Abstract
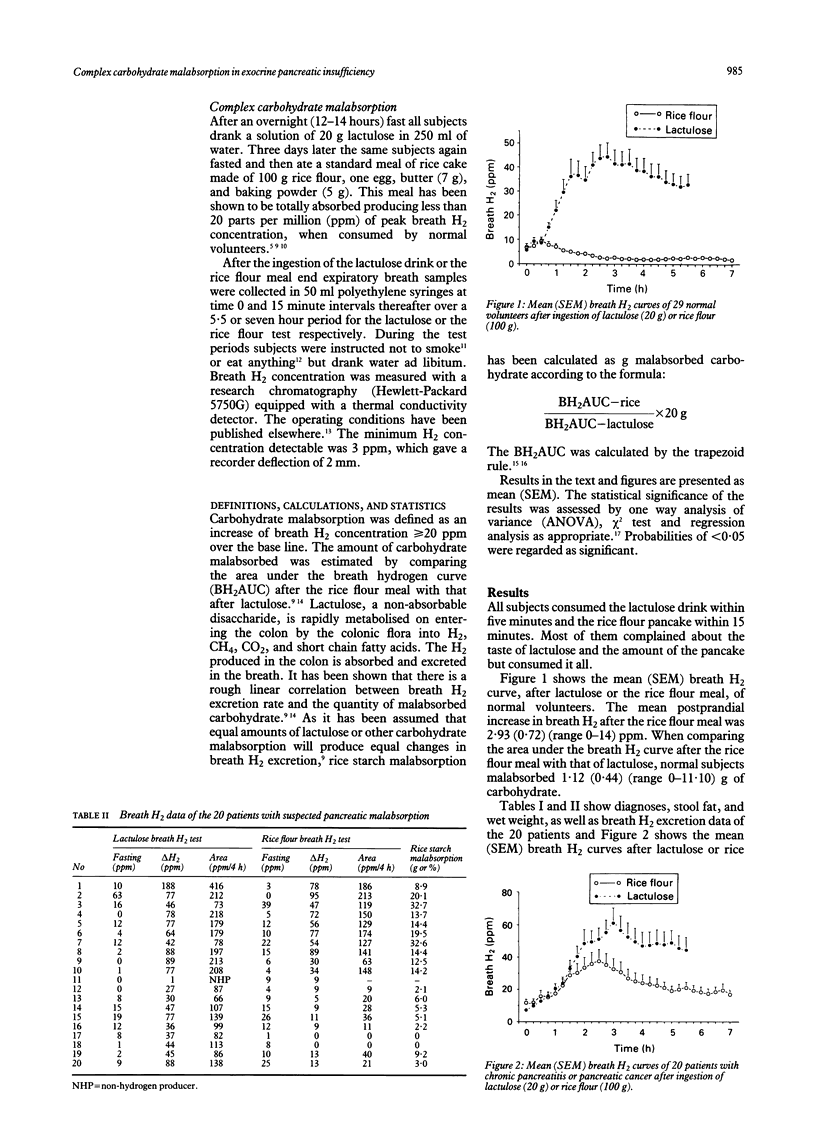
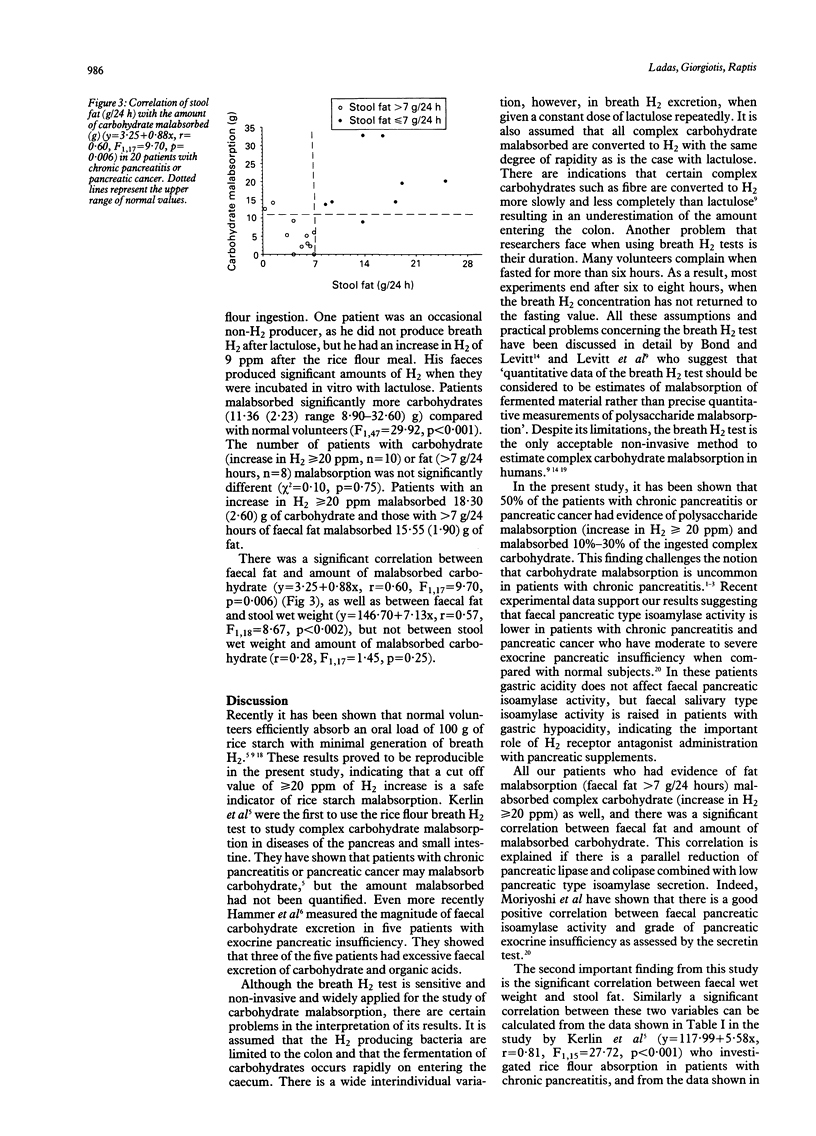
The magnitude of complex carbohydrate malabsorption in exocrine pancreatic insufficiency has not been well quantified in the past. The quantity of carbohydrate malabsorbed after a rice starch (100 g) meal in 20 patients with chronic pancreatitis (n = 10) or pancreatic cancer (n = 10) was therefore estimated. Patients had a three day stool fat collection (80 g/24 hour fat intake), a lactulose (20 g), and a rice flour (100 g) breath hydrogen test. Normal controls (n = 29) had a postprandial H2 increase < or = 14 ppm and malabsorbed (mean (SEM)) 1.12 (0.44) (range 0-11.10) g of the 100 g of carbohydrate ingested. Patients malabsorbed significantly more carbohydrate (11.36 (2.23) (range 8.90-32.60) g, F1.47 = 29.92, p < 0.001). The number of patients with fat (> 7 g, n = 8) or carbohydrate (increase in H2 > or = 20 ppm, n = 10) malabsorption was not different (chi 2 = 0.10, p = 0.75). There was a significant correlation between faecal fat and amount of malabsorbed carbohydrate (r = 0.60, F1.17 = 9.70, p = 0.006) and faecal fat and stool wet weight (r = 0.57, F1.18 = 8.67, p < 0.009), but not between stool wet weight and amount of malabsorbed carbohydrate (r = 0.28, F1.17 = 1.45, p = 0.25). Although patients with exocrine pancreatic insufficiency malabsorb 10%-30% of the ingested complex carbohydrate, the main determinant of stool wet weight could be faecal fat.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson I. H., Levine A. S., Levitt M. D. Incomplete absorption of the carbohydrate in all-purpose wheat flour. N Engl J Med. 1981 Apr 9;304(15):891–892. doi: 10.1056/NEJM198104093041507. [DOI] [PubMed] [Google Scholar]
- Bo-Linn G. W., Fordtran J. S. Fecal fat concentration in patients with steatorrhea. Gastroenterology. 1984 Aug;87(2):319–322. [PubMed] [Google Scholar]
- Bond J. H., Jr, Levitt M. D. Use of pulmonary hydrogen (H 2 ) measurements to quantitate carbohydrate absorption. Study of partially gastrectomized patients. J Clin Invest. 1972 May;51(5):1219–1225. doi: 10.1172/JCI106916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond J. H., Levitt M. D. Quantitative measurement of lactose absorption. Gastroenterology. 1976 Jun;70(6):1058–1062. [PubMed] [Google Scholar]
- Fogel M. R., Gray G. M. Starch hydrolysis in man: an intraluminal process not requiring membrane digestion. J Appl Physiol. 1973 Aug;35(2):263–267. doi: 10.1152/jappl.1973.35.2.263. [DOI] [PubMed] [Google Scholar]
- Grendell J. H. Nutrition and absorption in diseases of the pancreas. Clin Gastroenterol. 1983 May;12(2):551–562. [PubMed] [Google Scholar]
- Hammer H. F., Fine K. D., Santa Ana C. A., Porter J. L., Schiller L. R., Fordtran J. S. Carbohydrate malabsorption. Its measurement and its contribution to diarrhea. J Clin Invest. 1990 Dec;86(6):1936–1944. doi: 10.1172/JCI114927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiele M., Ghoos Y., Rutgeerts P., Vantrappen G. Starch digestion in normal subjects and patients with pancreatic disease, using a 13CO2 breath test. Gastroenterology. 1989 Feb;96(2 Pt 1):503–509. doi: 10.1016/0016-5085(89)91577-1. [DOI] [PubMed] [Google Scholar]
- Kerlin P., Wong L., Harris B., Capra S. Rice flour, breath hydrogen, and malabsorption. Gastroenterology. 1984 Sep;87(3):578–585. [PubMed] [Google Scholar]
- Ladas S., Papanikos J., Arapakis G. Lactose malabsorption in Greek adults: correlation of small bowel transit time with the severity of lactose intolerance. Gut. 1982 Nov;23(11):968–973. doi: 10.1136/gut.23.11.968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitt M. D., Hirsh P., Fetzer C. A., Sheahan M., Levine A. S. H2 excretion after ingestion of complex carbohydrates. Gastroenterology. 1987 Feb;92(2):383–389. doi: 10.1016/0016-5085(87)90132-6. [DOI] [PubMed] [Google Scholar]
- McNeil N. I., Cummings J. H., James W. P. Short chain fatty acid absorption by the human large intestine. Gut. 1978 Sep;19(9):819–822. doi: 10.1136/gut.19.9.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriyoshi Y., Takeuchi T., Shiratori K., Watanabe S. Fecal isoamylase activity in patients with pancreatic diseases. Pancreas. 1991 Jan;6(1):70–76. doi: 10.1097/00006676-199101000-00010. [DOI] [PubMed] [Google Scholar]
- Read N. W., Al-Janabi M. N., Bates T. E., Holgate A. M., Cann P. A., Kinsman R. I., McFarlane A., Brown C. Interpretation of the breath hydrogen profile obtained after ingesting a solid meal containing unabsorbable carbohydrate. Gut. 1985 Aug;26(8):834–842. doi: 10.1136/gut.26.8.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadesse K., Eastwood M. Breath-hydrogen test and smoking. Lancet. 1977 Jul 9;2(8028):91–91. doi: 10.1016/s0140-6736(77)90098-8. [DOI] [PubMed] [Google Scholar]
- Welsh J. D., Payne D. L., Manion C., Morrison R. D., Nichols M. A. Interval sampling of breath hydrogen (H2) as an index of lactose malabsorption in lactase-deficient subjects. Dig Dis Sci. 1981 Aug;26(8):681–685. doi: 10.1007/BF01316855. [DOI] [PubMed] [Google Scholar]


