Abstract
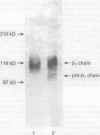
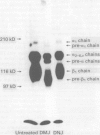
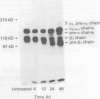
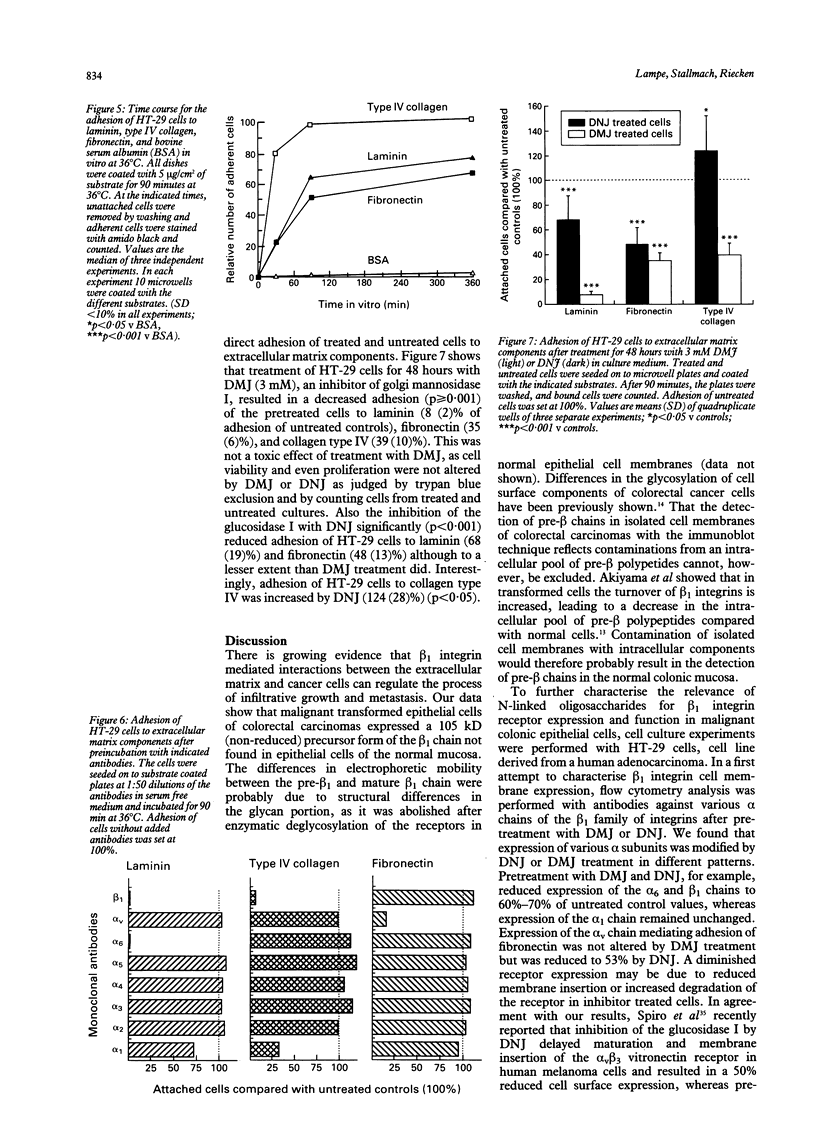
The integrin mediated interactions between tumour cells and the surrounding extracellular matrix are thought to play crucial parts in the complex process of invasion and metastasis. It has been previously shown that the expression of integrins is differently diminished in a chain-specific manner in human colorectal cancer. To further characterise the integrins still expressed in colorectal carcinomas, immunoblots with monoclonal antibodies against the beta 1 integrin subunit have been performed. In isolated cell membranes of colorectal cancers a second smaller beta 1 chain (105 kD non-reduced) was found as well as the mature beta 1 chain (116 kD non-reduced) present in normal mucosa of the colon. This smaller beta 1 chain comigrates with the diminished glycosylated precursor form of the beta 1 chain. The role of N-glycosylation for the function and expression of integrins in vitro was therefore investigated, with deoxymannojirimycin (DMJ) and deoxynojirimycin (DNJ) as specific inhibitors of N-glycan processing. Pretreatment of human colon adenocarcinoma derived HT-29 cells with DMJ resulted in an expression of the 105 kD beta 1 precursor chain and of smaller forms of the alpha 1, alpha 3, alpha 6, and alpha v integrin subunits in a time and dose dependent manner. HT-29 cells treated with DMJ adhered poorly to laminin (8% of untreated controls), collagen type IV (40%), and fibronectin (35%). Pretreatment of the cells with DNJ did not alter the molecular weight of the integrin chains expressed and reduced HT-29 adhesion to laminin and fibronectin only to 68% and 49% respectively. Adhesion to collagen type IV was increased to 124% by DNJ. These results show that N-glycan processing is essential for the function and expression of integrins in human colorectal cancer cells. An altered glycosylation of these adhesion receptors may contribute to a more invasive or metastatic phenotype in colorectal cancer.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams J. C., Watt F. M. Changes in keratinocyte adhesion during terminal differentiation: reduction in fibronectin binding precedes alpha 5 beta 1 integrin loss from the cell surface. Cell. 1990 Oct 19;63(2):425–435. doi: 10.1016/0092-8674(90)90175-e. [DOI] [PubMed] [Google Scholar]
- Akiyama S. K., Yamada S. S., Yamada K. M. Analysis of the role of glycosylation of the human fibronectin receptor. J Biol Chem. 1989 Oct 25;264(30):18011–18018. [PubMed] [Google Scholar]
- Albelda S. M., Buck C. A. Integrins and other cell adhesion molecules. FASEB J. 1990 Aug;4(11):2868–2880. [PubMed] [Google Scholar]
- Breitfeld P. P., Rup D., Schwartz A. L. Influence of the N-linked oligosaccharides on the biosynthesis, intracellular routing, and function of the human asialoglycoprotein receptor. J Biol Chem. 1984 Aug 25;259(16):10414–10421. [PubMed] [Google Scholar]
- Duronio V., Jacobs S., Romero P. A., Herscovics A. Effects of inhibitors of N-linked oligosaccharide processing on the biosynthesis and function of insulin and insulin-like growth factor-I receptors. J Biol Chem. 1988 Apr 15;263(11):5436–5445. [PubMed] [Google Scholar]
- Garcia M., Seigner C., Bastid C., Choux R., Payan M. J., Reggio H. Carcinoembryonic antigen has a different molecular weight in normal colon and in cancer cells due to N-glycosylation differences. Cancer Res. 1991 Oct 15;51(20):5679–5686. [PubMed] [Google Scholar]
- Hemler M. E., Huang C., Schwarz L. The VLA protein family. Characterization of five distinct cell surface heterodimers each with a common 130,000 molecular weight beta subunit. J Biol Chem. 1987 Mar 5;262(7):3300–3309. [PubMed] [Google Scholar]
- Hemler M. E., Huang C., Takada Y., Schwarz L., Strominger J. L., Clabby M. L. Characterization of the cell surface heterodimer VLA-4 and related peptides. J Biol Chem. 1987 Aug 25;262(24):11478–11485. [PubMed] [Google Scholar]
- Hemler M. E., Jacobson J. G., Strominger J. L. Biochemical characterization of VLA-1 and VLA-2. Cell surface heterodimers on activated T cells. J Biol Chem. 1985 Dec 5;260(28):15246–15252. [PubMed] [Google Scholar]
- Hessle H., Sakai L. Y., Hollister D. W., Burgeson R. E., Engvall E. Basement membrane diversity detected by monoclonal antibodies. Differentiation. 1984;26(1):49–54. doi: 10.1111/j.1432-0436.1984.tb01372.x. [DOI] [PubMed] [Google Scholar]
- Hildreth J. E., Gotch F. M., Hildreth P. D., McMichael A. J. A human lymphocyte-associated antigen involved in cell-mediated lympholysis. Eur J Immunol. 1983 Mar;13(3):202–208. doi: 10.1002/eji.1830130305. [DOI] [PubMed] [Google Scholar]
- Humphries M. J., Matsumoto K., White S. L., Olden K. Oligosaccharide modification by swainsonine treatment inhibits pulmonary colonization by B16-F10 murine melanoma cells. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1752–1756. doi: 10.1073/pnas.83.6.1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes R. O. Integrins: a family of cell surface receptors. Cell. 1987 Feb 27;48(4):549–554. doi: 10.1016/0092-8674(87)90233-9. [DOI] [PubMed] [Google Scholar]
- Hynes R. O., Wyke J. A. Alterations in surface proteins in chicken cells transformed by temperature-sensitive mutants of Rous sarcoma virus. Virology. 1975 Apr;64(2):492–504. doi: 10.1016/0042-6822(75)90126-9. [DOI] [PubMed] [Google Scholar]
- Koretz K., Schlag P., Boumsell L., Möller P. Expression of VLA-alpha 2, VLA-alpha 6, and VLA-beta 1 chains in normal mucosa and adenomas of the colon, and in colon carcinomas and their liver metastases. Am J Pathol. 1991 Mar;138(3):741–750. [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Liotta L. A., Rao C. N., Barsky S. H. Tumor invasion and the extracellular matrix. Lab Invest. 1983 Dec;49(6):636–649. [PubMed] [Google Scholar]
- Maeda T., Balakrishnan K., Mehdi S. Q. A simple and rapid method for the preparation of plasma membranes. Biochim Biophys Acta. 1983 May 26;731(1):115–120. doi: 10.1016/0005-2736(83)90404-2. [DOI] [PubMed] [Google Scholar]
- Morhenn V. B., Schreiber A. B., Soriero O., McMillan W., Allison A. C. A monoclonal antibody against basal cells of human epidermis. Potential use in the diagnosis of cervical neoplasia. J Clin Invest. 1985 Nov;76(5):1978–1983. doi: 10.1172/JCI112197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto C., Letvin N. L., Boyd A. W., Hagan M., Brown H. M., Kornacki M. M., Schlossman S. F. The isolation and characterization of the human helper inducer T cell subset. J Immunol. 1985 Jun;134(6):3762–3769. [PubMed] [Google Scholar]
- Newton S. A., White S. L., Humphries M. J., Olden K. Swainsonine inhibition of spontaneous metastasis. J Natl Cancer Inst. 1989 Jul 5;81(13):1024–1028. doi: 10.1093/jnci/81.13.1024. [DOI] [PubMed] [Google Scholar]
- Ogier-Denis E., Trugnan G., Sapin C., Aubery M., Codogno P. Dual effect of 1-deoxymannojirimycin on the mannose uptake and on the N-glycan processing of the human colon cancer cell line HT-29. J Biol Chem. 1990 Apr 5;265(10):5366–5369. [PubMed] [Google Scholar]
- Olson T. S., Lane M. D. Post-translational acquisition of insulin binding activity by the insulin proreceptor. Correlation to recognition by autoimmune antibody. J Biol Chem. 1987 May 15;262(14):6816–6822. [PubMed] [Google Scholar]
- Pignatelli M., Smith M. E., Bodmer W. F. Low expression of collagen receptors in moderate and poorly differentiated colorectal adenocarcinomas. Br J Cancer. 1990 Apr;61(4):636–638. doi: 10.1038/bjc.1990.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulverer G., Beuth J., Ko H. L., Yassin A., Ohshima Y., Roszkowski K., Uhlenbruck G. Glycoprotein modifications of sarcoma L-1 tumor cells by tunicamycin, swainsonine, bromoconduritol or 1-desoxynojirimycin treatment inhibits their metastatic lung colonization in Balb/c-mice. J Cancer Res Clin Oncol. 1988;114(2):217–220. doi: 10.1007/BF00417842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruoslahti E. Integrins. J Clin Invest. 1991 Jan;87(1):1–5. doi: 10.1172/JCI114957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoso S., Kiefel V., Mueller-Eckhardt C. Immunochemical characterization of the new platelet alloantigen system Bra/Brb. Br J Haematol. 1989 Jun;72(2):191–198. doi: 10.1111/j.1365-2141.1989.tb07682.x. [DOI] [PubMed] [Google Scholar]
- Sonnenberg A., Hogervorst F., Osterop A., Veltman F. E. Identification and characterization of a novel antigen complex on mouse mammary tumor cells using a monoclonal antibody against platelet glycoprotein Ic. J Biol Chem. 1988 Oct 5;263(28):14030–14038. [PubMed] [Google Scholar]
- Sonnenberg A., Linders C. J., Modderman P. W., Damsky C. H., Aumailley M., Timpl R. Integrin recognition of different cell-binding fragments of laminin (P1, E3, E8) and evidence that alpha 6 beta 1 but not alpha 6 beta 4 functions as a major receptor for fragment E8. J Cell Biol. 1990 Jun;110(6):2145–2155. doi: 10.1083/jcb.110.6.2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnenberg A., Modderman P. W., Hogervorst F. Laminin receptor on platelets is the integrin VLA-6. Nature. 1988 Dec 1;336(6198):487–489. doi: 10.1038/336487a0. [DOI] [PubMed] [Google Scholar]
- Spiro R. C., Laufer D. M., Perry S. K., Harper J. R. Effect of inhibitors of N-linked oligosaccharide processing on the cell surface expression of a melanoma integrin. J Cell Biochem. 1989 Sep;41(1):37–45. doi: 10.1002/jcb.240410105. [DOI] [PubMed] [Google Scholar]
- Stallmach A., Schuppan D., Dax J., Hanski C., Riecken E. O. Identification of laminin binding proteins in cell membranes of a human colon adenocarcinoma cell line. Gut. 1990 Jan;31(1):70–76. doi: 10.1136/gut.31.1.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stallmach A., von Lampe B., Matthes H., Bornhöft G., Riecken E. O. Diminished expression of integrin adhesion molecules on human colonic epithelial cells during the benign to malign tumour transformation. Gut. 1992 Mar;33(3):342–346. doi: 10.1136/gut.33.3.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symington B. E., Symington F. W., Rohrschneider L. R. Phorbol ester induces increased expression, altered glycosylation, and reduced adhesion of K562 erythroleukemia cell fibronectin receptors. J Biol Chem. 1989 Aug 5;264(22):13258–13266. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trudel G. C., Holland P. C. The glycoprotein-processing inhibitors bromoconduritol and N-methyl-1-deoxynojirimycin alter the adhesion phenotype of skeletal myoblasts. Biochem Cell Biol. 1990 Dec;68(12):1411–1418. doi: 10.1139/o90-204. [DOI] [PubMed] [Google Scholar]
- Vogel B. E., Tarone G., Giancotti F. G., Gailit J., Ruoslahti E. A novel fibronectin receptor with an unexpected subunit composition (alpha v beta 1). J Biol Chem. 1990 Apr 15;265(11):5934–5937. [PubMed] [Google Scholar]
- Wayner E. A., Carter W. G., Piotrowicz R. S., Kunicki T. J. The function of multiple extracellular matrix receptors in mediating cell adhesion to extracellular matrix: preparation of monoclonal antibodies to the fibronectin receptor that specifically inhibit cell adhesion to fibronectin and react with platelet glycoproteins Ic-IIa. J Cell Biol. 1988 Nov;107(5):1881–1891. doi: 10.1083/jcb.107.5.1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries J. E., Keizer G. D., te Velde A. A., Voordouw A., Ruiter D., Rümke P., Spits H., Figdor C. G. Characterization of melanoma-associated surface antigens involved in the adhesion and motility of human melanoma cells. Int J Cancer. 1986 Oct 15;38(4):465–473. doi: 10.1002/ijc.2910380403. [DOI] [PubMed] [Google Scholar]