Abstract
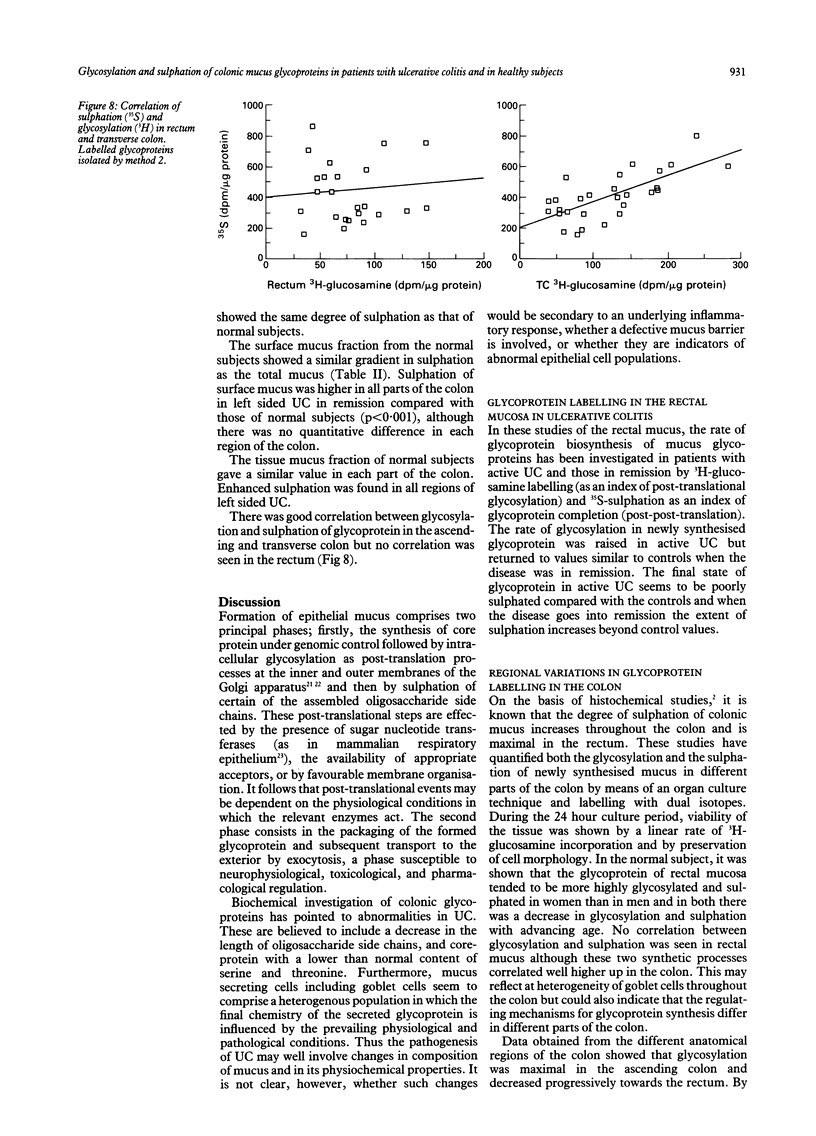
Studies have been made of mucus glycoprotein biosynthesis in different regions of the lower gastrointestinal tract in normal patients and those with ulcerative colitis (UC), active or inactive, by means of 3H-glucosamine (3H-GlcNH2)--35S-sulphate double labelling of epithelial biopsy specimens under culture conditions. The time based rate of 3H-GlcNH2 labelling of mucus in rectal tissue was similar to that in active or inactive UC whereas the rate of 35SO4(2) labelling was significantly increased in active disease. The 3H specific activities measuring the amount of isotopic incorporation into surface and tissue mucus glycoproteins were increased in patients with active UC compared with normal or inactive subjects. The 35S specific activities did not differ significantly between patients with active UC and those in remission. In the rectum, glycosylation of mucus glycoproteins decreases with the increasing age of the patient. Regional differences in 3H-labelling of mucus components are reported for ascending colon, transverse colon, sigmoid colon, and rectum. Sulphation (35S-labelling) was higher in all parts of the colon in left sided UC. Results point to accelerated glycosylation of core proteins in the active phase of UC.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allan A., Bristol J. B., Williamson R. C. Crypt cell production rate in ulcerative proctocolitis: differential increments in remission and relapse. Gut. 1985 Oct;26(10):999–1003. doi: 10.1136/gut.26.10.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boland C. R., Clapp N. K. Glycoconjugates in the colons of New World monkeys with spontaneous colitis. Association between inflammation and neoplasia. Gastroenterology. 1987 Mar;92(3):625–634. doi: 10.1016/0016-5085(87)90010-2. [DOI] [PubMed] [Google Scholar]
- Boland C. R., Lance P., Levin B., Riddell R. H., Kim Y. S. Abnormal goblet cell glycoconjugates in rectal biopsies associated with an increased risk of neoplasia in patients with ulcerative colitis: early results of a prospective study. Gut. 1984 Dec;25(12):1364–1371. doi: 10.1136/gut.25.12.1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clamp J. R., Fraser G., Read A. E. Study of the carbohydrate content of mucus glycoproteins from normal and diseased colons. Clin Sci (Lond) 1981 Aug;61(2):229–234. doi: 10.1042/cs0610229. [DOI] [PubMed] [Google Scholar]
- Cottrell J. M., Hall R. L., Sturton R. G., Kent P. W. Polypeptide N-acetylgalactosaminyltransferase activity in tracheal epithelial microsomes. Biochem J. 1992 Apr 1;283(Pt 1):299–305. doi: 10.1042/bj2830299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culling C. F., Reid P. E., Dunn W. L. A histochemical comparison of the O-acylated sialic acids of the epithelial mucins in ulcerative colitis, Crohn's disease, and normal controls. J Clin Pathol. 1979 Dec;32(12):1272–1277. doi: 10.1136/jcp.32.12.1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson P. A., Filipe M. I. Uptake of [35S]sulphate in human colonic mucosa associated with carcinoma: an autoradiographic analysis at the ultrastructural level. Histochem J. 1983 Jan;15(1):3–13. doi: 10.1007/BF01006067. [DOI] [PubMed] [Google Scholar]
- Draper P., Kent P. W. Biosynthesis of intestinal mucins. 4. Utilization of [1-C]glucose by sheep colonic mucosa in vitro. Biochem J. 1963 Feb;86(2):248–254. doi: 10.1042/bj0860248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastwood G. L., Trier J. S. Epithelial cell renewal in cultured rectal biopsies in ulcerative colitis. Gastroenterology. 1973 Mar;64(3):383–390. [PubMed] [Google Scholar]
- Ehsanullah M., Filipe M. I., Gazzard B. Mucin secretion in inflammatory bowel disease: correlation with disease activity and dysplasia. Gut. 1982 Jun;23(6):485–489. doi: 10.1136/gut.23.6.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forstner G., Wesley A., Forstner J. Clinical aspects of gastrointestinal mucus. Adv Exp Med Biol. 1982;144:199–224. doi: 10.1007/978-1-4615-9254-9_32. [DOI] [PubMed] [Google Scholar]
- Franzin G., Grigioni W. F., Dina R., Scarpa A., Zamboni G. Mucin secretion and morphological changes of the mucosa in non-neoplastic diseases of the colon. Histopathology. 1983 Sep;7(5):707–718. doi: 10.1111/j.1365-2559.1983.tb02283.x. [DOI] [PubMed] [Google Scholar]
- Goochee C. F., Gramer M. J., Andersen D. C., Bahr J. B., Rasmussen J. R. The oligosaccharides of glycoproteins: bioprocess factors affecting oligosaccharide structure and their effect on glycoprotein properties. Biotechnology (N Y) 1991 Dec;9(12):1347–1355. doi: 10.1038/nbt1291-1347. [DOI] [PubMed] [Google Scholar]
- Goodman M. J., Kent P. W., Truelove S. C. Glucosamine synthetase activity of the colonic mucosa in ulcerative colitis and Crohn's disease. Gut. 1977 Mar;18(3):219–228. doi: 10.1136/gut.18.3.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs L. R., Huber P. W. Regional distribution and alterations of lectin binding to colorectal mucin in mucosal biopsies from controls and subjects with inflammatory bowel diseases. J Clin Invest. 1985 Jan;75(1):112–118. doi: 10.1172/JCI111662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podolsky D. K., Fournier D. A. Alterations in mucosal content of colonic glycoconjugates in inflammatory bowel disease defined by monoclonal antibodies. Gastroenterology. 1988 Aug;95(2):379–387. doi: 10.1016/0016-5085(88)90494-5. [DOI] [PubMed] [Google Scholar]
- Podolsky D. K., Isselbacher K. J. Composition of human colonic mucin. Selective alteration in inflammatory bowel disease. J Clin Invest. 1983 Jul;72(1):142–153. doi: 10.1172/JCI110952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid P. E., Culling C. F., Dunn W. L., Ramey C. W., Clay M. G. Chemical and histochemical studies of normal and diseased human gastrointestinal tract. I. A comparison between histologically normal colon, colonic tumours, ulcerative colitis and diverticular disease of the colon. Histochem J. 1984 Mar;16(3):235–251. doi: 10.1007/BF01003608. [DOI] [PubMed] [Google Scholar]
- Rhodes J. M., Gallimore R., Elias E., Allan R. N., Kennedy J. F. Faecal mucus degrading glycosidases in ulcerative colitis and Crohn's disease. Gut. 1985 Aug;26(8):761–765. doi: 10.1136/gut.26.8.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes J. M., Gallimore R., Elias E., Kennedy J. F. Faecal sulphatase in health and in inflammatory bowel disease. Gut. 1985 May;26(5):466–469. doi: 10.1136/gut.26.5.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman J. E., Orci L. Molecular dissection of the secretory pathway. Nature. 1992 Jan 30;355(6359):409–415. doi: 10.1038/355409a0. [DOI] [PubMed] [Google Scholar]
- Teague R. H., Fraser D., Clamp J. R. Changes in monosaccharide content of mucous glycoproteins in ulcerative colitis. Br Med J. 1973 Jun 16;2(5867):645–646. doi: 10.1136/bmj.2.5867.645. [DOI] [PMC free article] [PubMed] [Google Scholar]


