Abstract
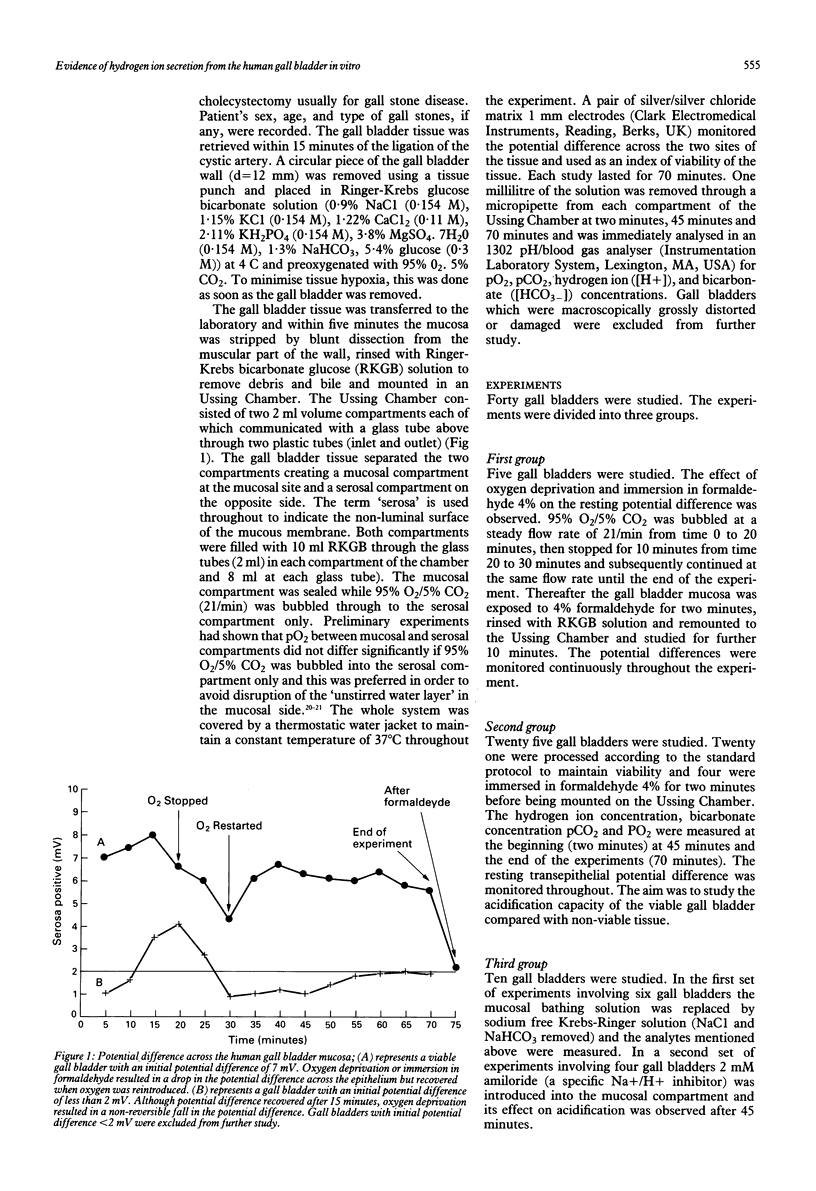
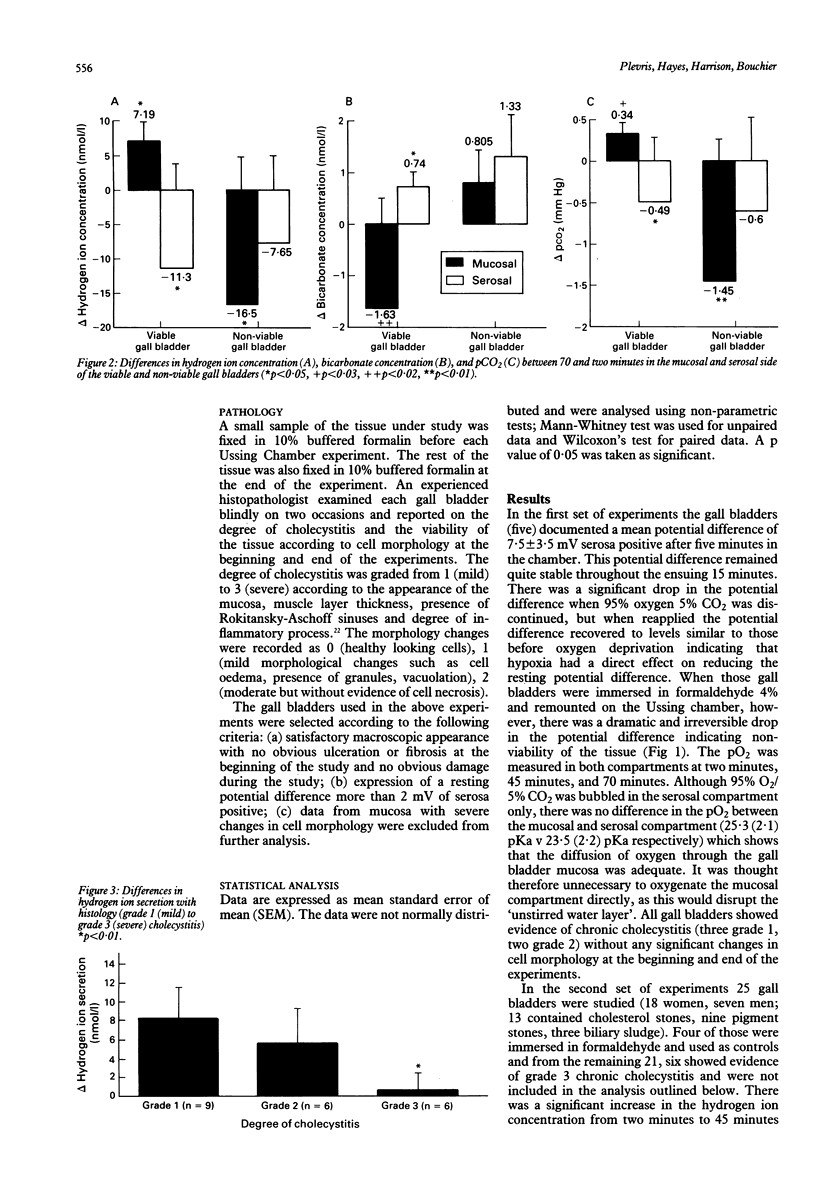
Gall bladder bile is more acid that hepatic bile and this has been attributed to bicarbonate absorption by the gall bladder epithelium. The aim of this study was to investigate in vitro the acid base changes that occur across the human gall bladder mucosa. Fresh gall bladder tissue was obtained at cholecystectomy and placed in an Ussing Chamber and perfused with Ringer-Krebs glucose bicarbonate solution. The viability of the gall bladder was assessed by measuring the potential differences across the epithelium and by the morphology of the epithelial cells at the end of the experiments. Aliquots from the solutions were taken at two, 45 and 70 minutes and pCO2, hydrogen ion and bicarbonate concentrations were measured. In the mucosal side of the chamber a consistent and significant decrease was observed from two minutes to 70 minutes in bicarbonate concentration while pCO2 and hydrogen ion concentrations significantly increased. The degree of inflammation correlated well with the ability for acidification, the more inflamed the tissue the less its ability to acidify. When the gall bladder was exposed to amiloride or sodium free solution acidification was abolished in the mucosal side. When tissue metabolism was irreversibly inhibited by exposure to formaldehyde, hydrogen ion concentration and pCO2 were significantly decreased in the mucosal side of the chamber compared with the viable gall bladder. The human gall bladder is capable of secreting acid and this may be an important mechanism for preventing calcium precipitation and gall stone formation.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Admirand W. H., Small D. M. The physicochemical basis of cholesterol gallstone formation in man. J Clin Invest. 1968 May;47(5):1043–1052. doi: 10.1172/JCI105794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altenberg G. A., Reuss L. Apical membrane Na+/H+ exchange in Necturus gallbladder epithelium. Its dependence on extracellular and intracellular pH and on external Na+ concentration. J Gen Physiol. 1990 Feb;95(2):369–392. doi: 10.1085/jgp.95.2.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Been J. M., Bills P. M., Lewis D. Microstructure of gallstones. Gastroenterology. 1979 Mar;76(3):548–555. [PubMed] [Google Scholar]
- Burnstein M. J., Ilson R. G., Petrunka C. N., Taylor R. D., Strasberg S. M. Evidence for a potent nucleating factor in the gallbladder bile of patients with cholesterol gallstones. Gastroenterology. 1983 Oct;85(4):801–807. [PubMed] [Google Scholar]
- Conter R. L., Roslyn J. J., Porter-Fink V., DenBesten L. Gallbladder absorption increases during early cholesterol gallstone formation. Am J Surg. 1986 Jan;151(1):184–191. doi: 10.1016/0002-9610(86)90030-9. [DOI] [PubMed] [Google Scholar]
- Cremaschi D., Hénin S., Meyer G. Stimulation by HCO3- of Na+ transport in rabbit gallbladder. J Membr Biol. 1979 May 21;47(2):145–170. doi: 10.1007/BF01876114. [DOI] [PubMed] [Google Scholar]
- DIAMOND J. M. THE MECHANISM OF ISOTONIC WATER TRANSPORT. J Gen Physiol. 1964 Sep;48:15–42. doi: 10.1085/jgp.48.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIAMOND J. M. TRANSPORT OF SALT AND WATER IN RABBIT AND GUINEA PIG GALL BLADDER. J Gen Physiol. 1964 Sep;48:1–14. doi: 10.1085/jgp.48.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond J. M. A rapid method for determining voltage-concentration relations across membranes. J Physiol. 1966 Mar;183(1):83–100. doi: 10.1113/jphysiol.1966.sp007852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heintze K., Petersen K. U., Olles P., Saverymuttu S. H., Wood J. R. Effects of bicarbonate on fluid and electrolyte transport by the guinea pig gallbladder: a bicarbonate-chloride exchange. J Membr Biol. 1979 Mar 28;45(1-2):43–59. doi: 10.1007/BF01869294. [DOI] [PubMed] [Google Scholar]
- Heintze K., Petersen K. U., Wood J. R. Effects of bicarbonate on fluid and electrolyte transport by guinea pig and rabbit gallbladder: stimulation of absorption. J Membr Biol. 1981;62(3):175–181. doi: 10.1007/BF01998163. [DOI] [PubMed] [Google Scholar]
- Hofmann A. F., Grundy S. M., Lachin J. M., Lan S. P., Baum R. A., Hanson R. F., Hersh T., Hightower N. C., Jr, Marks J. W., Mekhjian H. Pretreatment biliary lipid composition in white patients with radiolucent gallstones in the National Cooperative Gallstone Study. Gastroenterology. 1982 Oct;83(4):738–752. [PubMed] [Google Scholar]
- Holzbach R. T., Kibe A., Thiel E., Howell J. H., Marsh M., Hermann R. E. Biliary proteins. Unique inhibitors of cholesterol crystal nucleation in human gallbladder bile. J Clin Invest. 1984 Jan;73(1):35–45. doi: 10.1172/JCI111204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzbach R. T., Marsh M., Olszewski M., Holan K. Cholesterol solubility in bile. Evidence that supersaturated bile is frequent in healthy man. J Clin Invest. 1973 Jun;52(6):1467–1479. doi: 10.1172/JCI107321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopwood D., Kouroumalis E., Milne G., Bouchier I. A. Cholecystitis: a fine structural analysis. J Pathol. 1980 Jan;130(1):1–13. doi: 10.1002/path.1711300102. [DOI] [PubMed] [Google Scholar]
- Jacyna M. R., Ross P. E., Bakar M. A., Hopwood D., Bouchier I. A. Characteristics of cholesterol absorption by human gall bladder: relevance to cholesterolosis. J Clin Pathol. 1987 May;40(5):524–529. doi: 10.1136/jcp.40.5.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. P., LaMont J. T., Carey M. C. Role of gallbladder mucus hypersecretion in the evolution of cholesterol gallstones. J Clin Invest. 1981 Jun;67(6):1712–1723. doi: 10.1172/JCI110209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore E. W., Celic L., Ostrow J. D. Interactions between ionized calcium and sodium taurocholate: bile salts are important buffers for prevention of calcium-containing gallstones. Gastroenterology. 1982 Nov;83(5):1079–1089. [PubMed] [Google Scholar]
- Nahrwold D. L., Rose R. C., Ward S. P. Abnormalities in gallbladder morphology and function in patients with cholelithiasis. Ann Surg. 1976 Oct;184(4):415–421. doi: 10.1097/00000658-197610000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rege R. V., Moore E. W. Evidence for H+ secretion by the in vivo canine gallbladder. Gastroenterology. 1987 Feb;92(2):281–289. doi: 10.1016/0016-5085(87)90118-1. [DOI] [PubMed] [Google Scholar]
- Rege R. V., Moore E. W. Pathogenesis of calcium-containing gallstones. Canine ductular bile, but not gallbladder bile, is supersaturated with calcium carbonate. J Clin Invest. 1986 Jan;77(1):21–26. doi: 10.1172/JCI112278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rege R. V., Nahrwold D. L., Moore E. W. Absorption of biliary calcium from the canine gallbladder: protection against the formation of calcium-containing gallstones. J Lab Clin Med. 1987 Oct;110(4):381–386. [PubMed] [Google Scholar]
- Rose R. C., Gelarden R. T., Nahrwold D. L. Electrical properties of isolated human gallbladder. Am J Physiol. 1973 Jun;224(6):1320–1326. doi: 10.1152/ajplegacy.1973.224.6.1320. [DOI] [PubMed] [Google Scholar]
- Smith B. F., LaMont J. T., Small D. M. The sequence of events in gallstone formation. Lab Invest. 1987 Feb;56(2):125–126. [PubMed] [Google Scholar]
- Sutor D. J., Wilkie L. I. Calcium in bile and calcium salts in gallstones. Clin Chim Acta. 1977 Aug 15;79(1):119–127. doi: 10.1016/0009-8981(77)90469-7. [DOI] [PubMed] [Google Scholar]
- USSING H. H., ZERAHN K. Active transport of sodium as the source of electric current in the short-circuited isolated frog skin. Acta Physiol Scand. 1951 Aug 25;23(2-3):110–127. doi: 10.1111/j.1748-1716.1951.tb00800.x. [DOI] [PubMed] [Google Scholar]
- Wahlin T., Thornell E., Jivegård L., Svanvik J. Effects of intraluminal prostaglandin E2 in vivo on secretory behavior and ultrastructural changes in mouse gallbladder epithelium. Gastroenterology. 1988 Dec;95(6):1632–1635. doi: 10.1016/s0016-5085(88)80088-x. [DOI] [PubMed] [Google Scholar]
- Weinman S. A., Reuss L. Na+-H+ exchange at the apical membrane of Necturus gallbladder. Extracellular and intracellular pH studies. J Gen Physiol. 1982 Aug;80(2):299–321. doi: 10.1085/jgp.80.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitlock R. T., Wheeler H. O. Hydrogen ion transport by isolated rabbit gallbladder. Am J Physiol. 1969 Jul;217(1):310–316. doi: 10.1152/ajplegacy.1969.217.1.310. [DOI] [PubMed] [Google Scholar]
- Wood J. R., Svanvik J. Gall-bladder water and electrolyte transport and its regulation. Gut. 1983 Jun;24(6):579–593. doi: 10.1136/gut.24.6.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wosiewitz U. Limy bile and radiopaque, calcified gallstones: a combined analytical, radiographic, and micromorphologic investigation. Pathol Res Pract. 1980;167(2-4):273–286. doi: 10.1016/S0344-0338(80)80057-4. [DOI] [PubMed] [Google Scholar]



