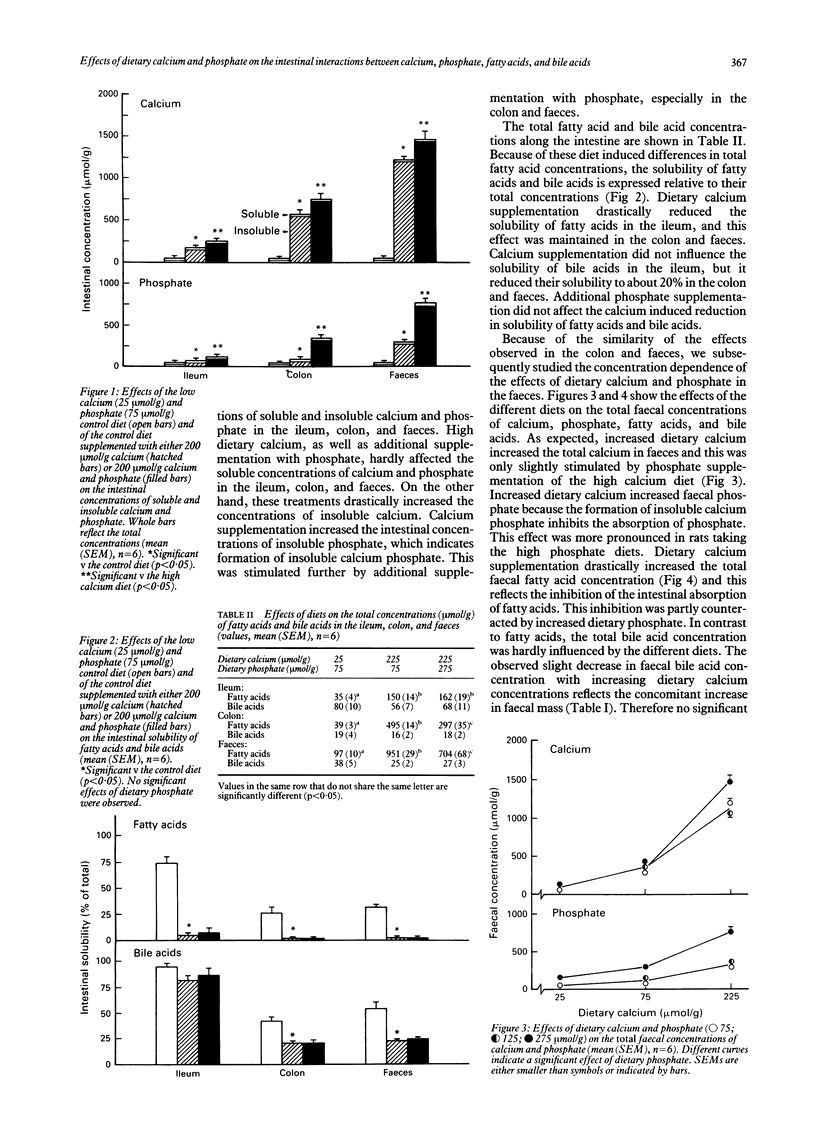
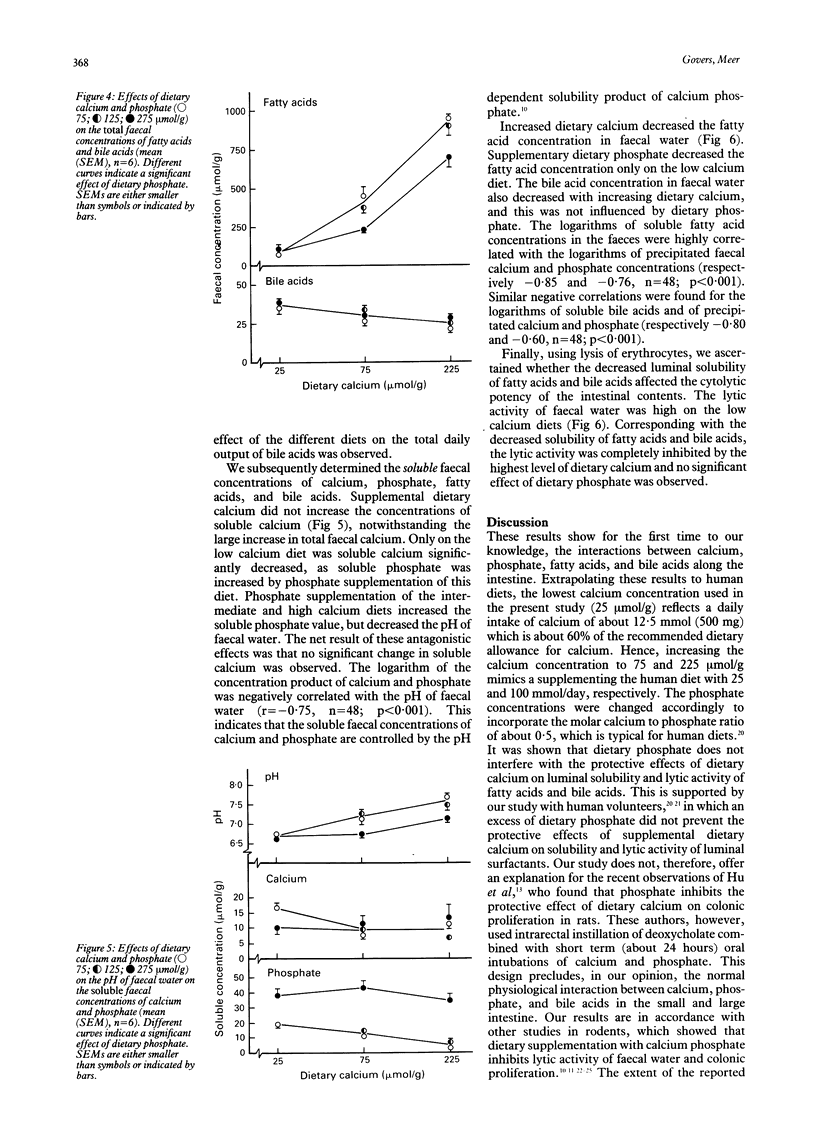
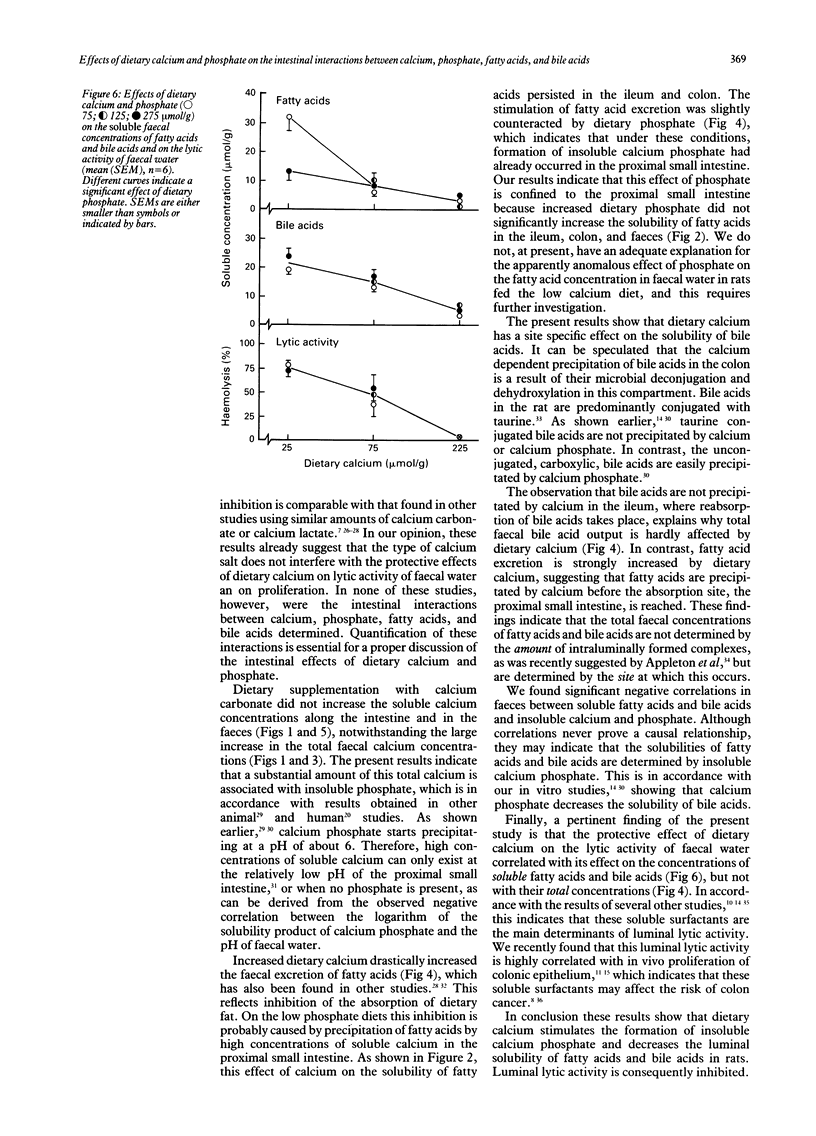
Abstract
Luminal free fatty acids and bile acids may damage the colonic epithelium and stimulate proliferation, which may increase the risk of colon cancer. It has been suggested that only soluble calcium ions (Ca2+) precipitate fatty acids and bile acids, thus reducing their lytic activity. Consequently, precipitation of luminal Ca2+ by dietary phosphate should inhibit these effects. To evaluate the proposed antagonistic effects of dietary calcium and phosphate, we studied the intestinal interactions between calcium, phosphate, fatty acids, and bile acids in rats fed purified diets that differed only in the concentrations of calcium and phosphate. Increased dietary calcium drastically decreased the solubility of fatty acids in the ileum, colon, and faeces, as well as the solubility of bile acids in the colon and faeces. Although dietary calcium strongly increased the total faecal fatty acid concentration and hardly affected the total faecal bile acid concentration, the fatty acid and bile acid concentrations in faecal water were drastically decreased by dietary calcium. Consequently, the lytic activity of faecal water was decreased. Dietary phosphate did not interfere with these intestinal effects of calcium. These results indicate that dietary phosphate does not inhibit the protective effects of dietary calcium on luminal solubility and the lytic activity of fatty and bile acids.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appleton G. V., Davies P. W., Bristol J. B., Williamson R. C. Inhibition of intestinal carcinogenesis by dietary supplementation with calcium. Br J Surg. 1987 Jun;74(6):523–525. doi: 10.1002/bjs.1800740635. [DOI] [PubMed] [Google Scholar]
- Appleton G. V., Owen R. W., Wheeler E. E., Challacombe D. N., Williamson R. C. Effect of dietary calcium on the colonic luminal environment. Gut. 1991 Nov;32(11):1374–1377. doi: 10.1136/gut.32.11.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird R. P. Effect of dietary components on the pathobiology of colonic epithelium: possible relationship with colon tumorigenesis. Lipids. 1986 Apr;21(4):289–291. doi: 10.1007/BF02536415. [DOI] [PubMed] [Google Scholar]
- Bird R. P., Schneider R., Stamp D., Bruce W. R. Effect of dietary calcium and cholic acid on the proliferative indices of murine colonic epithelium. Carcinogenesis. 1986 Oct;7(10):1657–1661. doi: 10.1093/carcin/7.10.1657. [DOI] [PubMed] [Google Scholar]
- Bruce W. R. Recent hypotheses for the origin of colon cancer. Cancer Res. 1987 Aug 15;47(16):4237–4242. [PubMed] [Google Scholar]
- Caderni G., Stuart E. W., Bruce W. R. Dietary factors affecting the proliferation of epithelial cells in the mouse colon. Nutr Cancer. 1988;11(3):147–153. doi: 10.1080/01635588809513982. [DOI] [PubMed] [Google Scholar]
- Coleman R., Iqbal S., Godfrey P. P., Billington D. Membranes and bile formation. Composition of several mammalian biles and their membrane-damaging properties. Biochem J. 1979 Jan 15;178(1):201–208. doi: 10.1042/bj1780201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fordtran J. S., Locklear T. W. Ionic constituents and osmolality of gastric and small-intestinal fluids after eating. Am J Dig Dis. 1966 Jul;11(7):503–521. doi: 10.1007/BF02233563. [DOI] [PubMed] [Google Scholar]
- Heuman D. M. Quantitative estimation of the hydrophilic-hydrophobic balance of mixed bile salt solutions. J Lipid Res. 1989 May;30(5):719–730. [PubMed] [Google Scholar]
- Lapré J. A., De Vries H. T., van der Meer R. Dietary calcium phosphate inhibits cytotoxicity of fecal water. Am J Physiol. 1991 Dec;261(6 Pt 1):G907–G912. doi: 10.1152/ajpgi.1991.261.6.G907. [DOI] [PubMed] [Google Scholar]
- Lapré J. A., Van der Meer R. Diet-induced increase of colonic bile acids stimulates lytic activity of fecal water and proliferation of colonic cells. Carcinogenesis. 1992 Jan;13(1):41–44. doi: 10.1093/carcin/13.1.41. [DOI] [PubMed] [Google Scholar]
- Lipkin M. Biomarkers of increased susceptibility to gastrointestinal cancer: new application to studies of cancer prevention in human subjects. Cancer Res. 1988 Jan 15;48(2):235–245. [PubMed] [Google Scholar]
- Newmark H. L., Wargovich M. J., Bruce W. R. Colon cancer and dietary fat, phosphate, and calcium: a hypothesis. J Natl Cancer Inst. 1984 Jun;72(6):1323–1325. [PubMed] [Google Scholar]
- Rafter J. J., Eng V. W., Furrer R., Medline A., Bruce W. R. Effects of calcium and pH on the mucosal damage produced by deoxycholic acid in the rat colon. Gut. 1986 Nov;27(11):1320–1329. doi: 10.1136/gut.27.11.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reshef R., Rozen P., Fireman Z., Fine N., Barzilai M., Shasha S. M., Shkolnik T. Effect of a calcium-enriched diet on the colonic epithelial hyperproliferation induced by N-methyl-N'-nitro-N-nitrosoguanidine in rats on a low calcium and fat diet. Cancer Res. 1990 Mar 15;50(6):1764–1767. [PubMed] [Google Scholar]
- Saunders D., Sillery J., Chapman R. Effect of calcium carbonate and aluminum hydroxide on human intestinal function. Dig Dis Sci. 1988 Apr;33(4):409–413. doi: 10.1007/BF01536023. [DOI] [PubMed] [Google Scholar]
- Shiga A., Sasaki T., Horii N. Correlations among pH and Mg, Ca, P, Na, K, Cl- and HCO3- contents of digesta in the gastro-intestinal tract of rats. Nihon Juigaku Zasshi. 1987 Dec;49(6):973–979. doi: 10.1292/jvms1939.49.973. [DOI] [PubMed] [Google Scholar]
- Skraastad O., Reichelt K. L. An endogenous colon mitosis inhibitor and dietary calcium inhibit the increased colonic cell proliferation induced by cholic acid. Scand J Gastroenterol. 1988 Sep;23(7):801–807. doi: 10.3109/00365528809090763. [DOI] [PubMed] [Google Scholar]
- Sorenson A. W., Slattery M. L., Ford M. H. Calcium and colon cancer: a review. Nutr Cancer. 1988;11(3):135–145. doi: 10.1080/01635588809513981. [DOI] [PubMed] [Google Scholar]
- Stadler J., Stern H. S., Yeung K. S., McGuire V., Furrer R., Marcon N., Bruce W. R. Effect of high fat consumption on cell proliferation activity of colorectal mucosa and on soluble faecal bile acids. Gut. 1988 Oct;29(10):1326–1331. doi: 10.1136/gut.29.10.1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Meer R., De Vries H. T. Differential binding of glycine- and taurine-conjugated bile acids to insoluble calcium phosphate. Biochem J. 1985 Jul 1;229(1):265–268. doi: 10.1042/bj2290265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Meer R., Termont D. S., De Vries H. T. Differential effects of calcium ions and calcium phosphate on cytotoxicity of bile acids. Am J Physiol. 1991 Jan;260(1 Pt 1):G142–G147. doi: 10.1152/ajpgi.1991.260.1.G142. [DOI] [PubMed] [Google Scholar]
- Van der Meer R., Welberg J. W., Kuipers F., Kleibeuker J. H., Mulder N. H., Termont D. S., Vonk R. J., De Vries H. T., De Vries E. G. Effects of supplemental dietary calcium on the intestinal association of calcium, phosphate, and bile acids. Gastroenterology. 1990 Dec;99(6):1653–1659. doi: 10.1016/0016-5085(90)90471-c. [DOI] [PubMed] [Google Scholar]
- Wargovich M. J., Allnutt D., Palmer C., Anaya P., Stephens L. C. Inhibition of the promotional phase of azoxymethane-induced colon carcinogenesis in the F344 rat by calcium lactate: effect of simulating two human nutrient density levels. Cancer Lett. 1990 Aug;53(1):17–25. doi: 10.1016/0304-3835(90)90005-i. [DOI] [PubMed] [Google Scholar]
- Wargovich M. J., Eng V. W., Newmark H. L., Bruce W. R. Calcium ameliorates the toxic effect of deoxycholic acid on colonic epithelium. Carcinogenesis. 1983 Sep;4(9):1205–1207. doi: 10.1093/carcin/4.9.1205. [DOI] [PubMed] [Google Scholar]
- Wargovich M. J., Eng V. W., Newmark H. L. Calcium inhibits the damaging and compensatory proliferative effects of fatty acids on mouse colon epithelium. Cancer Lett. 1984 Jul;23(3):253–258. doi: 10.1016/0304-3835(84)90091-0. [DOI] [PubMed] [Google Scholar]
- Weisburger J. H. Causes, relevant mechanisms, and prevention of large bowel cancer. Semin Oncol. 1991 Aug;18(4):316–336. [PubMed] [Google Scholar]
- Willett W. C., Stampfer M. J., Colditz G. A., Rosner B. A., Speizer F. E. Relation of meat, fat, and fiber intake to the risk of colon cancer in a prospective study among women. N Engl J Med. 1990 Dec 13;323(24):1664–1672. doi: 10.1056/NEJM199012133232404. [DOI] [PubMed] [Google Scholar]
- Willett W. The search for the causes of breast and colon cancer. Nature. 1989 Mar 30;338(6214):389–394. doi: 10.1038/338389a0. [DOI] [PubMed] [Google Scholar]


