos J. E., Blaser M. J. Campylobacter pylori antibodies in humans. Ann Intern Med. 1988 Jul 1;109(1):11–17. doi: 10.7326/0003-4819-109-1-11. [DOI] [PubMed] [Google Scholar]
- Peña A. S., Endtz H. P., Offerhaus G. J., Hoogenboom-Verdegaal A., van Duijn W., de Vargas N., den Hartog G., Kreuning J., van der Reyden J., Mouton R. P. Value of serology (ELISA and immunoblotting) for the diagnosis of Campylobacter pylori infection. Digestion. 1989;44(3):131–141. doi: 10.1159/000199902. [DOI] [PubMed] [Google Scholar]
- Rauws E. A., Langenberg W., Houthoff H. J., Zanen H. C., Tytgat G. N. Campylobacter pyloridis-associated chronic active antral gastritis. A prospective study of its prevalence and the effects of antibacterial and antiulcer treatment. Gastroenterology. 1988 Jan;94(1):33–40. [PubMed] [Google Scholar]
- Rotter J. I., Petersen G., Samloff I. M., McConnell R. B., Ellis A., Spence M. A., Rimoin D. L. Genetic heterogeneity of hyperpepsinogenemic I and normopepsinogenemic I duodenal ulcer disease. Ann Intern Med. 1979 Sep;91(3):372–377. doi: 10.7326/0003-4819-91-3-372. [DOI] [PubMed] [Google Scholar]
- Rotter J. I., Sones J. Q., Samloff I. M., Richardson C. T., Gursky J. M., Walsh J. H., Rimoin D. L. Duodenal-ulcer disease associated with elevated serum pepsinogen I: an inherited autosomal dominant disorder. N Engl J Med. 1979 Jan 11;300(2):63–66. doi: 10.1056/NEJM197901113000203. [DOI] [PubMed] [Google Scholar]
- Samloff I. M. Cellular localization of group I pepsinogens in human gastric mucosa by immunofluorescence. Gastroenterology. 1971 Aug;61(2):185–188. [PubMed] [Google Scholar]
- Samloff I. M., Liebman W. M. Cellular localization of the group II pepsinogens in human stomach and duodenum by immunofluorescence. Gastroenterology. 1973 Jul;65(1):36–42. [PubMed] [Google Scholar]
- Samloff I. M., Liebman W. M., Panitch N. M. Serum group I pepsinogens by radioimmunoassay in control subjects and patients with peptic ulcer. Gastroenterology. 1975 Jul;69(1):83–90. [PubMed] [Google Scholar]
- Samloff I. M. Peptic ulcer: the many proteinases of aggression. Gastroenterology. 1989 Feb;96(2 Pt 2 Suppl):586–595. doi: 10.1016/s0016-5085(89)80054-x. [DOI] [PubMed] [Google Scholar]
- Samloff I. M., Varis K., Ihamaki T., Siurala M., Rotter J. I. Relationships among serum pepsinogen I, serum pepsinogen II, and gastric mucosal histology. A study in relatives of patients with pernicious anemia. Gastroenterology. 1982 Jul;83(1 Pt 2):204–209. [PubMed] [Google Scholar]
- Sipponen P., Kekki M., Siurala M. The Sydney System: epidemiology and natural history of chronic gastritis. J Gastroenterol Hepatol. 1991 May-Jun;6(3):244–251. doi: 10.1111/j.1440-1746.1991.tb01472.x. [DOI] [PubMed] [Google Scholar]
- Villako K., Maards H., Tammur R., Keevallik R., Peetsalu M., Sipponen P., Kekki M., Siurala M. Helicobacter (Campylobacter) pylori infestation and the development and progression of chronic gastritis: results of long-term follow-up examinations of a random sample. Endoscopy. 1990 May;22(3):114–117. doi: 10.1055/s-2007-1012814. [DOI] [PubMed] [Google Scholar]
- Waldum H. L., Jorde R., Gunnes P. Renal excretion of and the effect of posture on serum group I pepsinogens. Scand J Gastroenterol. 1982 Mar;17(2):253–255. doi: 10.3109/00365528209182048. [DOI] [PubMed] [Google Scholar]
- Westerveld B. D., Pals G., Lamers C. B., Defize J., Pronk J. C., Frants R. R., Ooms E. C., Kreuning J., Kostense P. J., Eriksson A. W. Clinical significance of pepsinogen A isozymogens, serum pepsinogen A and C levels, and serum gastrin levels. Cancer. 1987 Mar 1;59(5):952–958. doi: 10.1002/1097-0142(19870301)59:5<952::aid-cncr2820590517>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]


