Abstract
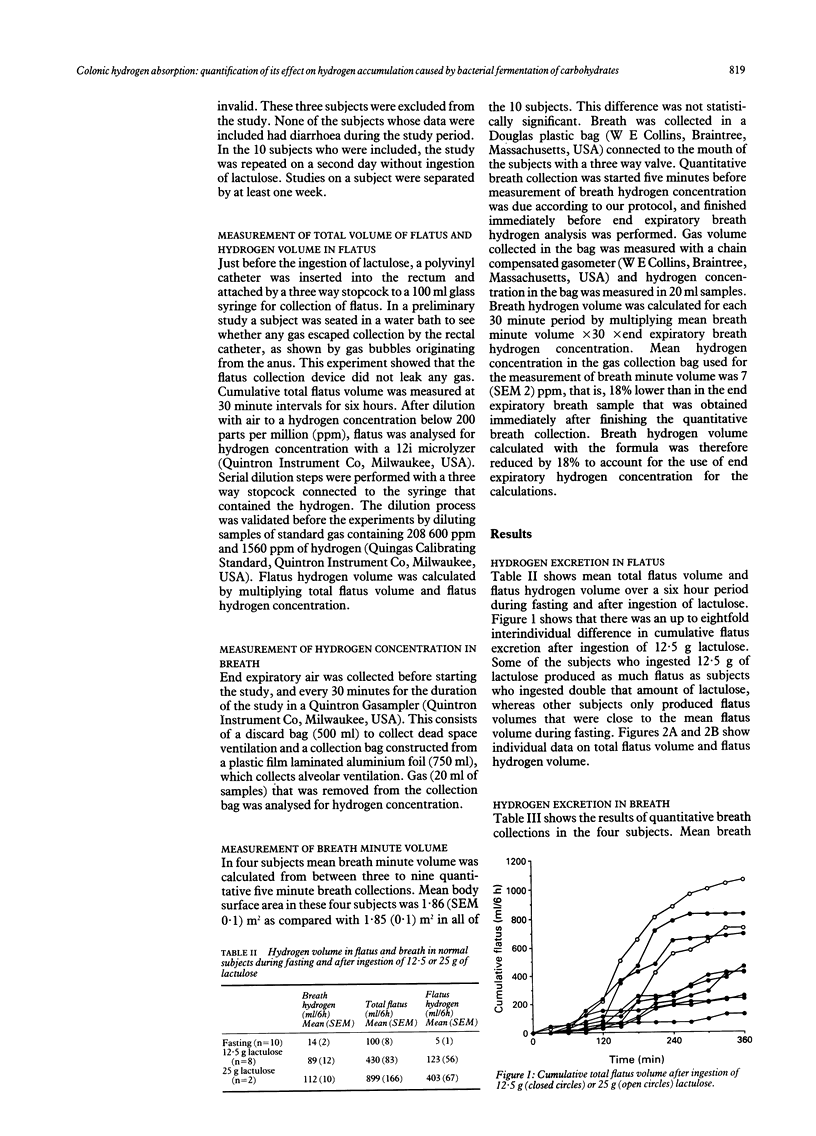
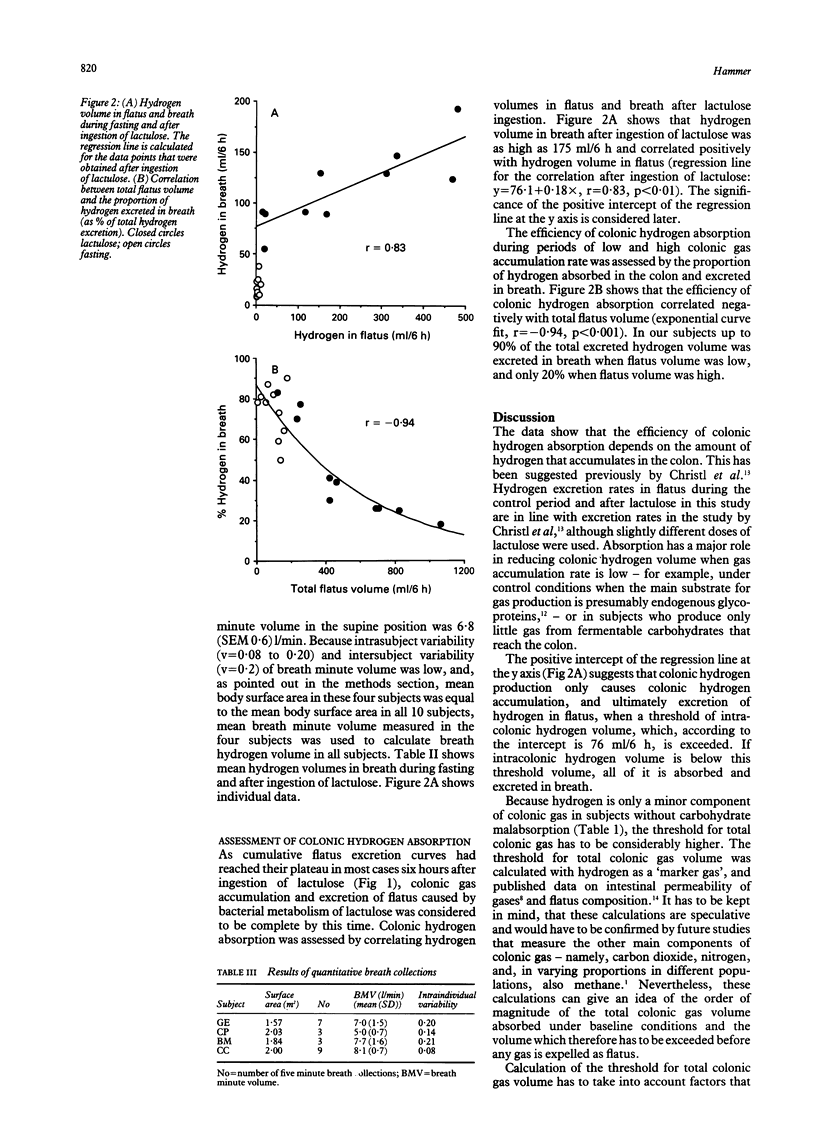
The aim of the study was to assess (quantitatively) colonic hydrogen absorption. Hydrogen volumes in flatus and breath were measured over periods of six hours in normal subjects during fasting and after ingestion of the non-absorbable carbohydrate lactulose to simulate the effect of fermentable dietary fibres. If less than 76 ml/6 h of hydrogen accumulated in the colon then all of it was absorbed, as suggested by the intercept of the regression line of the correlation between hydrogen volumes in flatus and breath after ingestion of lactulose. As total flatus volume increased, efficiency of colonic hydrogen absorption decreased from 90% to 20%. The positive correlation between hydrogen volumes of flatus and breath showed that the eightfold interindividual differences in flatus volume after ingestion of 12.5 g of lactulose were caused by differences in bacterial net gas production, not gas absorption. Differences in colonic gas emptying rate are the consequence rather than the cause of interindividual differences in flatus volume. In conclusion: (1) colonic hydrogen absorption is highly effective at low colonic hydrogen accumulation rates, but not at higher accumulation rates; (2) ineffective colonic gas absorption is the consequence and not the cause of high colonic gas accumulation rate after ingestion of non-absorbable carbohydrates; and (3) future therapeutic approaches to the large interindividual variability in colonic gas accumulation after ingestion of poorly absorbable fermentable carbohydrates, such as some kinds of dietary fibres, should be directed towards altering colonic bacterial metabolism.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bjørneklett A., Jenssen E. Measurement of pulmonary hydrogen (H2) and H2 diffusion from the small bowel and the colon. Scand J Gastroenterol. 1980;15(7):817–823. doi: 10.3109/00365528009181536. [DOI] [PubMed] [Google Scholar]
- Bown R. L., Gibson J. A., Sladen G. E., Hicks B., Dawson A. M. Effects of lactulose and other laxatives on ileal and colonic pH as measured by a radiotelemetry device. Gut. 1974 Dec;15(12):999–1004. doi: 10.1136/gut.15.12.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christl S. U., Murgatroyd P. R., Gibson G. R., Cummings J. H. Production, metabolism, and excretion of hydrogen in the large intestine. Gastroenterology. 1992 Apr;102(4 Pt 1):1269–1277. [PubMed] [Google Scholar]
- Connor W. E. Dietary fiber--nostrum or critical nutrient? N Engl J Med. 1990 Jan 18;322(3):193–195. doi: 10.1056/NEJM199001183220309. [DOI] [PubMed] [Google Scholar]
- Cummings J. H., Stephen A. M. The role of dietary fibre in the human colon. Can Med Assoc J. 1980 Dec 6;123(11):1109–1114. [PMC free article] [PubMed] [Google Scholar]
- Fleming S. E., Marthinsen D., Kuhnlein H. Colonic function and fermentation in men consuming high fiber diets. J Nutr. 1983 Dec;113(12):2535–2544. doi: 10.1093/jn/113.12.2535. [DOI] [PubMed] [Google Scholar]
- Flourié B., Etanchaud F., Florent C., Pellier P., Bouhnik Y., Rambaud J. C. Comparative study of hydrogen and methane production in the human colon using caecal and faecal homogenates. Gut. 1990 Jun;31(6):684–685. doi: 10.1136/gut.31.6.684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson G. R., Cummings J. H., Macfarlane G. T., Allison C., Segal I., Vorster H. H., Walker A. R. Alternative pathways for hydrogen disposal during fermentation in the human colon. Gut. 1990 Jun;31(6):679–683. doi: 10.1136/gut.31.6.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer H. F., Fine K. D., Santa Ana C. A., Porter J. L., Schiller L. R., Fordtran J. S. Carbohydrate malabsorption. Its measurement and its contribution to diarrhea. J Clin Invest. 1990 Dec;86(6):1936–1944. doi: 10.1172/JCI114927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer H. F., Santa Ana C. A., Schiller L. R., Fordtran J. S. Studies of osmotic diarrhea induced in normal subjects by ingestion of polyethylene glycol and lactulose. J Clin Invest. 1989 Oct;84(4):1056–1062. doi: 10.1172/JCI114267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menzies I. S., Laker M. F., Pounder R., Bull J., Heyer S., Wheeler P. G., Creamer B. Abnormal intestinal permeability to sugars in villous atrophy. Lancet. 1979 Nov 24;2(8152):1107–1109. doi: 10.1016/s0140-6736(79)92507-8. [DOI] [PubMed] [Google Scholar]
- Miller T. L., Wolin M. J. Fermentations by saccharolytic intestinal bacteria. Am J Clin Nutr. 1979 Jan;32(1):164–172. doi: 10.1093/ajcn/32.1.164. [DOI] [PubMed] [Google Scholar]
- Perman J. A., Modler S. Glycoproteins as substrates for production of hydrogen and methane by colonic bacterial flora. Gastroenterology. 1982 Aug;83(2):388–393. [PubMed] [Google Scholar]
- Swain J. F., Rouse I. L., Curley C. B., Sacks F. M. Comparison of the effects of oat bran and low-fiber wheat on serum lipoprotein levels and blood pressure. N Engl J Med. 1990 Jan 18;322(3):147–152. doi: 10.1056/NEJM199001183220302. [DOI] [PubMed] [Google Scholar]


