Abstract
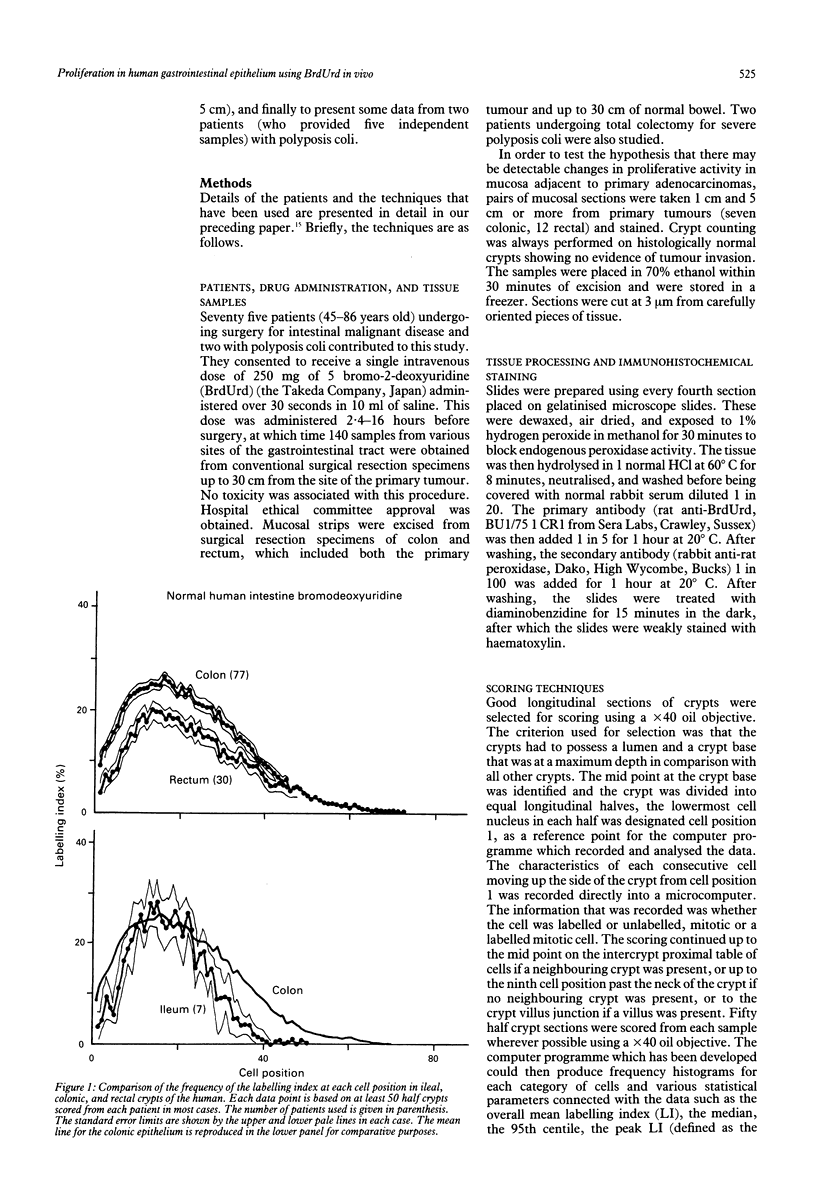
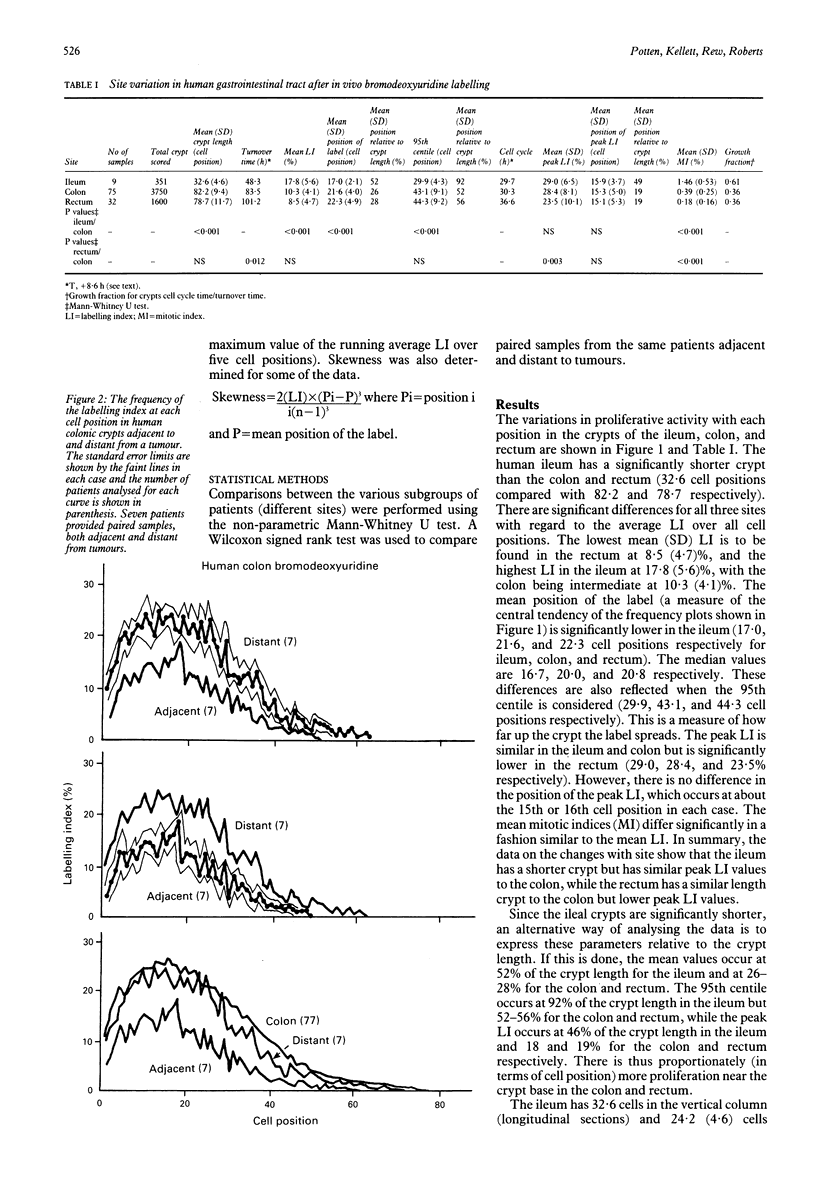
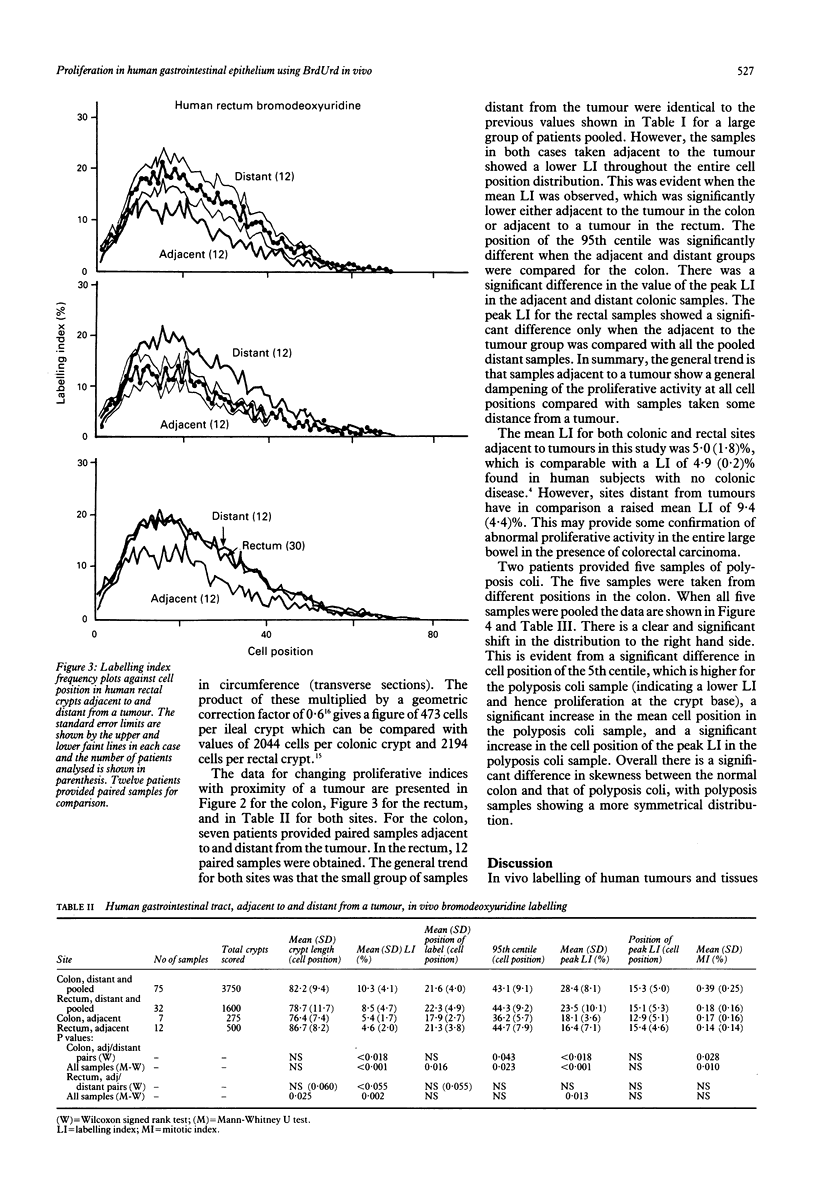
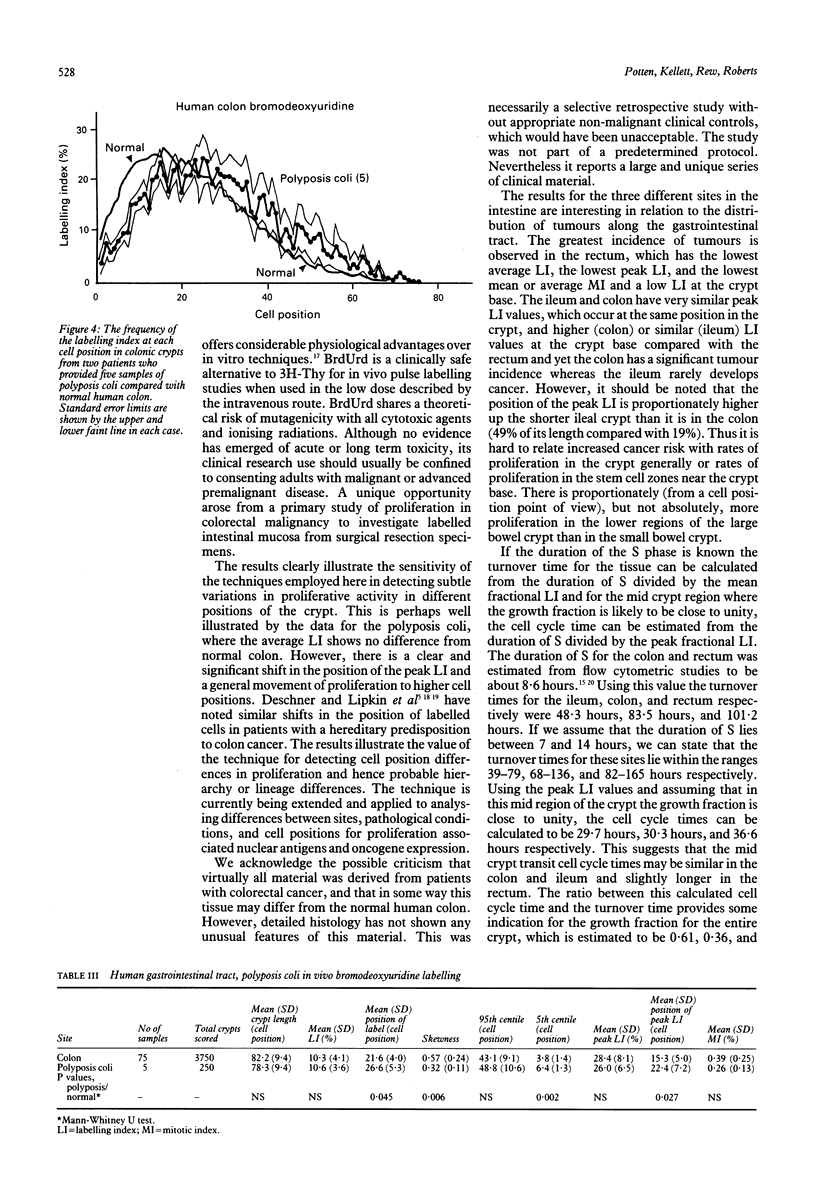
The distribution of DNA synthesising cells in the crypts of the epithelium in human small and large bowel after injection of bromodeoxyuridine into patients has been studied in relation to the position of the cells in the crypt using immunohistochemistry. Different sites of normal epithelium have been studied. The ileum has a shorter crypt and a very significantly smaller total cell population size. However, it has similar peak labelling index (LI) values to the colon, while the rectum has a lower peak LI value. The mean position of the label occurs at the 17th cell position in the ileum and at about the 22nd position in both the colon and rectum. The overall mean LI is significantly higher in the ileum at 17.8%, intermediate in the colon at 10.3%, and lowest in the rectum at 8.5%. There is thus an inverse relation between the likelihood of developing a tumour and the rate of cell proliferation as measured by the LI. Assuming a value of 8.6 hours for the duration of S, the data suggest that the cell cycle time in the mid crypt region is about 30 hours for the ileum and colon and about 37 hours for the rectum. Samples taken adjacent (within 1 cm) to a tumour show a general dampening of proliferative activity at all cell positions compared with samples taken more than 5 cm from a tumour. This is illustrated by the average LI, which is about 5.4% in the colon adjacent to a tumour compared with 10% distant; comparable values for the rectum are 4.6% and 8.5%. Samples taken from two patients with polyposis coli show distributions with a significant difference in skewness compared with normal colon and a general shifting of the distribution to the right, that is to higher cell positions. There is a significant increase in the mean cell position and the position of the peak LI in the polyposis coli samples.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bleiberg H., Galand P. In vitro autoradiographic determination of cell kinetic parameters in adenocarcinomas and adjacent healthy mucosa of the human colon and rectum. Cancer Res. 1976 Feb;36(2 Pt 1):325–328. [PubMed] [Google Scholar]
- Bleiberg H., Mainguet P., Galand P. Cell renewal in familial polyposis: comparison between polyps and adjacent healthy mucosa. Gastroenterology. 1972 Aug;63(2):240–245. [PubMed] [Google Scholar]
- Bleiberg H., Mainguet P., Galand P., Chretien J., Dupont-Mairesse N. Cell renewal in the human rectum. In vitro autoradiographic study on active ulcerative colitis. Gastroenterology. 1970 Jun;58(6):851–855. [PubMed] [Google Scholar]
- Bleiberg H., Salhadin A., Galand P. Cell cycle parameters in human colon: comparison between primary and recurrent adenocarcinomas, benign polyps and adjacent unaffected mucosa. Cancer. 1977 Mar;39(3):1190–1194. doi: 10.1002/1097-0142(197703)39:3<1190::aid-cncr2820390326>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Bussey H. J., Wallace M. H., Morson B. C. Metachronous carcinoma of the large intestine and intestinal polyps. Proc R Soc Med. 1967 Mar;60(3):208–210. doi: 10.1177/003591576706000302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DESCHNER E., LEWIS C. M., LIPKIN M. IN VITRO STUDY OF HUMAN RECTAL EPITHELIAL CELLS. I. ATYPICAL ZONE OF H3 THYMIDINE INCORPORATION IN MUCOSA OF MULTIPLE POLYPOSIS. J Clin Invest. 1963 Dec;42:1922–1928. doi: 10.1172/JCI104878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deschner E. E., Lipkin M. Proliferative patterns in colonic mucosa in familial polyposis. Cancer. 1975 Feb;35(2):413–418. doi: 10.1002/1097-0142(197502)35:2<413::aid-cncr2820350217>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Deschner E. E., Maskens A. P. Significance of the labeling index and labeling distribution as kinetic parameters in colorectal mucosa of cancer patients and DMH treated animals. Cancer. 1982 Sep 15;50(6):1136–1141. doi: 10.1002/1097-0142(19820915)50:6<1136::aid-cncr2820500617>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Lightdale C., Lipkin M., Deschner E. In vivo measurements in familial polyposis: kinetics and location of proliferating cells in colonic adenomas. Cancer Res. 1982 Oct;42(10):4280–4283. [PubMed] [Google Scholar]
- Lipkin M., Blattner W. E., Fraumeni J. F., Jr, Lynch H. T., Deschner E., Winawer S. Tritiated thymidine (phi p, phi h) labeling distribution as a marker for hereditary predisposition to colon cancer. Cancer Res. 1983 Apr;43(4):1899–1904. [PubMed] [Google Scholar]
- Potten C. S., Kellett M., Roberts S. A., Rew D. A., Wilson G. D. Measurement of in vivo proliferation in human colorectal mucosa using bromodeoxyuridine. Gut. 1992 Jan;33(1):71–78. doi: 10.1136/gut.33.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potten C. S., Roberts S. A., Chwalinski S., Loeffler M., Paulus U. Scoring mitotic activity in longitudinal sections of crypts of the small intestine. Cell Tissue Kinet. 1988 Jul;21(4):231–246. doi: 10.1111/j.1365-2184.1988.tb00783.x. [DOI] [PubMed] [Google Scholar]
- Rew D. A., Wilson G. D., Taylor I., Weaver P. C. Proliferation characteristics of human colorectal carcinomas measured in vivo. Br J Surg. 1991 Jan;78(1):60–66. doi: 10.1002/bjs.1800780120. [DOI] [PubMed] [Google Scholar]
- Romagnoli P., Filipponi F., Bandettini L., Brugnola D. Increase of mitotic activity in the colonic mucosa of patients with colorectal cancer. Dis Colon Rectum. 1984 May;27(5):305–308. doi: 10.1007/BF02555636. [DOI] [PubMed] [Google Scholar]
- Schottenfeld D., Berg J. W., Vitsky B. Incidence of multiple primary cancers. II. Index cancers arising in the stomach and lower digestive system. J Natl Cancer Inst. 1969 Jul;43(1):77–86. [PubMed] [Google Scholar]
- Serafini E. P., Kirk A. P., Chambers T. J. Rate and pattern of epithelial cell proliferation in ulcerative colitis. Gut. 1981 Aug;22(8):648–652. doi: 10.1136/gut.22.8.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunter J. P., Watson A. J., Wright N. A., Appleton D. R. Cell proliferation at different sites along the length of the rat colon. Virchows Arch B Cell Pathol Incl Mol Pathol. 1979 Dec;32(1):75–87. doi: 10.1007/BF02889015. [DOI] [PubMed] [Google Scholar]
- Sunter J. P., Wright N. A., Appleton D. R. Cell population kinetics in the epithelium of the colon of the male rat. Virchows Arch B Cell Pathol. 1978 Jan 18;26(3):275–287. doi: 10.1007/BF02889556. [DOI] [PubMed] [Google Scholar]
- Terpstra O. T., van Blankenstein M., Dees J., Eilers G. A. Abnormal pattern of cell proliferation in the entire colonic mucosa of patients with colon adenoma or cancer. Gastroenterology. 1987 Mar;92(3):704–708. doi: 10.1016/0016-5085(87)90021-7. [DOI] [PubMed] [Google Scholar]
- Tudor E. M., Hopper J. L., Hannah M. C. An autoradiographic study of the developing parietal cell population in neonatal pigs. Cell Tissue Kinet. 1988 Jan;21(1):3–14. doi: 10.1111/j.1365-2184.1988.tb00766.x. [DOI] [PubMed] [Google Scholar]



