Abstract
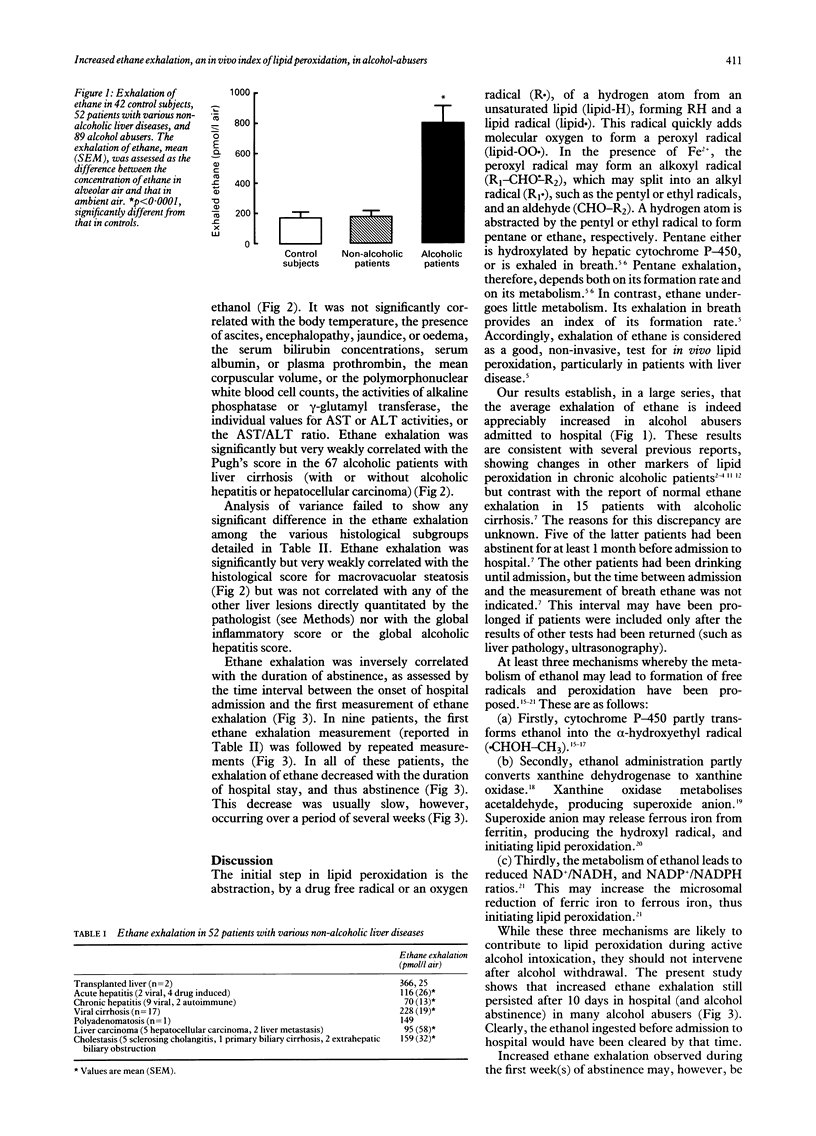
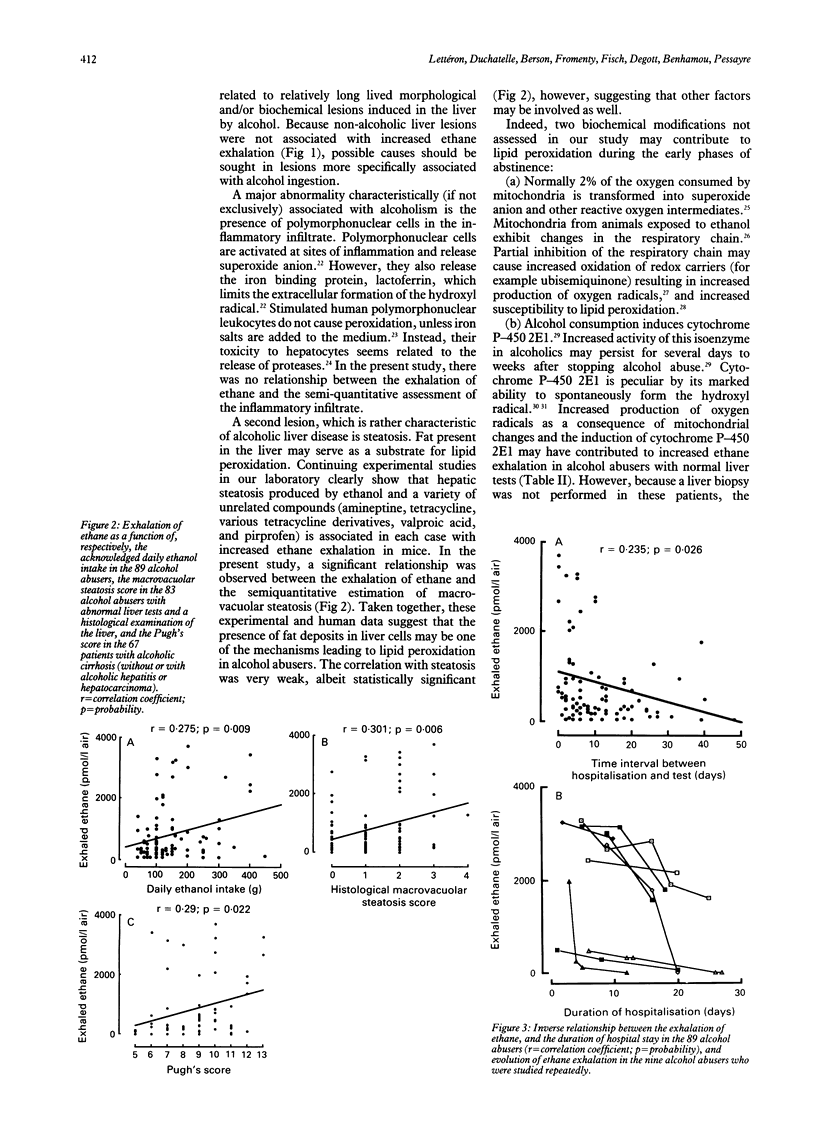
Ethane exhalation was measured in 42 control subjects, 52 patients with various non-alcoholic liver diseases, and 89 alcohol abusers who had been admitted to hospital for alcohol withdrawal and assessment of liver disease (six with normal liver tests, 10 with steatosis with or without fibrosis, six with alcoholic hepatitis, 29 with cirrhosis, 34 with both cirrhosis and alcoholic hepatitis, and four with both cirrhosis and a hepatocellular carcinoma). Ethane exhalation was similar in control subjects and in patients with non-alcoholic liver diseases, but was five times higher in alcohol abusers. Ethane exhalation in alcohol abusers was significantly, but very weakly, correlated with the daily ethanol intake before hospital admission, and the histological score for steatosis, but not with the inflammation or alcoholic hepatitis scores. Ethane exhalation was inversely correlated with the duration of abstinence before the test. In nine alcoholic patients, the exhalation of ethane was measured repeatedly, and showed slow improvement during abstinence. Ethane exhalation was significantly but weakly correlated with the Pugh's score in patients with alcoholic cirrhosis. It is concluded that the mean ethane exhalation is increased in alcohol abusers. One of the possible mechanisms may be the presence of oxidizable fat in the liver. The weak correlation with the Pugh's score is consistent with the contribution of many other factors in the progression to severe liver disease.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albano E., Tomasi A., Goria-Gatti L., Dianzani M. U. Spin trapping of free radical species produced during the microsomal metabolism of ethanol. Chem Biol Interact. 1988;65(3):223–234. doi: 10.1016/0009-2797(88)90108-1. [DOI] [PubMed] [Google Scholar]
- Allerheiligen S. R., Ludden T. M., Burk R. F. The pharmacokinetics of pentane, a by-product of lipid peroxidation. Drug Metab Dispos. 1987 Nov-Dec;15(6):794–800. [PubMed] [Google Scholar]
- Biemond P., Swaak A. J., Beindorff C. M., Koster J. F. Superoxide-dependent and -independent mechanisms of iron mobilization from ferritin by xanthine oxidase. Implications for oxygen-free-radical-induced tissue destruction during ischaemia and inflammation. Biochem J. 1986 Oct 1;239(1):169–173. doi: 10.1042/bj2390169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borowitz S. M., Montgomery C. The role of phospholipase A2 in microsomal lipid peroxidation induced with t-butyl hydroperoxide. Biochem Biophys Res Commun. 1989 Feb 15;158(3):1021–1028. doi: 10.1016/0006-291x(89)92824-6. [DOI] [PubMed] [Google Scholar]
- Boveris A., Oshino N., Chance B. The cellular production of hydrogen peroxide. Biochem J. 1972 Jul;128(3):617–630. doi: 10.1042/bj1280617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britigan B. E., Rosen G. M., Thompson B. Y., Chai Y., Cohen M. S. Stimulated human neutrophils limit iron-catalyzed hydroxyl radical formation as detected by spin-trapping techniques. J Biol Chem. 1986 Dec 25;261(36):17026–17032. [PubMed] [Google Scholar]
- Cederbaum A. I. Role of lipid peroxidation and oxidative stress in alcohol toxicity. Free Radic Biol Med. 1989;7(5):537–539. doi: 10.1016/0891-5849(89)90029-4. [DOI] [PubMed] [Google Scholar]
- Drevon C. A. Absorption, transport and metabolism of vitamin E. Free Radic Res Commun. 1991;14(4):229–246. doi: 10.3109/10715769109088952. [DOI] [PubMed] [Google Scholar]
- Girre C., Hispard E., Therond P., Guedj S., Bourdon R., Dally S. Effect of abstinence from alcohol on the depression of glutathione peroxidase activity and selenium and vitamin E levels in chronic alcoholic patients. Alcohol Clin Exp Res. 1990 Dec;14(6):909–912. doi: 10.1111/j.1530-0277.1990.tb01836.x. [DOI] [PubMed] [Google Scholar]
- Jeffrey G. P., Muller D. P., Burroughs A. K., Matthews S., Kemp C., Epstein O., Metcalfe T. A., Southam E., Tazir-Melboucy M., Thomas P. K. Vitamin E deficiency and its clinical significance in adults with primary biliary cirrhosis and other forms of chronic liver disease. J Hepatol. 1987 Jun;4(3):307–317. doi: 10.1016/s0168-8278(87)80539-1. [DOI] [PubMed] [Google Scholar]
- Knecht K. T., Bradford B. U., Mason R. P., Thurman R. G. In vivo formation of a free radical metabolite of ethanol. Mol Pharmacol. 1990 Jul;38(1):26–30. [PubMed] [Google Scholar]
- Krikun G., Lieber C. S., Cederbaum A. I. Increased microsomal oxidation of ethanol by cytochrome P-450 and hydroxyl radical-dependent pathways after chronic ethanol consumption. Biochem Pharmacol. 1984 Oct 15;33(20):3306–3309. doi: 10.1016/0006-2952(84)90097-2. [DOI] [PubMed] [Google Scholar]
- Kukiełka E., Cederbaum A. I. NADH-dependent microsomal interaction with ferric complexes and production of reactive oxygen intermediates. Arch Biochem Biophys. 1989 Dec;275(2):540–550. doi: 10.1016/0003-9861(89)90400-1. [DOI] [PubMed] [Google Scholar]
- Lauterburg B. H. Early disturbance of calcium translocation across the plasma membrane in toxic liver injury. Hepatology. 1987 Nov-Dec;7(6):1179–1183. doi: 10.1002/hep.1840070602. [DOI] [PubMed] [Google Scholar]
- Lettéron P., Labbe G., Degott C., Berson A., Fromenty B., Delaforge M., Larrey D., Pessayre D. Mechanism for the protective effects of silymarin against carbon tetrachloride-induced lipid peroxidation and hepatotoxicity in mice. Evidence that silymarin acts both as an inhibitor of metabolic activation and as a chain-breaking antioxidant. Biochem Pharmacol. 1990 Jun 15;39(12):2027–2034. doi: 10.1016/0006-2952(90)90625-u. [DOI] [PubMed] [Google Scholar]
- Lieber C. S. Biochemical and molecular basis of alcohol-induced injury to liver and other tissues. N Engl J Med. 1988 Dec 22;319(25):1639–1650. doi: 10.1056/NEJM198812223192505. [DOI] [PubMed] [Google Scholar]
- Lieber C. S. Interaction of alcohol with other drugs and nutrients. Implication for the therapy of alcoholic liver disease. Drugs. 1990;40 (Suppl 3):23–44. doi: 10.2165/00003495-199000403-00004. [DOI] [PubMed] [Google Scholar]
- Lieber C. S. Mechanism of ethanol induced hepatic injury. Pharmacol Ther. 1990;46(1):1–41. doi: 10.1016/0163-7258(90)90032-w. [DOI] [PubMed] [Google Scholar]
- Masini A., Trenti T., Ventura E., Ceccarelli D., Muscatello U. The effect of ferric iron complex on Ca2+ transport in isolated rat liver mitochondria. Biochem Biophys Res Commun. 1985 Jul 16;130(1):207–213. doi: 10.1016/0006-291x(85)90403-6. [DOI] [PubMed] [Google Scholar]
- Mavier P., Preaux A. M., Guigui B., Lescs M. C., Zafrani E. S., Dhumeaux D. In vitro toxicity of polymorphonuclear neutrophils to rat hepatocytes: evidence for a proteinase-mediated mechanism. Hepatology. 1988 Mar-Apr;8(2):254–258. doi: 10.1002/hep.1840080211. [DOI] [PubMed] [Google Scholar]
- Mazzanti R., Moscarella S., Bensi G., Altavilla E., Gentilini P. Hepatic lipid peroxidation and aldehyde dehydrogenase activity in alcoholic and non alcoholic liver disease. Alcohol Alcohol. 1989;24(2):121–128. doi: 10.1093/oxfordjournals.alcalc.a044875. [DOI] [PubMed] [Google Scholar]
- Moscarella S., Laffi G., Buzzelli G., Mazzanti R., Caramelli L., Gentilini P. Expired hydrocarbons in patients with chronic liver disease. Hepatogastroenterology. 1984 Apr;31(2):60–63. [PubMed] [Google Scholar]
- Nordmann R., Ribière C., Rouach H. Implication of free radical mechanisms in ethanol-induced cellular injury. Free Radic Biol Med. 1992;12(3):219–240. doi: 10.1016/0891-5849(92)90030-k. [DOI] [PubMed] [Google Scholar]
- Pencil S. D., Glende E. A., Jr, Recknagel R. O. Loss of calcium sequestration capacity in endoplasmic reticulum of isolated hepatocytes treated with carbon tetrachloride. Res Commun Chem Pathol Pharmacol. 1982 Jun;36(3):413–428. [PubMed] [Google Scholar]
- Persson J. O., Terelius Y., Ingelman-Sundberg M. Cytochrome P-450-dependent formation of reactive oxygen radicals: isozyme-specific inhibition of P-450-mediated reduction of oxygen and carbon tetrachloride. Xenobiotica. 1990 Sep;20(9):887–900. doi: 10.3109/00498259009046904. [DOI] [PubMed] [Google Scholar]
- Reinke L. A., Lai E. K., DuBose C. M., McCay P. B. Reactive free radical generation in vivo in heart and liver of ethanol-fed rats: correlation with radical formation in vitro. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9223–9227. doi: 10.1073/pnas.84.24.9223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouach H., Clément M., Orfanelli M. T., Janvier B., Nordmann J., Nordmann R. Hepatic lipid peroxidation and mitochondrial susceptibility to peroxidative attacks during ethanol inhalation and withdrawal. Biochim Biophys Acta. 1983 Oct 11;753(3):439–444. doi: 10.1016/0005-2760(83)90068-1. [DOI] [PubMed] [Google Scholar]
- Shaw S., Jayatilleke E. The role of aldehyde oxidase in ethanol-induced hepatic lipid peroxidation in the rat. Biochem J. 1990 Jun 15;268(3):579–583. doi: 10.1042/bj2680579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw S., Rubin K. P., Lieber C. S. Depressed hepatic glutathione and increased diene conjugates in alcoholic liver disease. Evidence of lipid peroxidation. Dig Dis Sci. 1983 Jul;28(7):585–589. doi: 10.1007/BF01299917. [DOI] [PubMed] [Google Scholar]
- Sokol R. J., Kim Y. S., Hoofnagle J. H., Heubi J. E., Jones E. A., Balistreri W. F. Intestinal malabsorption of vitamin E in primary biliary cirrhosis. Gastroenterology. 1989 Feb;96(2 Pt 1):479–486. doi: 10.1016/0016-5085(89)91574-6. [DOI] [PubMed] [Google Scholar]
- Sokol R. J. Vitamin E and neurologic function in man. Free Radic Biol Med. 1989;6(2):189–207. doi: 10.1016/0891-5849(89)90117-2. [DOI] [PubMed] [Google Scholar]
- Spach P. I., Cunningham C. C. Control of state 3 respiration in liver mitochondria from rats subjected to chronic ethanol consumption. Biochim Biophys Acta. 1987 Dec 17;894(3):460–467. doi: 10.1016/0005-2728(87)90125-3. [DOI] [PubMed] [Google Scholar]
- Suematsu T., Matsumura T., Sato N., Miyamoto T., Ooka T., Kamada T., Abe H. Lipid peroxidation in alcoholic liver disease in humans. Alcohol Clin Exp Res. 1981 Summer;5(3):427–430. doi: 10.1111/j.1530-0277.1981.tb04926.x. [DOI] [PubMed] [Google Scholar]
- Sultatos L. G. Effects of acute ethanol administration on the hepatic xanthine dehydrogenase/oxidase system in the rat. J Pharmacol Exp Ther. 1988 Sep;246(3):946–949. [PubMed] [Google Scholar]
- Tanner A. R., Bantock I., Hinks L., Lloyd B., Turner N. R., Wright R. Depressed selenium and vitamin E levels in an alcoholic population. Possible relationship to hepatic injury through increased lipid peroxidation. Dig Dis Sci. 1986 Dec;31(12):1307–1312. doi: 10.1007/BF01299808. [DOI] [PubMed] [Google Scholar]
- Thomas M. J., Shirley P. S., Hedrick C. C., DeChatelet L. R. Role of free radical processes in stimulated human polymorphonuclear leukocytes. Biochemistry. 1986 Dec 2;25(24):8042–8048. doi: 10.1021/bi00372a037. [DOI] [PubMed] [Google Scholar]
- Van Gossum A., Decuyper J. Breath alkanes as an index of lipid peroxidation. Eur Respir J. 1989 Sep;2(8):787–791. [PubMed] [Google Scholar]
- Younes M., Siegers C. P. Interrelation between lipid peroxidation and other hepatotoxic events. Biochem Pharmacol. 1984 Jul 1;33(13):2001–2003. doi: 10.1016/0006-2952(84)90564-1. [DOI] [PubMed] [Google Scholar]



